ISSN: 0973-7510
E-ISSN: 2581-690X
Bacterial leaf blight (BLB), caused by Xanthomonas oryzae pv. oryzae (Xoo), is a major threat to rice cultivation globally, including in Indonesia. Conventional control methods, such as the use of resistant varieties, copper-based compounds, pesticides, and antibiotics, have limitations, including environmental concerns and the development of resistance. Bacteriophages offer a promising alternative as biological control agents due to their high specificity to target pathogens without adverse effects on plants or humans. This study aimed to isolate and characterize the bacteriophages infecting X. oryzae pv. oryzae. Phages were isolated from samples including infected rice leaves, soil from rice fields, and irrigation water in Malang. A bacteriophage designated Xoo-Tp1 was successfully isolated from soil and shown to infect Xanthomonas oryzae pv. oryzae. Xoo-Tp1 exhibited stability at pH 6 to 8, with the highest titer observed at pH 7.0, reaching 4.76 log10 PFU/mL (approximately 5.75 × 104 PFU/mL). No phage activity was detected at pH 5 and pH 9. Temperature stability was observed at 4, 30, 40, and 60 °C, but phage infectivity was no longer detected at 90 °C. Transmission Electron Microscopy (TEM) revealed that phage Xoo-Tp1 possesses an isometric head measuring 171.8 nm in diameter and a tail 94.6 nm in length, consistent with the morphological characteristics of the Myoviridae family. Based on its large size, phage Xoo-Tp1 is suspected to belong to the jumbo phage group. In liquid culture, phage Xoo-Tp1 inhibited the growth of Xanthomonas oryzae pv. oryzae. The 7:1 phage-to-host ratio treatment resulted in the most significant reduction of bacterial cell concentration (44.23%) compared to the untreated control. This study demonstrated that isolated phage Xoo-Tp1 has the potential to be used as a biological control agent in the control of BLB in rice. Further studies are recommended to evaluate its efficacy under field conditions, including phage formulation stability, delivery methods, and host range assessment.
Bacterial Leaf Blight, Characterization, Isolation, Phages, Xanthomonas oryzae
Rice is an important commodity for the whole world, with approximately 90% of total global production and consumption contributing immensely to nutritional and food security.1 Today, rice is grown in hundreds of countries and produces 715 million tons of rice per year. The Asian region is the highest rice producer and consumer in the world. Indonesia is the 4th largest rice-producing country in the world.2 However, rice production in Indonesia continues to decline from 81.2 million tons in 2017 to 53.98 million tons in 2023.3 This decline in production can be caused by a variety of factors, one of which is caused by plant pathogenic bacteria. One of the bacteria that can attack the plant is Xanthomonas oryzae pv. oryzae (Xoo), which is a pathogenic bacterium causing bacterial leaf blight (BLB) disease in rice crops that is often found in rice-producing countries, including Indonesia. The disease caused by Xoo is considered very harmful because it can attack plants from the seed phase to the adult plant and can even reduce the harvest by 50%-70%.2,4
The success of agricultural production is not only determined by the quality of seeds, but disease management strategies also play an important role. Conventional strategies for managing BLB rely on the use of high-quality seedlings, physical interventions such as crop rotation and removal of infected crops, as well as chemical treatment involving the extensive use of pesticides and antibiotics.5 However, these measures were deemed unsuccessful in preventing the spread of Xanthomonas spp. infection. Copper-based pesticides and antibiotics have long been used in plant disease management strategies. However, as a consequence of antibiotic or copper excessive usage, resistant strains of these chemicals have been frequently reported in numerous plant-pathogenic bacteria, raising significant concerns. Moreover, the accumulation of these chemicals in the environment can affect human health through the food chain.6,7 Streptomycin has been tested for bacterial illness control since the 1950s, but it has led to a new problem, which can lead to the development of antibiotic resistance. There was a report of Erwina, Pseudomonas, and Xanthomonas becoming resistant after excessive use of streptomycin.7 The pathogen’s resistance to copper-based fungicides poses another problem for treatment, and it has been discovered in several kinds of plant disease pathogens, including Xanthomonas spp. and Pseudomonas.8 Long-term use of chemical compounds can also damage the ecosystems around plants. Pesticides also leave residues that can disturb microbial metabolism, harm other microorganisms, and reduce microbial activity, which leads to a reduction in plant nutrient availability. Additionally, pesticide residues can also enter the food chain and harm both human and animal health.9
Efforts to promote sustainable agriculture and avoid chemical pesticides have led to the development of newer control methods. Biocontrol presents a viable alternative for managing plant diseases.7,10 The interest in biocontrol agents is substantial due to their lower toxicity compared to chemical pesticides and antibiotics. Most studies on biocontrol focus on utilizing bacteria, fungi, and their metabolic products to combat plant pathogens.10 Nevertheless, there is also significant research exploring the potential of using bacteriophages as biocontrol agents.10-12
The use of bacteriophages as biocontrol agents is considered to have great potential to replace existing chemical compounds. Bacteriophages are a type of virus that only infects bacteria and replicates within the bacterial host. Their specificity, rapid growth, capability to overcome resistance, omnipresence, and ease of biosynthesis are just some of their unique and appealing characteristics that make them an ideal biocontrol agent.12,13 Phages are self-replicating; they reproduce only as long as their host bacterium is present in the environment, but at the same time, they are self-limiting; without host bacteria, phages quickly degrade in the absence of a host. Their extremely host-specific ability makes them harmless to eukaryotic cells and other beneficial microorganisms found in the typical microbiota. As a result, they have minimal effects on the environmental ecosystems.11,14,15
The potential of phages to control pathogenic bacteria has been gaining popularity in recent years due to reports demonstrating their ability to effectively control the bacterial host. This approach has led to their widespread application across various fields, including agriculture, food safety, human and veterinary medicine, also wastewater treatment.13,16,17 Bacteriophages have demonstrated the ability to inhibit a broad spectrum of Xanthomonas-caused plant diseases, such as citrus cancer by X. citri,18 onion leaf blight by X. axonopodis pv. allii,19 and black rot by X. campestris pv. campestris.20 A bacteriophage-containing pesticide called AgriphageTM from OmniLytics has been certified by the US Environmental Protection Agency for the biological control of plant bacterial diseases, including bacterial leaf spot (BLS) on peppers and tomatoes caused by Xanthomonas campestris pv. vesicatoria.14 This indicates that plant diseases caused by bacteria, particularly those caused by Xanthomonas, might be controlled by bacteriophages. In order to develop bacteriophages as an efficient biopesticide for the management of bacterial blight disease in rice, an attempt has been made in the current work to assess their potential as biological control agents against Xanthomonas oryzae pv. oryzae.
Materials
The materials used in this study include samples from rice leaves that have symptoms of blight, rice field irrigation water, and rice field soil taken from Randigading Village, Tajinan District, Malang Regency (Coordinates: 8.052977, 112.675209), and the bacterium Xanthomonas oryzae pv. oryze (InaCC B16) obtained from the Indonesian Culture Collection (InaCC) BRIN, Indonesia. Chemicals used in this study include LB media, agar media, peptone water, SM buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, 8 mM MgSO4.7H2O, and 0.02% gelatin), HCl, NaOH, PEG 6,000, chloroform, and glycerol.
While the tools used in this study include Laminar Air Flow, TEM (JEOL 1010,
80.0 KV), autoclave, incubator (Binder), shaker waterbath (Memmert), centrifuge (Thermo Scientific), micro centrifuge (Benchmark), pH meter, spectrophotometer (Thermo Scientific), Viral Gene-spin™ Viral DNA/RNA Extraction Kit, vortex (Thermo Scientific), analytical scales (Scout Pro), electric stove (Maspion), micropipette (Thermo Scientific).
Bacteriophages isolation and purification
Bacteriophages are isolated based on the method in a previous study with modifications.21 Samples included infected rice leaves, soil, and irrigation water collected from paddy fields in Malang. For leaf samples, infected tissue was cut into small pieces and suspended in 5 mL of 1% (w/v) peptone water, while 1 g of soil was suspended in 5 mL of SM buffer (50 mM Tris-HCl, 100 mM NaCl, 8 mM MgSO4, pH 7.5). Irrigation water samples (5 mL) were used directly. Leaf and soil suspensions were incubated at 27 °C with shaking at 120 rpm for 2 h to release phages. All samples were then centrifuged at 500x g for 30 min, and the supernatants were collected for phage detection.
To check for the presence of bacteriophages, the agar plaque assay method was employed using LB (Luria-Bertani) medium. First, Xanthomonas oryzae pv. oryzae (Xoo) bacterial culture was prepared by inoculating 1 mL of a 24 hour old bacterial culture into 10 mL of fresh LB, followed by incubation for 2 hours at 27 °C to reach the exponential phase. Next, two types of LB agar were prepared: hard LB agar (1.5%) and soft LB agar (0.7%). For the assay, a mixture of 100 µL of Xoo culture and 100 µL of test sample was added to 5 mL of soft agar. The mixture was then poured as an overlay onto the hard LB agar. Plates were then incubated at 27 °C for 18-24 hours. The appearance of clear plaques indicated the presence of bacteriophages capable of infecting Xoo.
After plaque formation, individual plaques differing in size and clarity were carefully picked using a sterile Pasteur pipette tip and transferred into 1 mL of SM buffer. Subsequently, 100 µL of chloroform was added, and the suspension was incubated at 4 °C for 24 hours. This single-plaque isolation procedure was repeated three times to obtain a pure bacteriophage culture. For long-term storage, 50% glycerol and 10% chloroform were added to the purified phage suspension in SM buffer, and the bacteriophage stock was stored at -80 °C.
Morphological characterization of bacteriophage by Transmission Electron Microscopy (TEM)
This procedure was adapted from a previously reported method, with modifications.22 The PEG-purified bacteriophage suspension was centrifuged at 25,000x g for 60 min. The suspension was then washed twice with ammonium acetate (0.1 mol/L, pH 7). The pellet was removed and deposited on a copper grid with Formvar film-coated carbon. Samples were stained using 2% (w/v) uranyl acetate (pH 4.2), followed by observation under the microscope.
pH stability test
With certain adjustments, this method was carried out based on the previous study.23 The stability test at various pHs is done by incubating bacteriophages in SM buffer with different pHs in the range of pH 5-9 for 1 hour at 27 °C. The agar plaque assay method was used to determine the concentration of bacteriophages, expressed as plaque-forming units per milliliter (PFU/mL).
Temperature stability test
To test the temperature stability of bacteriophages, this method was conducted based on the methodology described in a previous study with some adjustments.24 Mixture of X. oryzae pv. oryzae (Xoo) and Tp1 phage were incubated at various temperatures using a water bath at 4 °C, 30 °C, 40 °C, 60 °C, and 90 °C. Bacteriophage lytic ability was tested by measuring the absorbance value of the lysed host bacteria. The absorbance of the samples was measured every hour for 7 hours and once more at 24 hours.
Phage lytic activity
The procedure followed a previously described method with modifications.25 Inhibition activity based on OD was carried out to compare the control with the non-control. The control used only the bacterial culture, with no bacteriophage added, whereas the treatment involved a mixture of bacterial and bacteriophage isolates. In this assay, four samples were prepared with different ratios of bacteriophage Tp1 to X. oryzae pv. oryzae (Xoo) (1:5, 1:7, 5:1, and 7:1), and incubated at 27 °C. Xoo cell growth was monitored by measuring the absorbance at 600 nm every hour for 7 hours. The results of the four samples were then compared with the control.
Bacteriophages isolation
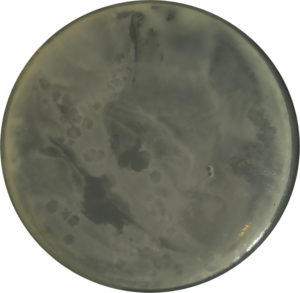
The bacteriophage isolation procedure aims to obtain bacteriophages that specifically infect Xoo. In the isolation stage, three samples were used, namely rice leaves attacked by Xoo, paddy field soil, and rice field’s irrigation water. The presence of phages was only visible in soil samples, while no plaque formation was observed in water and leaf samples. The isolate obtained was then named Tp1. The appearance of Tp1 plaques is shown in Figure 1.
Figure 1. Plaques formed by bacteriophage Tp1 on Xanthomonas oryzae pv. oryzae (Xoo) using the agar plaque assay on LB (Luria-Bertani), after 18 hours of incubation at 27 °C
Morphological characterization of bacteriophage by Transmission Electron Microscopy (TEM)
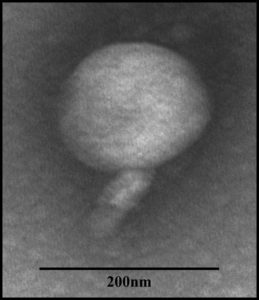
Bacteriophage Tp1 morphology was observed using TEM at 30,000x magnification. The results showed that the morphology of bacteriophage Tp1 head is isometric and has a short tail (Figure 2). Bacteriophage Tp1 has an isometric head with a diameter of 171.8 nm and a tail with a length of 94.6 nm.
Figure 2. Morphology of bacteriophage Tp1 observed under transmission electron microscopy (TEM) at 30,000 × magnification. The phage exhibits an isometric head and a short tail. The sample was negatively stained with 2% uranyl acetate, and a scale bar of 200 nm is included
Effect of pH on bacteriophage stability
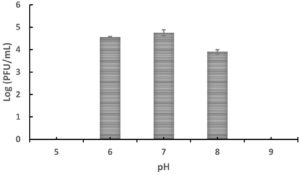
To determine the effect of pH on the stability and infectivity of bacteriophages, Tp1 was exposed to various pH levels. Plaques were observed at pH values between 6 and 8, whereas no plaques formed at pH 5 or 9 (Figure 3). These results suggest that phage Tp1 is pH-sensitive, maintaining infectivity only within a narrow pH range of 6-8, and becoming inactivated under more extreme acidic or alkaline conditions.
Figure 3. Stability of bacteriophage Tp1 at different pH levels after 1-hour incubation at 37 °C, measured by agar plaque assay (Log PFU/mL)
Effect of temperature on bacteriophage stability
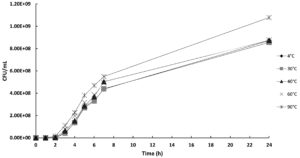
In this study, a turbidity test was used to assess the effect of temperature on the stability of phage Tp1. The result of the turbidity test was determined by measuring the absorbance of the bacterial samples. A high absorbance value indicates that the bacteriophage became inactive during the temperature treatment and was unable to infect the bacterial cells. On the other hand, low absorbance values indicate that the bacteriophage remained active during the temperature treatment and was able to infect and lyse the bacterial cells. Phage samples were exposed to different temperatures, 4, 30, 40, 60, and 90 °C. Figure 4 shows that increasing the temperature treatment resulted in higher absorbance values of the bacterial samples. At 4, 30, 40, and 60 °C, phage lytic activity was similar; however, at 90 °C, there was a decrease in lytic activity, which caused an increase in absorbance value.
Figure 4. The effect of temperature treatment on bacteriophage Tp1 activity against host bacteria, measured by an agar assay (CFU/mL)
Phage lytic activity (Turbidity Test)
The turbidity test was intended to observe and confirm the decrease in the number of bacterial cells that were lysed due to bacteriophage infection. The number of host bacterial cells will decrease when lysed by bacteriophages. The decrease in the number of host bacterial cells can be observed by measuring the absorbance value of the sample.
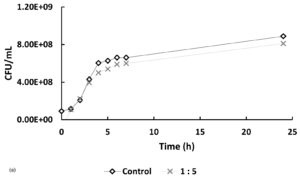
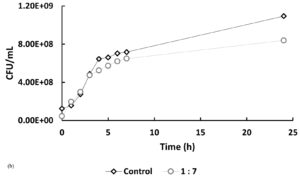
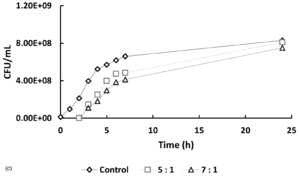
In Figure 5, at hour 0, the initial bacterial concentrations in the 1:5 and 1:7 treatments (Figure 5a and 5b) were slightly higher than those in the 5:1 and 7:1 treatments (Figure 5c), likely due to their higher host-to-phage ratios. Over time, bacterial counts in all phage-treated samples decreased compared to the controls, indicating phage-induced lysis. The 1:5 and 1:7 treatments showed average reductions of 10.92% and 20.73%, respectively, in bacterial numbers, while the 5:1 and 7:1 treatments exhibited more pronounced inhibitory effects, with reductions of 42.62% and 44.23%. If no lytic activity had occurred, bacterial counts in treated samples would have remained similar to or higher than those in the controls. These findings indicate that phage treatment is effective across all tested MOIs, with significantly enhanced lytic activity observed at higher phage-to-host ratios (5:1 and 7:1).
Figure 5. Effect of different multiplicities of infection (MOI) on the growth of Xanthomonas oryzae pv. oryzae (CFU/mL) over 24 hours compared to the untreated control. The bacteriophage Tp1-to-host cell ratios tested were: (a) 1:5, (b) 1:7, (c) 5:1 & 7:1
*Control treatment shows Xoo culture in liquid media without the addition of bacteriophage
In this study, Tp1 phage was isolated from paddy field soil taken from Ngembal Village, Tajinan District, Malang Regency. The presence of the phage was seen in the formation of the plaque. Plaque itself is a clear zone in bacterial cultures that grow on a bacterial lawn. The clear zone is formed due to bacteriophage activity that lyses bacteria around that area. The mechanism of plaque formation is initiated by a phage that infects and lyses one host bacterial cell. New phages that come out of the lysed bacterial cell will then infect neighboring bacteria. Eventually, after enough bacterial cells were killed, a clear area of cell-free agar could be observed.26
Morphology observation using TEM showed Tp1 has an isometric head and a short tail (Figure 2). Based on the short tail and shape of the head, Tp1 can be classified into the Myoviridae family. One of the prominent characteristics of the Myoviridae family is an icosahedral head and a thick tail.27 However, genome analysis is still needed to confirm that phage Tp1 belongs to the Myoviridae family. Tp1 is a bacteriophage with a larger capsid size when compared to previous Xoo-specific phages. Several previous studies that isolated Myoviridae phages obtained bacteriophages with an average head diameter of around 51.37 nm to 65.82 nm.4,5,21,28 Whereas in this study, the head diameter of Tp1 is 171.8 nm. Based on its large size, it is suspected that Tp1 belongs to the jumbo phage group.
Bacteriophages with tails that have genome sizes that exceed 200 kb and large virion sizes are called jumbo phages.29 In terms of capsid size, according to data compiled in a previous study, the size of the capsid that is included in the jumbo phage ranges from 115 to 160 nm.30,31 Based on virion size, Tp1 isolate has a larger capsid size than the biggest known phage, Bacillus megaterium phage G, which has a capsid size of 160 nm in length.31 However, to determine whether the Tp1 isolate qualifies as a jumbo phage, its genome size must be measured, as one of the defining criteria for jumbo phages is a genome larger than 200 kilobase pairs (kbp). In a previous study, Xoo–specific jumbo phage, Xoo-sp13 and Xoo-sp14, were found to consist of a linear double-stranded DNA molecule with a length of 309,023 and 232,104 bp, respectively.30,32
The large size of the virion has disadvantages, such as the small number of virions produced. For example, the bacterium Bacillus megaterium, which is the host of bacteriophage G, can only hold 30 virions in one cell.30 It is also more difficult for jumbo phages to infect host bacteria due to the huge virion size, which limits their ability to move on semisolid substrates.27
Environmental conditions with inappropriate pH and temperature can inhibit bacteriophage growth.26 Therefore, a pH stability test was carried out to determine the stability of bacteriophages at different pH values. The result in this study showed that plaque is observed at pH 6 to 8, but no plaque appears at pH 5 or 9 (Figure 3). Results obtained in this study have similarities with several studies that have been previously conducted on pH stability tests on Xoo phages. Phages XooX1IDN and XooX2IDN obtained in a previous study also have optimum stability at pH 6-8.4 Another study that also conducted pH tests on Xoo–phages ϕXOF4 and NR08 obtained similar results.14,33 However, in those studies, the bacteriophage was still viable in pH 4 and pH 9, although it was drastically reduced. Unlike the bacteriophage Tp1, which can only survive at pH 6-8.
For proper handling of the preservation, usage, and application of the phage during phage therapy, it is important to examine the effect of different pH values on the viability of the isolated phages. pH plays an important role in the success of phage therapy in agricultural soils, where acidic and alkaline pH dynamics can change over time.10,11,34 Numerous studies conducted in both field and laboratory environments have shown that exposure to high and low pH levels can easily inactivate bacteriophages because pH can damage their structural elements, cause phage aggregate formation, and inhibit the phage attachment process.18,33,35
In this study, the turbidity test was used to determine the effect of temperature on phage TP1 stability. The result of the turbidity test is determined by the absorbance value of the bacterial sample. If the absorbance value of the bacterium is high, it means that the bacteriophage becomes inactive during the temperature treatment and is unable to infect the bacterial sample. On the other hand, low absorbance values indicate the presence of an active bacteriophage that can infect bacteria during the temperature treatment.
Based on Figure 4, it can be concluded that bacteriophage Tp1 has good temperature stability. If Tp1 is indeed a jumbo phage, this is the main reason for the phage’s high temperature stability. Jumbo phage is more resilient and stable when exposed to high temperatures. Jumbo phage has a large genome (>200 kb) compared to other phages, which enables it to encode proteins that can help repair its DNA. This ability makes jumbo phage more resistant and stable to exposure to extreme temperatures.8 In addition to being able to repair DNA, the large genome of the jumbo phage is thought to be able to code for proteins that can withstand extreme temperatures. The size of the capsid is another important component that contributes to the resilience of jumbo phages. It has been suggested that the jumbo phage’s large, thick capsid size offers better protection against exposure to high temperatures.36
In recent years, phages have been an attractive alternative for controlling pathogenic bacteria through the lytic replication cycle.12,13 Therefore, it is necessary to perform a lytic activity test to determine the ability of phages to inhibit their hosts. In Figure 5, at hour 0, the bacterial counts in the 5:1, 7:1, and control groups were similar, while the 1:5 and 1:7 samples showed slightly higher initial bacterial concentrations. This difference is likely due to the higher host-to-phage ratios in these samples. Overall, the data indicate that the phage-treated samples had lower bacterial counts compared to the controls. However, the reductions observed in the 1:5 and 1:7 samples were not notably different from the control, suggesting a less pronounced lytic effect at these ratios. If it is assumed that there is no lytic activity, then the number of cells in the sample should be the same as the control or even exceed the control. However, the graphs show that the samples have a lower cell number compared to the control, especially in samples 5:1 and 7:1, which showed a significant difference in cell number when compared to the control.
Based on the data, the phages demonstrated the ability to infect Xanthomonas oryzae pv. oryzae (Xoo), but were not able to eliminate the host population. Instead, they primarily inhibited bacterial growth. This partial effectiveness may be attributed to several factors, including suboptimal preservation or storage conditions, insufficient phage concentration, reduced lytic activity, development of bacterial resistance, or a low burst size of the phage. Virions with large capsid sizes often exhibit lower burst size capabilities. This is likely due to the fact that larger virions occupy more physical space within the host cell during replication, thereby limiting the number of progeny that can be produced per cycle. Additionally, the synthesis of larger virions requires greater amounts of resources and energy, which are derived from the host bacterium. As a result, the metabolic burden placed on the host restricts the efficiency of phage assembly and ultimately reduces the overall burst size. Bacterial hosts that are unable to provide sufficient resources and energy for the phage to replicate result in low phage production.37
A low burst size limits the number of virions produced per infected host cell, thereby reducing the overall phage replication rate and its capacity to effectively control bacterial populations. This reduced infectivity is reflected in Figure 5, where the host bacterial cell count continues to increase despite phage treatment, indicating that the phage was unable to fully suppress bacterial growth. To effectively reduce the increasing bacterial population, a higher concentration of bacteriophage is required. A greater phage concentration increases the likelihood of successful infection and subsequent lysis of host cells, as more phage particles are available to encounter and infect the bacteria. This is supported by the results shown in Figure 5, where treatments with higher phage-to-host ratios (e.g., 5:1 and 7:1) demonstrated a greater inhibitory effect on bacterial growth compared to treatments with lower ratios.
A bacteriophage specific to Xanthomonas oryzae pv. oryzae (Xoo) was successfully isolated from Xoo-infected rice leaves, irrigation water, and soil samples. In this study, bacteriophage Tp1 was obtained from soil and further characterized. Tp1 exhibited good stability at pH levels 6 to 8 and maintained infectivity at temperatures of 4 °C,
30 °C, 40 °C, and 60 °C, but was inactivated at 90 °C. Transmission electron microscopy revealed that Tp1 possesses an isometric capsid measuring 171.8 nm in diameter and a short tail measuring 94.6 nm, consistent with the morphological characteristics of the Myoviridae family.
Functionally, Tp1 demonstrated the ability to inhibit the growth of Xoo in liquid culture, although it did not eliminate the host. These findings suggest that Tp1 holds potential as a biological control agent against bacterial leaf blight in rice. However, further studies are required to optimize its efficacy, particularly regarding phage concentration, application methods, and environmental stability under field conditions.
ACKNOWLEDGMENTS
The authors would like to thank Universitas Brawijaya, Malang, Indonesia, for the financial support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
AKW conceptualized the study and applied methodology. AKW performed funding acquisition. RAF and FRR performed investigation and data curation. AKW, RAF and FRR performed data analysis. KF and AKW performed supervision. AKW and RAF wrote the manuscript. HT, AKW, KF and FNEP reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This work was supported by Universitas Brawijaya under contract number 1622/UN10.F10/PN/2021
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
- Mohidem NA, Hashim N, Shamsudin R, Che Man H. Rice for food security: Revisiting its production, diversity, rice milling process and nutrient content. Agriculture. 2022;12(6):741.
Crossref - Food and Agriculture Organization of the United Nations (FAO). Food Outlook – Biannual Report on Global Food Markets. FAO; 2024.
Crossref - Statistics Indonesia (Badan Pusat Statistik). Official statistical news No. 20/03/Th. XXVII: Harvested area and rice production in Indonesia 2023. Jakarta: Statistics Indonesia; March 1, 2024.
- Rejeki D, Addy HS, Narulita E. Partial Characterization of bacteriophages from Indonesia and its potency as biocontrol of Xanthomonas oryzae pv. oryzae. Int J Agric Biol. 2021;25(01):75-80.
Crossref - Zhang M, Xu X, Lv L, et al. Genomic characterization of phage ZP3 and its endolysin LysZP with antimicrobial potential against Xanthomonas oryzae pv. oryzae. Viruses. 2024;16(9):1450.
Crossref - Kumar A, Kumar R, Sengupta D, et al. Deployment of Genetic and Genomic Tools Toward Gaining a Better Understanding of Rice-Xanthomonas oryzae pv. oryzae Interactions for Development of Durable Bacterial Blight Resistant Rice. Front Plant Sci. 2020;11:1152.
Crossref - Jiang H, Li C, Huang X, et al. Phage combination alleviates bacterial leaf blight of rice (Oryza sativa L.). Front Plant Sci. 2023;14:1147351.
Crossref - Kering KK, Zhang X, Nyaruaba R, Yu J, Wei H. Application of Adaptive Evolution to Improve the Stability of Bacteriophages during Storage. Viruses. 2020;12(4):423.
Crossref - Shahid M, Khan MS. Ecotoxicological implications of residual pesticides to beneficial soil bacteria: A review. Pestic Biochem Physiol. 2022;188:105272.
Crossref - Ul Haq I, Khan M, Khan I. Phytopathological management through bacteriophages: enhancing food security amidst climate change. J Ind Microbiol Biotechnol. 2024;51:kuae031.
Crossref - Korniienko N, Kharina A, Budzanivska I, Burketova L, Kalachova T. Phages of phytopathogenic bacteria: High potential, but challenging application. Plant Protection Science. 2022;58(2):81-91.
Crossref - Wang X, Wang S, Huang M, et al. Phages enhance both phytopathogen density control and rhizosphere microbiome suppressiveness. mBio. 2024;15(6):e0301623.
Crossref - Nhung TTT, Verma S, Ponne S, Meghwanshi GK, Schone T, Kumar R. Bacteriophage-based strategies for biocontrol and treatment of infectious diseases. Comput Struct Biotechnol J. 2025;27:2924-2932.
Crossref - Ranjani P, Gowthami Y, Gnanamanickam S, Palani P. Bacteriophages: A new weapon for the control of bacterial blight disease in rice caused by Xanthomonas oryzae. Microbiol Biotechnol Lett. 2018;46(4):346-359.
Crossref - Vu NT, Oh CS. Bacteriophage usage for bacterial disease management and diagnosis in plants. Plant Pathol J. 2020;36(3):204-217.
Crossref - Premaratne A, Zhang H, Wang R, Chinivasagam N, Billington C. Phage biotechnology to mitigate antimicrobial resistance in agriculture. In: Panwar H, Lichtfouse E, Sharma C, eds. Sustainable Agriculture Reviews 49: Mitigation of Antimicrobial Resistance Vol 2. Natural and Synthetic Approaches. Springer, Cham; 2021:313-345.
Crossref - Wardani AK, Buana EOGHN, Sutrisno A. The potency of bacteriophages isolated from chicken intestine and beef tribe to control biofilm-forming bacteria, Bacillus subtilis. Sci Rep. 2023;13(1):1-8.
Crossref - Marquioni V, Rossi FPN, Mendonחa DC, et al. Isolation and characterization of vB_XciM_LucasX, a new jumbo phage that infects Xanthomonas citri and Xanthomonas fuscans. PLoS One. 2022;17(4):e0266891.
Crossref - Nga NTT, Tran TN, Holtappels D, et al. Phage biocontrol of bacterial leaf blight disease on welsh onion caused by Xanthomonas axonopodis pv. allii. Antibiotics. 2021;10(5):517.
Crossref - Holtappels D, Fortuna KJ, Moons L, et al. The potential of bacteriophages to control Xanthomonas campestris pv. campestris at different stages of disease development. Microb Biotechnol. 2022;15(6):1762-1782.
Crossref - Ogunyemi SO, Chen J, Zhang M, et al. Identification and characterization of five new OP2-related Myoviridae bacteriophages infecting different strains of Xanthomonas oryzae pv. oryzae. J Plant Pathol. 2019;101:263-273.
Crossref - Hardanti S, Wardani AK, Rukmi WD. Isolation and Identification of bacteriophage specific Salmonella typhi from chicken skin. Jurnal Teknologi Pertanian. 2018;19(2):107-116.
Crossref - Khawaja KA, Abbas Z, Rehman S ur. Isolation and characterization of lytic phages TSE1-3 against Enterobacter cloacae. Open Life Sci. 2016;11(1):287-292.
Crossref - Phothichaisri W, Ounjai P, Phetruen T, et al. Characterization of bacteriophages infecting clinical isolates of Clostridium difficile. Front Microbiol. 2018;9.
Crossref - Wardani AK, Nurbayu IR, Qodriyah NL. Isolation of lytic bacteriophages and their potential to control Cronobacter spp. – Opportunistic food-borne pathogens. IOP Conf Ser Earth Environ Sci. 2020;475(1).
Crossref - ֱAcs N, Gambino M, Brondsted L. Bacteriophage enumeration and detection methods. Front Microbiol. 2020;11(594868).
Crossref - Weinheimer AR, Aylward FO. Infection strategy and biogeography distinguish cosmopolitan groups of marine jumbo bacteriophages. ISME J. 2022;16(6):1657-1667.
Crossref - Chae JC, Hung NB, Yu SM, Lee HK, Lee YH. Diversity of bacteriophages infecting Xanthomonas oryzae pv. oryzae in paddy fields and its potential to control bacterial leaf blight of rice. J Microbiol Biotechnol. 2014;24(6):740-747.
Crossref - Yuping L, Guan L, Becher I, et al. Jumbo phage killer immune system targets early infection of nucleus-forming phages. Cell. 2025;188(8):2127-2140.e21.
Crossref - Yuan Y, Gao M. Jumbo Bacteriophages: an Overview. Front Microbiol. 2017;8:403.
Crossref - Nazir A, Ali A, Qing H, Tong Y. Emerging aspects of jumbo bacteriophages. Infect Drug Resist. 2021;14:5041-5055.
Crossref - Nazir A, Dong Z, Liu J, et al. Sequence analysis of a jumbo bacteriophage, Xoo-sp14, that infects Xanthomonas oryzae pv. oryzae. Microbiol Resour Announc. 2020;9(48):1072-20.
Crossref - Jain L, Kumar V, Jain SK, Kushal P, Ghosh K. Isolation of bacteriophages infecting Xanthomonas oryzae pv. oryzae and genomic characterization of novel phage vB_XooS_NR08 for biocontrol of bacterial leaf blight of rice. Front Microbiol. 2023;14:1084025.
Crossref - Gasik K, Kuzmanovic V, Ivanovic M, et al. Complete genome of the Xanthomonas euvesicatoria specific bacteriophage KF1, its survival and potential in control of pepper bacterial spot. Front Microbiol. 2018;9:2021.
Crossref - Baginska N, Grygiel I, Orwat F, et al. Stability study in selected conditions and biofilm-reducing activity of phages active against drug-resistant Acinetobacter baumannii. Sci Rep. 2024;14(1):1-16.
Crossref - Hu M, Xing B, Yang M, et al. Characterization of a novel genus of jumbo phages and their application in wastewater treatment. iScience. 2023;26(6):106947.
Crossref - Edwards KF, Steward GF, Schvarcz CR. Making sense of virus size and the tradeoffs shaping viral fitness. Ostling A, ed. Ecol Lett. 2020;24(2):363-373.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.