ISSN: 0973-7510
E-ISSN: 2581-690X
In search of novel endophytic bacteria capable of producing plant growth promoting phytohormones and providing tolerance against biotic and abiotic stress to the plant, present study was carried out during 2021-22. For this, endophytic bacteria were isolated from halophytic Suaeda nigra at salt stress areas of Srikakulam district, Andhra Pradesh. Total of sixteen endophytic bacteria were isolated from roots and aerial parts of Suaeda nigra. Isolates were enumerated, purified and preserved for subsequent studies. All isolates were analyzed for their phenotypic, biochemical, enzymatic assay and molecular characterization was carried out by 16S rRNA molecular method. Isolates were tested for their ability in producing plant growth promoting phytohormones, siderophores, exo enzymes and ability to solubilize the phosphate molecules. Among total isolates extracted, bacteria which was labeled as “SNA7” isolated from aerial parts of Suaeda nigra showed better characters in producing catalytic enzymes like catalase, amylase, protease, phosphate solubilization ability and Indole-3-acetic acid (IAA) production. Isolate SNA7 was gram negative, motile, aerobic, rod shaped, non-spore forming, and no pigmentation which grows best at 42°C, pH 8.3 with tolerate of 8% NaCl nutrient agar. Based on phenotypic, biochemical, nucleotide homology and phylogenetic analysis isolate SNA7showed higher relationship with Pseudomonas pseudoalcaligenes Pseudomonas spp. was characterized as an effective organism to explore its ability in various research fields. In this current study, isolate SNA7 showed higher potential in producing wide range of enzymes and bioactive secondary metabolites and was first of its kind reported and isolated from halophytic Suaeda nigra.
Catalytic Activity, Endophytic Bacteria, Halophytes, Plant Growth Promoters, Pseudomonas, Secondary Metabolites
Endophytic bacteria are the organisms that has the capacity to harbor inter and intra cellular spaces of the host plant and they can spread systemically.1,2 Their exact functions for the plants are still uncertain.2 Endophytes encourages the growth of the plant and yields and also acts as antagonists bacteria.3
Halophytes are the plants which can tolerate salt and grows well in water with extreme salinity like mangrove forest, marsh lands, sea shores, saline deserts and coastal areas of arid and semi arid regions. Halophytes exists in no more than 2% of the entire kingdom of plants establish on earth. They can tolerate high salinity by adopting diverse techniques such as resistance, tolerance and avoidance, and also they had less opposition in halo environmental surroundings.
Halophytes grows in areas where there had regular floods. Suaeda monica grows more vigorously in degraded soils when compare to other halophytes. As salt extractors, they carry out major functions like decreasing the salinity of the soils creating conditions more suitable to growing mangrove plant species. During the course of ecological time halophytes are gradually replaced by mangrove ecosystem. In absence of halophytic plants the rejuvenation of mangrove ecosystem will be very difficult in sea shore areas.
Microorganism interacting in rhizosphere, phyllosphere and endosphere of the host plant are exceptionally diverse.4 Symbiotic relationship of microbes with host plant plays a important key role in maintaining plant health, plant growth promotion, resistant to diseases and survival of the host plant.5,6 In the midst of diverse microflora, endophytic bacteria shares same environment as that of plant pathogens and makes them as ideal models of antagonistic agents.7 There are several studies are made which illustrate that endophytes has the ability to resist plant pathogens, insects and nematodes in addition to promoting plant growth and establishment under stress environments.8,9
Currently, researchers have recognized more than hundreds of endophytic bacteria from medicinal plants of South Indian which showed promising characters against anti-tumour and antimicrobial agents.10 Suaeda nigra formerly called as Suaeda amoquinii is a species of flowering plant belonging to family Amaranthaceae. It grows well in saline and alkaline habitats regularly occurs in inland and occasionally in coastal ecosystems. Suaeda nigra grows as shrub from a woody base with several branches growing upto 5.0 feet in tall.
Pseudomonas sp. isolated from soils are of particular attention because of their root forming ability, catabolic adaptability and capability of producing vast ranges of proteins, enzymes and secondary metabolites which helps host plant to tolerate diverse biotic and abiotic stress environments.11 Pseudomonas pseudoalcaligenes KF707 isolated at Biphenyl manufacturing industry in Japan12 is a soil born bacteria. It is well known for its ability to aerobically degrade polychlorinated biphenyls13 using biphenyl as secondary metabolites.
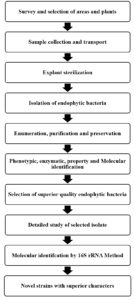
In search of novel endophytic bacteria capable of producing promising bioactive molecules, current study was undertaken to screen and identify endoyphytic bacteria isolated from halophytic Suaeda nigra. Brief report of work done is presented as flowchart (Figure 2).
This current investigation was carried out during 2020-21 and 2021-22 at Department of Botany, Andhra University, Visakhapatnam, Andhra Pradesh.
Endophytic salt tolerant bacteria isolation
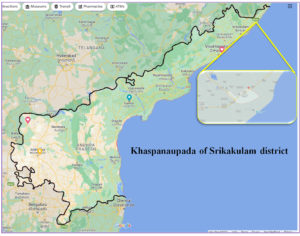
Explants were collected from aerial and root parts of Suaeda nigra located at holophytic zone of Khaspanaupada of Srikakulam district within latitude of 18°34’25.6″N and longitude of 84°18’50.4″E (18.573986, 84.313925) (Figure 3). The explants were sealed in sterile container for further studies.
Surface sterilization of explants
The explants were first washed with sterile water, blotted dry with filter paper dipped in 70% ethanol and kept for about 1min. The sample was removed and again washed in sterile water. After washing the sample was then dipped in 4% Sodium hypochlorite solution and kept for about 5 minutes and again washed in sterile water. Followed by dipped in 70% ethanol and kept for about 1min. The sample was removed and again washed in sterile water for final preparation of sample into paste.
To test the sterility of the procedure, the explants were kept and rolled on plates containing nutrient agar. 0.1 ml of the water from last wash was also inoculated to nutrient broth. After incubation period if any colonies developed, then all the samples were discarded and again surface sterilization of explants was carried out.
Endophytic bacteria isolated from explants of Suaeda nigra
After the surface sterilization of the explants, about 1g of each sample was weighed, cut into small pieces and well grounded into paste using sterile saline water in a mortar. Make the sample pastes into about 10ml using saline water. Each stock sample is further diluted 4 times, ten parts each time. The highest diluted solutions (10-4) were spread (50μl) on agar plates containing Zobell marine agar (ZM agar) (Composition – Zobell-Marine Broth 2216 Himedia @ 5.5 % + Agar agar @ 1.5 %) with the aid of ‘L’ shaped glass spreader, and the plates were incubated. Colonies with different morphology were isolated and streaked on ZM agar plates with assigned codes and kept for 24 hours at 32°C. In order to obtain pure culture the streaked cultures were again quaternary streaked on individual ZM agar plate and kept for 24 hours at 32°C for incubation. After 24 hours, the cultures were obtained from individual colonies were subjected to further studies like biochemical, molecular, property and salt tests.
Preservation and Maintenance of endophytic bacterial isolates
All the test isolates were streaked on nutrient agar plates and stored with 20% glycerol at -20°C. Viability of the cultures was retrieved by periodic sub-culturing into new media at monthly intervals.
Morphological and Microscopic observations of endophytic isolates
The following morphological and microscopic examination which is cell shape, colony morphology, Gram’s staining and motility were done to characterize the tentative identity of endophytic bacteria.
All sixteen isolates were inoculated on 3% NaCl Nutrient agar and the phenotypic, colony morphology was observed.14 According to Aneja, 200615 the cells from purified cultures were observed for their morphological characters like cell shape, cell size, endospore forming ability, reaction to gram’s stain, pigmentation and cell motility.
Growth at different NaCl concentration
The young cultures were streaked on Nutrient agar plates with different salt percentages i.e. 8% NaCl, 10% NaCl and 15% NaCl. The streaked plated were incubated at 32°C and observation of growth was made for 24hrs and 48hrs.
Oxygen requirement of endophytic bacterial isolates
Oxygen requirement was tested by growing bacteria in thioglycolate broth. The tubes were inoculated and incubated at 32°C. Bacterial population concentrate in the test tube where the O2 concentration is best suited for that particular microorganisms. Strict anaerobes concentrate at the bottom of the test tube, strict aerobes at the top to the test tube, aerotolerant organisms found evenly spread throughout the test tubes.
Biochemical enumeration of endophytic bacterial isolates
Catalase activity16
Loopfull of 24 hours old cultures of endophytic bacterial test isolates were transport to test tube containing 0.5 ml of sterile water. 3% solution of hydrogen peroxide (0.5ml) was added and thoroughly mixed. Observation was recorded for the production of effervescence give positive test for catalase production.
Oxidase test17
Trypticase Soy agar medium was used for this test. The test isolates were streaked on petri plates containing media and incubated for 48 hours at 32°C temperature in an upturn position. After incubation few drops of para amino dimethyl aniline oxalate were added. Observation was recorded for any colour changes from pink to maroon within few seconds indicates oxidase positive.
KOH test
Similar to Gram staining reaction, this test is also based on differences in the arrangement of the bacterial cell wall. Small amount of colony was mixed with little amount of 3% KOH. If the cell breaks, the cellular DNA makes the composition viscous or stringy in appearance. If KOH test is positive it indicates a gram negative microorganisms or If KOH test is negative then it indicates a gram positive microorganisms.
IndoleTest17
Glucose Tryptone broth was used to conduct Indole test. The test isolates was inoculated in sterile test tube containing sterile broth and kept for incubation for 48 hours at 32°C. After incubation time, 0.3 ml of Kovac’s reagent was added and thoroughly mixed. Observation was recorded by the formation of red colour alcohol ring at the top of the test tube indicates positive test for indole production.
Methyl Red &Voges Proskauer Test17
Glucose phosphate broth (MR-VP medium) was used to test both Methyl red & Voges Proskauer. Two sets of sterilized broth was inoculated with test organisms and incubated at 32°C. After 48 hours of incubation period few drops of methyl red indicator were added, development of red colour indicates Methy red test was positive. To another set of test tubes 5-6 drops of 5% a-naphthol and 2-3 drops of 40% potassium hydroxide solution were added and mixed well. The positive reaction of acetylmethylcarbinol from glucose fermentation was converted into diacetyl in presence of a-naphthol and potassium hydroxide with the development of red colour at the surface within few minutes.
Citrate Test18
Simmon’s Citrate Agar was used to test the consumption of Citrate as only carbon source and ammonium salts as only nitrogen source. All the isolates were inoculated in slants containing simmons citrate agar and incubated for 48 hours at 32°C. The positive test was indicated by the change of its colour from green to blue because of the change in pH.
Property studies of endophytic isolates
The isolated bacteria were subjected to different property studies like Protease activity (Gelatin hydrolysis), Amylase Production (Starch hydrolysis), IAA production and Phosphate solubilizing ability.
Gelatin and Starch hydrolysis
Gelatin and Starch hydrolysis test was conducted by growing the organism on 1% Gelatin Nutrient Agar and 1% Starch Nutrient agar plates in that order. After 48 hours of incubation the plates were flooded with saturated solution of Ammonium Chloride (5g/10ml) and diluted Iodine solution for Gelatin and Starch utilization respectively.
IAA production and Phosphate solubilizing ability
Bacteria isolated from plant rhizosphere produce various phytohormones in the form of secondary metabolites, the most common of which is Indole-3-acetic acid (IAA). IAA production was screened for the presence of Indole using Salkowski reagent and colour developed was measured spectrophotometrically at 536 nm using UV Spectrophotometer.
Phosphate solubilization ability was carried out by Pikovskaya’s broth and the presence of Phosphate was tested by adding 750 µl of phosphate reagent (Molybdenum Blue) and colour development was measured spectrophotometrically at 680 nm by using UV Spectrophotometer.
Microbial Identification using 16S rRNA gene based molecular method
Nucleic acid was extracted from test isolates using Silica based membrane technology and its quality was detected on 1 per cent agarose gel, a high molecular weight containing single band of DNA was recorded. Fragments of 16 S rRNAgene was multiplied by using 16S rRNA-Forward and 16S rRNA-Reverse primers. A distinct PCR amplicon (a piece of RNA or DNA which is the source for amplification or replication reaction) of 1500 base pairs band was observed which was determined on agarose gel and purified. Forward and Reverse DNA sequencing of PCR amplicon was determined by using Big Dye Terminator Version 3.1 cycle on Applied Bio systems 3730 X 1 genetic Analyzer. By using Aligner software tool complete consensus sequence of 16S ribosomal-RNA gene sequence was generated and BLAST was carried out with nucleotide collection database from NCBI GenBank. Primary 10 sequence were selected and aligned by using Clustal W alignment programme. By using Molecular Evolutionary Genetics Analysis (MEGA 10) phylogenetic tree and distance matrix was constructed.
Enumeration of endophytic bacterial isolates in aerial and roots of halophytic plant Suaeda nigra
The endophytic bacterial population in aerial parts and roots of halophytic plant Suaeda nigra are 3.7 × 105cfu/g and 4.9× 105cfu/g respectively. Total sixteen isolates from both aerial and root parts of Suaeda nigra were isolated and purified by quaternary streak plate method and they were coded according to their host plant followed by part (aerial and root) they are collected and finally by numbering in Serial Number (Table 1). Among 16 isolates, strain SNA-7 showed distinct morphology, phenotypic and biochemical characters which was selected for further studies. The results of cultural, biochemical and enzymatic characters are recorded in Table 2.
Table (1):
Coding of endophytic bacterial isolates.
| Plant | Plant part | Isolates |
|---|---|---|
| Suaeda nigra | Aerial parts (08 isolates) | SNA1, SNA2, SNA3, SNA4, SNA5, SNA6, SNA7, SNA8 |
| Root (08 isolates) | SNR1, SNR2, SNR3, SNR4, SNR5, SNR6, SNR7, SNR8 |
*S-Suaeda N-nigraA-aerial parts R-roots
Table (2):
Comparative study of isolated SNA7 strain with other pseudomonas spp.
SNA7 strain |
P. alcaligenes |
P. pseudo alcaligenes |
Pseudomonas pseudo alcaligenes strain TPS8 |
Pseudomonas putida BP25 |
|
|---|---|---|---|---|---|
Morphological identification |
|||||
1) Cell shape |
Bacillus |
Bacillus |
Bacillus |
Bacillus |
Bacillus |
2) Gram’s staining |
– |
– |
– |
– |
– |
3) Motility |
+ |
+ |
+ |
– |
+ |
4) NaCl concentration |
8% |
* |
* |
* |
* |
5) Oxygen requirement |
Aerobic |
Aerobic |
Aerobic |
Aerobic |
Aerobic |
6) Pigmentation |
– |
+ |
– |
– |
* |
Biochemical Tests |
|||||
1) KOH |
+ |
+ |
+ |
+ |
+ |
2) Oxidase |
+ |
+ |
+ |
+ |
+ |
3) Catalase |
+ |
* |
* |
+ |
+ |
4) Indole |
+ |
– |
– |
– |
+ |
5) Methyl Red |
+ |
+ |
+ |
+ |
– |
6) Voges-Proskauer |
– |
– |
– |
– |
– |
7) Citrate |
+ |
+ |
+ |
+ |
+ |
Enzymatic activity |
|||||
1) Gelatin |
+ |
+ |
+ |
– |
– |
2) Starch |
+ |
– |
– |
* |
– |
3) IAA production |
+ |
* |
* |
* |
+ |
4) Phosphate solubilisation |
+ |
* |
* |
* |
|
5) Siderophore production |
+ |
* |
* |
* |
+ |
*Not reported
Colony and cell morphology
The colonies of SNA7 strain on agar medium were medium, round in shape, colourless, convex in elevation. The cells when viewed microscopically are single rods and motile. Gram’s staining was carried out and the cells were gram negative in appearance. When grow in nutrient broth cells showed aerobic in nature. Grows well at temperature 25-30°C with 3% NaCl supplement, pH ranges 05-10 and salt tolerance upto 8% NaCl concentration.
Biochemical characteristic of endophytic bacterial isolates
Biochemical studies were carried out by the isolate SNA7and compare with strain of other Pseudomonas species are presented in Table 2. The results showed that the SNA7 isolate were KOH positive with stringy appearance. Catalase positive when 3% H2O2 was added and oxidase positive when para aminodimethyl aniline oxalate solution were added. IMViC test showed Indolepositive with red colour when Kovac’s reagent was added, Methyl red positive with the production of red colour, Voges-Proskauer test negative with no colour and Citrate utilization positive with production of blue colour due to the change pH in the medium.
Hydrolysis of Gelatin (Protease production)
Agar plates inoculated with SNA7 was tested for gelatinase production. The plates were flooded with saturated solution of Ammonium sulphate (5g/10ml) and kept for 30 min. Gelatin hydrolysis was observed by clear zone around the culture inoculated showed positive result.
Hydrolysis of Starch (Amylase production)
48 hrs of SNA7 plates were flooded with diluted Iodine solution. The iodine reacts with the starch present in the agar plate and produces blue colour. Clear zone was observed around the inoculated culture showed positive reaction to starch hydrolysis.
Production of Indole-3-acetic acid (IAA)
IAA production was screened for the presence of Indole compounds by colorimetric assay using Salkowski reagent. Strain SNA7 was cultured and inoculated in 4ml of 3% Nutrient broth in ria vials as detailed in material and methods. After 48 hrs incubation the culture were subjected to centrifugation and the liquid from supernatant was pippetted and mixed with 1.5 ml of Salkowski’s reagent. The colour intensities were measured by using UV Spectrophotometer at 536 nm. The OD values were compared with standard graph of IAA and 43μg/l of IAA production was recorded with strain SNA7.
Phosphate solubilizing ability
SNA7 culture were inoculated in 4ml of 3% pikovskaya’s borth in ria vials and kept for 48 hrs incubation as mentioned in material and methods. After incubation 750 µl of phosphate reagent to the 4ml of the sample and blue colour intensities were measured by using UV Spectrophotometer at 680 nm wavelength. 2.3 μg/l was recorded with strain SNA7.
Production of Siderophore
To test the siderophore production, all the test isolates were inoculated on blue agar for Chrome Azurol S and incubated for 72 hours at 30°C. After incubation period the plates inoculated with SNA7 shows colour change from blue to orange which indicates the positive for siderophore production.
Microbial Identification using 16S rRNA gene based molecular method
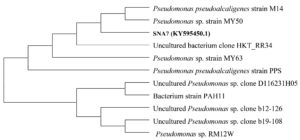
Nucleic acid from the isolate SNA7 (accession number KY595450.1) was isolated and evaluated on 1% agarose gel and obtained single band of DNA with high molecular weight. Fragments of 16S rRNA gene was multiplied by 16S rRNA-Forward-5′-GCCGTTGGGTTCCTTGAGAAC-3′ and 16S rRNA-Reverse-5′-CTTAATGCGTTAGCTGCGCC-3′ primers using BDT version 3.1 Cycle sequencing kit on ABI 3730 X l Genetic Analyzer. A comparison using 16S ribosomal-RNA gene sequences from the databases revealed that the 16S rRNA gene sequence of the type strain of SNA7 displayed high levels of similarity to those of Pseudomonas pseudoalcaligenes. The percentage of 16S rRNA sequence similarity between strain SNA-7 and Pseudomonas pseudoalcaligenes was 100%. The phylogenetic tree reconstructed using the neighbour-joining algorithm also confirmed that the strain SNA7 and Pseudomonas pseudoalcaligenes clustered together and constituted a separate group from the other closely related species (Figure 1).
Similar studies were carried out by different research workers on endophytic bacterial interaction with the host plant especially on endophytic bacteria belonging to the genus Pseudomonas and halophytic Suaeda family are discussed in this session.
Jha et al.19 studied on genus pseudomonas and stated that combination of endophytic Pseudomonas pseudoalcaligenes and rhizospheric Bacillus pumilus in paddy has an ability to promote resistance to the host plant from biotic and abiotic stress condition by inducing osmoprotectant and antioxidant proteins rather than by endophytic or rhizospheric bacteria alone at the early stages of development.
Setiawati et. al.,20 in their study isolated strain Pseudomonas stutzeri K10P4 from Karawang increase Nitrogen uptake (3.5%), dry weight (20.93 mg) and chlorophyll (67.51 µmol) in rice crops under saline environments.
Riva et al.,21 in their study revealed that the P. pseudoalcaligenes activate changes in plant defense mechanism and sugar metabolism leading to better tolerance against Sclerotium rolfsii.
Ashengroph et al.,22 isolated strain P. pseudoalcaligenes TPS8, was screened from tea plantation soil, which has high ability for caffeine tolerance (upto 15g/l) and caffeine degradation (80.2%) without any external carbon/nitrogen source addition.
Recent research findings was also correlated with present study, according to de Vilhena et. al.23 Endophytes have been associated with the production of secondary metabolites that may confer environmental advantages to the host. Similar findings was also studied by Fouda et al.24 that Endophytes promote plant growth and fitness through the production of phytohormones or biofertilizers or by alleviating abiotic and biotic stress tolerance and strengthening plant immune system.
Jabborova et al.25 isolated four endophytic bacterial isolates (GS2, GS5, GS8 and GS10) from Ginger (Zingiber officinal Rosc) showed positive for IAA production, siderophore production and phosphate solubilization activity and production of enzymes.
Similar results were obtained by Abdelshafy et al,26 in their study showed that endophytic bacteria exhibited several plant growth-promoting activities in vitro, including auxin synthesis, diazotrophy, phosphate solubilization, siderophore production, and production of lytic enzymes (i.e., chitinase, cellulase, protease, and lipase)
Papik et al.27 finds that Endophytic bacteria promote plant growth and protect their host plant against pathogens, herbivores, and abiotic stresses including drought, increased salinity and pollution.
Pseudomonas spp. having vast potential in production of siderophores, ACC deaminase, variety of bioactive secondary metabolites, phosphate and potassium solubilization and are effective aerobic degraders of polychlorinated biphenyls (PCB) and also used as bio-inoculants in many biological applications such as bioremediation, biological nitrogen fixation, antagonism. Hence, Pseudomonas spp. was characterized as an effective organism to explore its ability in various research fields. In this present study, an attempt was made to study Pseudomonas pseudoalcaligenes isolated from Suaeda nigra which had higher potential in producing bioactive secondary metabolites and was first of its kind reported in halophytic Suaeda nigra.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in this manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Hurek T, Reinhold-Hurek B, Van Montagu M, Kellenberger E. Root colonization and systemic spreading of Azoarcus sp. strain BH72 in grasses. J Bacteriol. 1994;176(7):1913-1923.
Crossref - Olivares FL, James EK, Baldani JI, Dobereiner J. Infection of mottled stripe disease-susceptible and resistant sugar cane varieties by the endophytic diazotroph Herbaspirillum. The New Phytologist. 1997;135(4):723-737.
Crossref - Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW. Bacterial endophytes in agricultural crops. Can J Microbiol. 1997;43(10):895-914.
Crossref - Turner TR, Ramakrishnan K, Walshaw J, et al. Comparative metatranscriptomics reveals kingdom level changes in the rhizosphere microbiome of plants. The ISME Journal. 2013;7(12):2248-2258.
Crossref - Bulgarelli D, Garrido-Oter R, Munch PC, et al. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe. 2015;17(3):392-403.
Crossref - Vorholt JA. Microbial life in the phyllosphere. Nat Rev Microbiol. 2012;10(12):828-840.
Crossref - Berg G, Eberl L, Hartmann A. The rhizosphere as a reservoir for opportunistic human pathogenic bacteria. Environ Microbiol. 2005;7(11):1673-1685.
Crossref - Aravind R, Eapen SJ, Kumar A, Dinu A, Ramana KV. Screening of endophytic bacteria and evaluation of selected isolates for suppression of burrowing nematode (Radopholus similis Thorne) using three varieties of black pepper (Piper nigrum L.). Crop Protection. 2010;29(4):318-324.
Crossref - Azevedo JL, Maccheroni Jr W, Pereira JO, De Araujo WL. Endophytic microorganisms: a review on insect control and recent advances on tropical plants. Electronic Journal of Biotechnology. 2000;3(1):40-65.
Crossref - Gangadevi V, Muthumary J. A novel endophytic taxol-producing fungus Chaetomella raphigera isolated from a medicinal plant, Terminalia arjuna. Appl Biochem Biotechnol. 2009;158(3):675-684.
Crossref - Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil. 2003;255(2):571-586.
Crossref - Furukawa K, Miyazaki T. Cloning of a gene cluster encoding biphenyl and chlorobiphenyl degradation in Pseudomonas pseudoalcaligenes. J Bacteriol. 1986;166(2):392-398.
Crossref - Fedi S, Carnevali M, Fava F, Andracchio A, Zappoli S, Zannoni D. Polychlorinated biphenyl degradation activities and hybridization analyses of fifteen aerobic strains isolated from a PCB-contaminated site. Res Microbiol. 2001;152(6):583-592.
Crossref - Gyaneshwar P, James EK, Mathan N, Reddy PM, Reinhold-Hurek B, Ladha JK. Endophytic colonization of rice by a diazotrophic strain of Serratia marcescens. J Bacteriol. 2001;183(8):2634-2645.
Crossref - Hawksworth DL, Pitt JI. A new taxonomy for Monascus species based on cultural and microscopical characters. Australian Journal of Botany. 1983;31(1):51-61.
Crossref - Aneja KR. Experiments in Microbiology, Plant Pathology and Biotechnology. 4th Edition. New Delhi. 2006;245-275.
- Cappuccino JG, Sherman N. Microbiology: A Laboratory Manual, 10th Edition. 1996.
- Seeley HW, Vandemark PJ. Microbes in action. A Laboratory Manual of Microbiology. Freeman and Company, San Francisco, USA. 1981;388.
- Jha Y, Subramanian RB. Effect of root-associated bacteria on soluble sugar metabolism in plant under environmental stress. Plant Metabolites and Regulation Under Environmental Stress. 2018;231-240.
Crossref - Setiawati MR, Sugiyono L, Kamaluddin NN, Simarmata T. The use of endophytic growth-promoting bacteriato alleviate salinity impact and enhance the chlorophyll, N uptake, and growth of rice seedling. Open Agriculture 2021;6:798-806.
Crossref - Riva DS, Ribaudo CM. Inoculation with Pseudomonas Pseudoalcaligenes Lead to Changes in Plant Sugar Metabolism and Defense That Enhance Tolerance Against the Pathogenic Fungus Sclerotium Rolfsii. American Academic Scientific Research Journal for Engineering, Technology and Sciences. 2020;69(1):89-104.
- Ashengroph M, Ababaf S. Biodecaffeination by Pseudomonas pseudoalcaligenes TPS8, an Isolated Strain from Tea Plantation Soil. Journal of Sciences, Islamic Republic of Iran. 2013;24(4):305-312.
- de Vilhena Araujo E, de Moraes Pontes JG, da Silva SN, da Silva Amaral L, Fill TP. The chemical warfare involved in endophytic microorganisms-plant associations. Microbial Endophytes. 2020;125-159.
Crossref - Eid AM, Fouda A, Abdel-Rahman MA, et al. Harnessing bacterial endophytes for promotion of plant growth and biotechnological applications: an overview. Plants. 2021;10(5):935.
Crossref - Jabborova D, Annapurna K, Fayzullaeva M,et al. Isolation and characterization of endophytic bacteria from ginger (Zingiber officinale Rosc.). Ann Phytomed. 2020;9(1):116-121.
Crossref - Mohamad OAA, Ma JB, Liu YH, et al. Beneficial endophytic bacterial populations associated with medicinal plant Thymus vulgaris alleviate salt stress and confer resistance to Fusarium oxysporum. Front Plant Sci. 2020;11:47.
Crossref - Papik J, Folkmanova M, Polivkova-Majorova M, Suman J, Uhlik O. The invisible life inside plants: Deciphering the riddles of endophytic bacterial diversity. Biotechnol Adv. 2020;44:107614.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.