ISSN: 0973-7510
E-ISSN: 2581-690X
We applied biosynthesized titanium and selenium nanoparticles, prepared using a fungal water extract of Trichoderma harzianum (T. harzianum), to eradicate tomota wilt infection. Transmission electron microscopy (TEM), Scanning electron microscopy (SEM), Energy Dispersive X-Ray analysis, and Transmission electron microscopy/X-ray diffraction (TEM/XRD) techniques were used to characterize the spherical metal nanoparticles, whose diameters were 16.0 nm for selenium nanoparticles (SeNPs) and 50.0 nm for titanium nanoparticles (TiNPs). This confirmed the efficient biosynthesis of the nanoparticles. Under greenhouse conditions, the effectiveness of TiNPs and SeNPs produced by nonpathogenic fungi (T. harzianum) against the pathogen responsible for the tomato wilt disease, Fusarium oxysporum (F. oxysporum), was studied. Based on the results, the most efficient method for combating the pathogen that causes tomato wilt was used in open fields, whereas pot studies were conducted in greenhouse conditions. All tested treatments considerably lowered tomato plant wilt disease in both the greenhouse and the open field. The disease severity was reduced by 20.4% using TiNPs at high concentrations of 150 ppm and by 41.5% using SeNPs. Compared to conventional antibiotics, the antibacterial activity assessment of the biosynthesized TiNPs and SeNPs revealed a significant effect versus pathogenic bacteria and fungi, with a negligible influence on the examined human and animal microflora. The findings showed that biosynthesized TiNPs and SeNPs can be applied to suppress the plant pathogen F. oxysporum in a way that is safe for the microflora of humans and animals. This is the first instance where the nanocidal activity of biological TiNPs and SeNPs has been used against the pathogen that causes tomato wilt.
Biocontrol, Nano-biosynthesis, Titanium Nanoparticles, Selenium Nanoparticles, Tomato Wilt
One of the most significant nutritious vegetable crops globally is the tomato (Solanum lycopersicum L., syn., Lycopersicon esculentum Mill), which is also nutritious.1 In most nations, it is present as either an open-field or protected crop. It ranks among the greatest significance in Egypt’s vegetable production and is applied to both industrial and food purposes.2 In 2014, Egypt ranked fourth worldwide in tomato production, producing 4.86% of the world’s tomatoes. Recently, Egypt’s tomato output grew and surpassed 6.6 million tons.3 Tomato plants are prone to infection by several soil-borne fungi, primarily Fusarium species and Rhizoctonia solani that result in significant diseases such as root rot and wilt which lowers harvest yields yearly.4,5 The well-known fungal pathogen found in soil, “Fusarium oxysporum f. sp. lycopersici (Sacc.)” is responsible for the Fusarium wilt, which affects only tomatoes. Wilted plants, yellowed foliage, and finally decreased yield quality and quantity are all signs of this disease.6 While fungicides have historically been primarily used to combat tomato wilt disease, there is growing concern about biological and environmental safety. Alternative methods of controlling plant diseases that are also safe for people, animals, and the environment are increasingly needed.
An innovative solution to this crisis was provided by nanotechnology, which offers a platform to modify and enhance the fundamental metal’s nanoparticle characteristics with hopeful applications in the fields of biological scanning controllers, tranquillizer cellular structures, nanomedicines, and treating various infections. There are numerous ways to acquire nanomaterials, but the biosynthetic route of nanoparticles has great potential and needs to be explored due to the growing demand to secure reliable and environmentally friendly innovations for the integration of nanostructures.7 Due to the vast array of possible applications in agriculture, biomedicine, electrical engineering, and optics, there is currently a large amount of scientific curiosity about nanoparticles.8 Using titanium dioxide (TiO2) as a photocatalyst and photoactive material for environmental cleanup purposes, including wastewater treatment, antifouling, deodorizing, and antibacterial purposes, has received substantial research. Due to their substantially higher photoactivity compared to micro-TiO2 powders, they have a far more extensive range of potential applications than nano-TiO2 powders.9
Selenium nanoparticles were utilized in bioremediation as a biosensor and exhibit antioxidant, anticancer, and antibacterial properties.10-12 Bacteria and Mariannaea sp. can biosynthesize selenium nanoparticles.11,13 This research project aimed to examine the impact of biologically generated titanium and selenium nanoparticles on tomato plants infected with Fusarium wilt.
Microbial cultures
Trichoderma harzianum (RCMB 017 009), a fungal strain, was used in this research for the biosynthesis of titanium and selenium nanomaterials. The Regional Centre for Mycology & Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt, kindly provided the microbial cultures utilised for the antimicrobial action. These cultures include; Salmonella typhimurium ATTC 14028, Bacillus subtilis NRRL B-543, Pseudomonas aeruginosa ATTC 27853, Methicillin Resistant-Staphylococcus aureus (MRSA) RCMB011001, Staphylococcus aureus ATTC25923, Escherichia coli ATTC 25955 and Enterococcus faecalis ATCC 29212 as well as some pathogenic filamentous fungi; Aspergillus niger ATCC 16404, Aspergillus flavus RCMB 002002 (5), Aspergillus fumigatus RCMB 002008 (3), Penicillium marnefeii RCMB 001022 and unicellular yeast-fungi; Candida albicans ATCC 10231 and Candida lipolytica RCMB 005007. Individual fungal colonies were selected and sub-cultured on potato dextrose agar (PDA): 100 mL of potato infusion, 20 g/l of dextrose, 0.1 g/l of yeast extract , and 20.0 g/l of agar. To preserve a stock fungal culture, sub-cultivating was done on slants of malt extract agar medium (MEA) containing: malt extract; 20.0 g/l, glucose; 20.0 g/l, peptone; 5.0 g/l, agar 15.0 g/l, the slants were kept at -20°C for a prolonged time. While bacterial cultures were activated, purified, and kept preserved using nutrient agar (NA) medium containing; Beef extract; 3.0 g, Peptone; 5.0 g, Agar; 15.0 g.
Titanium nanoparticles preparation
A Freshly T. harzianum culture was grown in 250 mL Erlenmeyer flask containing 100 mL of MGYP broth medium comprising; Yeast extract; 3.0 g/l, Malt extract; 3.0 g/l, Peptone; 5.0 g/l, Dextrose 10.0 g/l, and the final pH was customized to 6.5±0.4 using 1N HCl. The fungal culture was cultivated for 72 h while being continuously shaken at 150 rpm on a rotary shaker at 28°C. After incubation, the mycelia’s fungal pellets were taken out of the culture broth by centrifuging (4,000 rpm) at 4°C for 10 minutes. They were then given two thorough rinses with sterile distilled water. In a 250 mL Erlenmeyer flask, the collected fungal biomass (around 15 g wet weight) was reconstituted in 100 mL sterile deionized distilled water and then shaken (at 150 rpm) for further 72 h at 28°C. Following incubation, 0.1 M TiO2 metal salt was added in a concentration of 1:1 (v/v) to the water cell-free filtrate, which had been obtained through filtration. The whole mixture was shaken at 150 rpm at 28°C to allow the reaction to occur for a period of 48 h. Bio-transformed products of TiNPs were collected periodically and monitored for a further purification.14
Selenium nanoparticles preparation
SeNPs were prepared as described by Assadawy et al.15 To reduce 10 mM of the inorganic metal salt of sodium selenite (Na2SeO3) to selenium nanoparticles, they used a fungal cell-free aqueous extract of Trichoderma harzianum that contained the lowering enzyme Nicotinamide Adenine Dinucleotide Phosphate (NADPH)-dependent dehydrogenase.
Purification of TiNPs and SeNPs
To obtain the dry, pure powder of TiNPs and SeNPs. After centrifuging the titanium or selenium nanoparticle solution at 12,000 rpm for 20 min., the pellet was dispersed again in clean, distilled, deionized water to discard any debris. Three cycles of centrifugation in sterile, purified water were used to guarantee higher segregation of the free components from the metal nanoparticles. The sterilized pellets were then subjected to a 30 min. sonication process via an ultrasonicator (Jeveriy Instrument Supplies, Italy) for increased dissimilarity. The specimens were then thoroughly dried using a Lyophilized (Thermo Electron Corporation, Micro Modulyo 230 stop drier, USA). Purified titanium or selenium nanoparticles were collected and subjected to further characterization.16
Titanium and selenium nanoparticles characterization
The biotransformation processes of TiO2 or Na2SeO3 metal ion-salts into titanium and selenium nanoparticles, respectively, were assessed using different analytical techniques. The variation in reaction color was recorded. The particle size and shape analyzing systems of (TEM) (TEM JEOL 1010, Japan) and (EDX) connected to SEM (SEM/EDX, JSM-5500 LV JEOL, SEM, Japan) and X-ray diffraction (XRD) connected to (TEM/XRD, TEM JEOL 1010, Japan) were used for measuring zeta potential for tested Nano-metals techniques.
Antimicrobial activity of biosynthesized titanium and selenium nanoparticles
The potency of the screened biosynthesized titanium nanomaterials was tested using agar well diffusion versus test microorganisms. The region of inhibition around the formed material was used to measure the inhibition capacity of titanium and selenium nanoparticles in each well.17
Preparation of wilt disease inoculums
In order to identify the pathogen responsible for the tomato wilt disease, samples of sick tomatoes were gathered from several sites in various governorates around Egypt. Fungus was checked based on morphological characteristics using a microscope.18, 19
Pathogenicity test
We identified nine fungal spp. potential for disease in a pot test conducted in a greenhouse setting at the Unit of Recognition of Microbes, in Plant Pathology Research Institute, Agricultural Research Centre (A.R.C.), Giza, Egypt, F. oxysporum was tested on a tomato sensitive cultivar (Super Strain B). Three tomato seedlings that were 4-5 ‘weeks old were moved into plastic pots with soil that was infected with isolates of the fungus F. oxysporum or Fusarium oxysporum f. sp. lycopersici. Using a scale from 1-6, the severity of the wilt at 21 days following transplantation was assessed.20
Greenhouse experiments
The efficacy of different concentrations of titanium and selenium nanoparticles biosynthesized by antagonistic fungi (T. harzianum) was tested against the tomato wilt pathogen under greenhouse conditions. Tomato seedlings were prone to Fusarium wilt disease were obtained from the Agricultural Research Center in Giza. Seedlings of tomatoes were submerged in the tested SeNPs and TiNPs solutions at concentrations of 0.0, 50.0, 100.0, and 150.0 ppm. The chemical forms of selenium (Se) and titanium (Ti) were considered the negative control treatments. Before experimentation, every treatment was carried out by dipping the seedling roots for 6 hours. 5.0 kg of soil in plastic pots with a 25 cm diameter were intentionally contaminated with pathogenic F. oxysporum. Three tomato seedlings per pot of healthy, 40 days old Super Strain B seedlings were transplanted into plastic containers as part of three duplicates of each treatment, along with two control treatments using soil that had been infected with the pathogen and control soil that had not been inoculated. The positive control treatment for disease severity was the fungicide Topsin-M (1.5 g/l). Using a visual scale from 1 to 6, the incidence of the wilt illness was determined.20
The open-field experiment with titanium and selenium nanoparticles
Tomato seedlings were prone to the Fusarium wilt disease were obtained from the Agricultural Research Center in Giza. Based on our observation, outdoor pot trials resulted in the majority of the most successful treatments for tomato wilt illness. Various concentrations of SeNPs and TiNPs at 0.0, 50.0, 100.0, and 150.0 ppm, in addition to 10 mM of selenium salts (Na2SeO3) and 0.1M of titanium salt (TiO2) in their chemical form as well as Topsin-M (1.5 g/l) used as a solution for soaking seedlings for six hours to examine their impact on the severity of the tomato wilt disease, plant growth metrics, and yield-related components. The studies were carried out using a randomized block design across two growing seasons, 2018–2019 and 2019–2020, in a field at Giza Station, Agriculture Research Centre, Egypt, that was naturally infected with the organism that causes tomato wilt disease. 40-day-old tomato seedlings of the Super Strain B variety were sacked for 6 h at a rate of 100 transplants per 100 mL. The field plots (15 m2) had three rows that were each 5 m long with a 1 m space in between. One seedling per hill was grown, with hills spaced 50 cm apart. As a control, untreated seedlings were used. For four months, every 30 days, the severity of the disease was noted. At the end of the growing season, measurements of the plants’ height, number of fruits produced per plant, fruit weight (kg), fruit weight (g), fruit yield (T/ton), fresh weight, and dry weight were made. Tomato plants were assessed for residual selenium and titanium TiNPs and SeNPs-treated tomato fruits were dried at room temperature and put into 15 mL spin tubes, and then mixed with 3 mL of 68% HNO3. The specimens were then digested for 40 minutes at 115°C. The samples were then cooled, 500 ml of water was incorporated, and they were left to stand at 115°C for 20 min. The digests were diluted 14 times with deionized water, put through Whatman no. 42 filter paper, and then had total Ti and Se determined by ICP-MS analysis using Agilent triple quadrupole ICP-MS, standard (ICP-QQQ – 8800, Tokyo, Japan).21, 22
High performance liquid chromatography analysis of phenolic compounds of tomato plants
The high-performance liquid chromatography analysis was performed in the current work using a pump, an automated specimen injector, and a related Dell-compatible computer program that made up the Thermo-ultimate 3,000 system. The DAD-3000 diode array detection system was employed. At 25°C, the 2.5 x 30 cm Thermo-hypersil reversed phase C18 column was in operation. Distilled water and methanol made up the mobile phase (solvents A and B). The UV spectral absorption of the samples and the standards were captured between 230 and 400 nm. Degassing was done before the mobile phase, and standards solutions, and 0.45 µm Millipore membrane filters were used for filtering. By contrasting the retention duration and ultraviolet (UV) absorption spectra of the compounds with those of the standards, the compounds were identified.23
Carbohydrate assessment of tomato plants
Total carbohydrates were determined according to.24
Statistical analysis
All experiments were done in triplicate, and the results were represented as means.
Nanotechnology can provide environmentally safe and green alternatives for the control of plant diseases. Fungi are employed as bio-manufacturing nanoparticles since they are not only environmentally friendly but also easier to use than other bacteria. The ability to mass-produce nanoparticles will be enhanced by the nonpathogenic characteristics of specific fungal species and their ease of handling and manufacture. Recently, a wide variety of fungi have been tested for their capacity to produce various kinds of nanoparticles. The myco-synthesis of nanoparticles made of metals has also been documented in some studies. There is little data about the use of nanotechnology in plant pathology. For instance, agriculture operations frequently employ nanofungicides and nanopesticides. Future agricultural growers may benefit from the faraway deployment and tracking of smart nano-delivery systems to decrease the use of fungicides and pesticides.25
In this work, a report on the extracellular production of TiNPs and SeNPs by the fungus T. harzianum was completed. After being incubated, filtration was used to separate the fungal biomass after 72 hours of sterile deionized distilled water. The water cell-free filtrate was exposed to a 48 hours to complete reaction incubation with 0.1 M TiO2 ion and 10 mM Na2SeO3 salt. During incubation, the flasks exhibited a steady change in the medium’s hue to a colloidal whitish solution, which increased in intensity over time. The rise in intensity may be caused by the formation of more nanoparticles as a result of the TiO2 and Na2SeO3 ions’ reduction to nanoform in the aqueous solution. The results demonstrated the creation of titanium nanoparticles from the reduction of TiO2 and Na2SeO3 ions by the reducing agent, which is typically an enzyme of a proteinic nature obtained from the water-free filtrate of the investigated T. harzianum fungal species, as shown in (Figures 1, 3).
Figure 1. (Right): T. harzianum cell-free water filtrate with 0.1 M TiO2 salt solution at the beginning of the reaction; and (left): color change after 72 hrs. of completing the reaction
Figure 2. (Right): T. harzianum cell-free water filtrate with 10 mM of sodium selenite (Na2SeO3) salt solution at the beginning of the reaction; and (left): color change after 72 hrs. of completing the reaction
It is generally known that fungi secrete significant amounts of proteins that are essential to their life cycle. Amylases, cellulases, and proteases are among the hydrolytic enzymes found in the majority of these proteins.26 The majority of proteome research has concentrated on the proteins engaged in metabolic pathways; however, little is known about the function of other proteins. A research group27 used the SDS-PAGE protocol to analyze the cell-free filtrate’s protein composition from several fungal cultures, including Penicillium aurantiogresium, Penicillium roqueforti, and Trichoderma longibranchiatum, to find out which fungus contributes to the production nanoparticles. The reduction of silver ions to AgNPs was successful in all of these examined fungal cell-free filtrates. They discovered that each fungal extract employed included two distinct bands, ranging in size from 30 to 80 kD depending on the kind of fungal protein, which was visible in the protein fractions that were the outcome. These proteins may be responsible for both the nanobiosynthesis and the monodisperse nanoparticles’ stability against aggregation and oxidation. Similarly, investigation using Fusarium oxysporum revealed two extracellular proteins with molecular weights of 24 and 28 kDa that were in charge of producing and stabilizing zirconia nanoparticles. The visual observation was the first to notice the medium’s shift in color.28 Titanium nanoparticles (TiNPs) in a bio-transformed colloidal solution were periodically collected, purified, and characterized according to published reports.16,27
Nanoparticles are often created using a variety of chemical and physical techniques. However, all of these techniques are resource and energy intensive, use hazardous chemicals, and frequently produce particles in non-polar organic solutions, inhibiting their potential use. As a result, the necessity for the creation of safe, dependable, biocompatible, and efficient methods to make nanoparticles drives an increasing number of researchers to consider using biological systems as potential eco-friendly “nanofactories. Due to their innate ability to reduce and oxidize metal ions into metallic oxide nanoparticles, microorganisms like fungi and bacteria behave as nanofactories.29 Se-nanoparticles (SeNPs) for the current investigation were made in accordance with Assadawy &
Helmy 15. We utilized a fungal cell-free water extract of T. harzianum featuring the precise reducing enzyme Nicotinamide Adenine Dinucleotide Phosphate (NADPH)-dependent dehydrogenase to reduce 10 mM of the inorganic metal salt of sodium selenite (Na2SeO3) to selenium nanospheres of 16 nm in diameter, as shown in (Figures 2, 4).
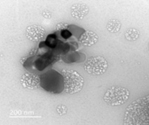
Figure 3. Transmission electron microscopy image of TiNPs nanospheres synthesized by the extract of T. harzianum with an average diameter of 50 nm
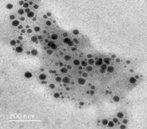
Figure 4. Transmission electron microscopy image of SeNPs nanospheres synthesized by the extract of T. harzianum with an average diameter of 16 nm
Industries have recently manufactured nanoparticles for commercial use, and these particles have various advantages. Researchers and businesses are interested in microorganisms as the ideal biological system to generate various nanoparticles because of the biosynthesis of nanoparticles. These microorganisms’ metabolic activity permits the extracellular or intracellular creation of nanoparticles using several modes of synthesis.8 Through bio-reduction, or the production of colloidal particles from the accumulation of non-soluble complexes with metal ions, microorganisms can reduce the toxicity of metal ions.8 The optimized crystal growth caused by stable reaction kinetics, which lowers the overall investment necessary in nanoparticle synthesis, causes biologically manufactured nanomaterials to be more precise in size than chemically synthesized ones. In addition to being less expensive overall, this approach is also more environmentally friendly because it does not employ hazardous chemicals, which are costly and bad for the environment when used in non-biological synthetic processes that result in nanoparticles with poor morphology, which can be used to optimize the culture conditions to produce nanoparticles with distinctive form.30
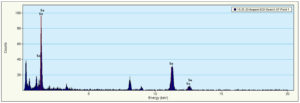
The more applications made possible by nanobiotechnology, the smaller the generated particle size. According to the transmission electron microscopy examination and the Energy Dispersive X-Ray spectra produced from (Figures 5, 6), the myco-formed spherical TiNPs and SeNPs from T. harzianum had average sizes of 16 nm in the case of SeNPs and 50 nm in the case of TiNPs (Figures 7, 8). Due to their smaller size, the bio-transformed nanomaterials can be used for a variety of purposes. For this reason, their specific properties at the nanoscale make them suitable for a variety of applications, including the prevention of microbial-induced plant diseases, particularly the F. oxysporum caused tomato wilt disease.
Titanium dioxide is a well-liked photocatalyst and is applied to make pigments.14 While selenium nanoparticles have antioxidant, anticancer, antibacterial, and are used in the treatment of diabetes.12,13 Bacteria and Mariannaea sp. can biosynthesize selenium nanoparticles.11
A fungal culture may be easily controlled during nanoparticle synthesis, as opposed to conventional methods. The use of extracellularly released enzymes has the benefit of allowing for the production of huge amounts of relatively pure enzymes. In accordance with that, a similar report29 demonstrated the synthesis of Zn, and Ti nanoparticles using the cell-free filtrate obtained from different soil fungi. It was discovered that maximum proteins were secreted by the isolate CZR-1 of Aspergillus terreus, and because of this more protein synthesis are effective in nanoparticle synthesis. These proteins, measuring 32 kDa, showed their intense band in the potential fungal species, upon SDS-PAGE polyacrylamide gel electrophoresis. Although other bands were also discovered, they play a function in the encapsulating of metal nanoparticles. This protein is crucial in the reduction of precursor metal into nano form. The real mechanism of metal biotransformation, however, has not yet been fully clarified, although numerous researchers have tried various approaches to support a potential mechanism.14
For the antimicrobial testing: TiO2 and Na2SeO3 were employed as a negative control experiment for the biosynthesized TiNPs and SeNPs, respectively, to adjust the control experiments. While Gentamycin and ketoconazole, respectively, served as the positive controls in commercial medication experiments for the growth of bacteria and fungus. The antibacterial efficacy of the biosynthesized TiNPs and SeNPs was confirmed using the Agar-Well Diffusion Assay. The average zone of inhibition (6 mm) was obtained for a variety of harmful bacteria. The results are shown in (Table 1).
Table (1):
Antimicrobial activity of biosynthesized TiNPs and SeNPs
Sample code Tested microorganisms |
TiNPs |
TiO2 (-ve control) |
SeNPs |
Na2SeO3 (-ve control) |
Positive Control of Commercial Drug |
|---|---|---|---|---|---|
FUNGI |
Ketoconazole |
||||
Aspergillus fumigatus |
8 |
NA |
12 |
NA |
17 |
Aspergillus niger |
9 |
NA |
13 |
NA |
20 |
Aspergillus flavus |
8 |
NA |
11 |
NA |
15 |
Penicillium marnefeii |
8 |
NA |
12 |
NA |
16 |
Candida albicans |
8 |
NA |
10 |
NA |
18 |
Candida lipolytica |
4 |
NA |
3 |
NA |
21 |
Bacteria: |
Gentamycin |
||||
Staphylococcus aureus |
10 |
NA |
12 |
NA |
24 |
Methicillin Resistant-Staphylococcus aureus |
10 |
NA |
14 |
NA |
20 |
Salmonella typhimurium |
11 |
NA |
15 |
NA |
22 |
Pseudomonas aeruginosa |
9 |
NA |
13 |
NA |
25 |
Escherichia coli |
3 |
NA |
3 |
NA |
30 |
Enterococcus faecalis |
3 |
NA |
4 |
NA |
27 |
Bacillus subtilis |
2 |
NA |
3 |
NA |
26 |
TiNPs: Titanium nanoparticles; SeNPS: selenium nanoparticles
To address the two most urgent problems in global public health the rise in antibiotic resistance and the decline in microbial incidence that causes infectious diseases, new antimicrobial nanostructures must be found and synthesized. In this way, the research by Dicastillo et al.17 demonstrated the antibacterial property of the chemically established Cesium-Titanium (CSTiO2) hollow nanospheres against various bacteria, including resistant E. coli and S. aureus, and demonstrated that CSTiO2 nanospheres performed better than commercial TiO2 nanoparticles. In the current study, many microbial strains comprising some bacterial pathogens, common intestinal flora, some pathogenic filamentous fungi, and unicellular yeast fungi were used to investigate the antibacterial activity of biosynthesized TiNPs and SeNPs. According to the study’s findings, the majority of the tested pathogenic strains were either moderately or significantly affected by the nano formula of both TiNPs and SeNPs such as Penicillium marnefeii, and Candida albicans or greatly affected, like in the case of Salmonella typhimurium, Pseudomonas aeruginosa, Staphylococcus aureus, Aspergillus niger, A. flavus, and A. fumigatus. While intestinal flora like Bacillus subtilis, Escherichia coli, Enterococcus faecalis and Candida lipolytica seemed not to be affected by applying the nanoformula of both TiNPs and SeNPs. Remarkably, no effect of the tested chemical salts of both TiNPs and SeNPs used as a negative control was observed. Additionally, SeNPs showed a slightly more powerful impact on the investigated strains of both fungi and bacteria compared to TiNPs. The effects against the variety of the tested cultures reflected the ability of the investigated formula of TiNPs and SeNPs to treat infections caused by some bacterial, fungal, and yeast causative agents. Additionally, they reflect a new, secure formula for treating the normal gut flora of the human body.17
On tomato plants of the cv. Super Strain B variety, the purified isolates’ pathogenic potential was examined in a greenhouse setting. The findings revealed that, under greenhouse circumstances, the investigated fungal isolates’ capacity to infect tomato plants with wilt greatly differed (Table 2). The isolate F9 of the most aggressive mould, F. oxysporum, was measured at 66.3 direct dispersal. In the meantime, 8.6 direct dispresal. of tomato plants were least likely to become infected by F. oxysporum (isolate F1). Additionally, in a greenhouse setting, two concentrations of SeNPs and TiNPs produced by T. harzianum were investigated for their ability to combat the wilt disease. Results in (Table 3) showed that tomato plant wilt disease was considerably (Pd™0.05) reduced at all tested doses of treatments, with disease severity decreasing by 13.3% to 51.2%. TiNPs, when used at concentrations of 100 and 150 ppm, respectively, lowered the disease severity by 30.3% and 20.4%, making them one of the best therapies. While SeNPs at a concentration of 150 ppm reduced the disease severity by 41.5%. Meanwhile, other concentrations showed a moderate effect. On the other hand, Topsin-M reduced disease severity by 11.1 % compared to those recorded with TiNPs.
Table (2):
Test of F. oxysporum’s isolates pathogenicity on tomato plants in different locations
| Isolates | Governorate | location | Direct dispersal % | Virulence level |
|---|---|---|---|---|
| F1 | El- Qalyubia | Qaha | 8.6 | L |
| F2 | Sharqia | Faqus | 21.2 | L |
| F3 | Beheira | El-Nubaryia | 53.4 | H |
| F4 | Giza | Giza | 26.2 | L |
| F5 | Giza | Bdrasheen | 32.2 | M |
| F6 | Ismailia | Ismailia | 40.1 | M |
| F7 | Kafr El-Sheikh | Sakha | 40.5 | M |
| F8 | Kafr El-Sheikh | Kafr El-Sheikh | 26.0 | M |
| F9 | Kafr El-Sheikh | Qellin | 66.3 | H |
| Control | 0 | NP | ||
Table (3):
Treatment effectiveness in reducing wilt severity in tomato plants (cv. super strain) grown in greenhouses
| Treatments | Concentrations (ppm) | Infection severity % |
|---|---|---|
| TiNPs | 100 | 30.3 |
| 150 | 20.4 | |
| SeNPs | 100 | 52.7 |
| 150 | 41.5 | |
| Ti | 44.3 | |
| Se | 61.4 | |
| Fungicide | 11.1 | |
| Control (infected) | 75.9 | |
| Control (uninfected) | 100 | |
In the Giza Governorate Station, the effects of nanoparticles on tomato plant growth parameters and the severity of the wilt disease were investigated. As shown in (Tables 4 and 5), all treatments in the tested concentrations significantly protect against wilt illnesses when compared to the control in the growing seasons 2018-2019 and 2019-2020. However, TiNPs at 150 ppm reduced growth by 13.43 and 12.30% in both growing seasons, making it the most successful treatment. The lowest protection against wilt illnesses was, however, demonstrated by tomato seedlings treated with SeNPs at 150 ppm, which resulted in reductions of 21.40 and 21.54%, respectively. According to (Tables 4 and 5), there was a significant difference between the treatments and the control in terms of the number of fruits produced per plant, fruit weight per plant-1 (kg), fruit weight (g), and total yield per field (Tonne), plant height, and dry plant weight. The most effective treatments were TiNPs at 150 ppm recorded a high number of fruit plant-1 (48.67 and 56.33), fruit weight plant-1 (kg) (3.47 and 3.60 kg), and total yield per field (Tonne) (24.67and 23.28 tonne), plant height (cm) (74.0 and 74.9) compared with 31.67, 35.33 and 1.23, 1.31 and 7.62, 7.80 and 47.0, 47.1 in the control treatment in both seasons, respectively. Contrarily, compared to the others, selenium‘s impact on raising the growth parameter was less significant.
Table (4):
Efficacy of interventions on tomato plants with the “cv. super strain” wilt under field conditions during the first season
Treatments |
Disease severity% |
No. of fruits plan |
Fruit weight plant-1 (kg) |
Fruit weight (gm) |
Total yield fed (Ton) |
Plant high |
Dry plant weight |
|---|---|---|---|---|---|---|---|
TiNPs |
13.43 |
48.67 |
3.47 |
71.30 |
24.67 |
74 |
21.4 |
SeNPs |
21.20 |
33.50 |
1.84 |
54.90 |
12.32 |
62 |
19.3 |
Ti |
25.33 |
26.50 |
1.35 |
50.80 |
9.31 |
51 |
13.9 |
Se |
29.64 |
19.25 |
0.75 |
39.24 |
5.36 |
49 |
11.6 |
Fungicide |
8.73 |
67.67 |
4.55 |
67.30 |
24.47 |
85 |
23.6 |
Control |
40. 90 |
31.76 |
1.23 |
38.70 |
7.62 |
47 |
12.0 |
LSD at 5% |
0.0622 |
0.0476 |
0.0334 |
0.2419 |
0.0144 |
0.8165 |
0.3559 |
TiNPs: titanium nanoparticles, selenium nanoparticles, Ti: titanium, Se: selenium LSD: Least significant difference
Table (5):
Effects of treatments on the severity of the wilt disease in tomato plants (cv. super strain) in a field setting at the second season
Treatments |
Disease severity% |
No. of fruits plant |
Fruit weight plant-1 (kg) |
Fruit weight (gm) |
Total yield fed -1 (Ton) |
Plant high |
Dry plant weigh |
|---|---|---|---|---|---|---|---|
TiNPs |
12.30 |
56.33 |
3.60 |
63.90 |
25.28 |
74.9 |
23.6 |
SeNPs |
21.54 |
37.33 |
1.99 |
50.89 |
12.98 |
63.0 |
20.4 |
Ti |
24.64 |
28.33 |
1.43 |
50.47 |
9.73 |
52.2 |
15.0 |
Se |
30.40 |
21.33 |
1.00 |
42.86 |
5.62 |
49.9 |
12.0 |
Fungicide |
9.47 |
71.67 |
4.68 |
65.29 |
24.94 |
85.3 |
24.6 |
Control |
39.80 |
35.33 |
1.31 |
37.07 |
7.80 |
48.1 |
11.6 |
L.S.D at 0.05% |
0.3452 |
0.476 |
0.0398 |
0.0346 |
0.2039 |
0.6733 |
0.8455 |
The presented data in (Table 6) showed measurements of titanium and selenium in tomato fruits treated with chemically and biologically synthesized nanoparticles. The results revealed that residual titanium and selenium in treatment with nanoparticles is lower than treatment with metal. Besides, the data in (Table 7) clearly showed a difference in measurements of total carbohydrates in tomato fruits treated with chemically and biologically synthesized nanoparticles. The results revealed that total carbohydrates in treatment with nanoparticles are higher than in treatment with metal.
Table (6):
Titanium and selenium concentrations in control and treated tomatoes were determined using Inductively coupled plasma mass spectrometry (ICP-MS)
Ti (ppm) |
Se (ppm) |
|
|---|---|---|
Control |
– |
– |
TiNPs |
109 |
– |
SeNPs |
– |
23 |
Ti |
414 |
– |
Se |
– |
71 |
ppm: Part per million
Table (7):
Total carbohydrates in tomato plants
Total carbohydrates mg/g control |
|
|---|---|
1.67 |
Control |
1.6 |
Ti |
1.34 |
Se |
1.81 |
TiNPs |
1.79 |
SeNPs |
High performance liquid chromatography chromatograms for methanol extracts of control and treated tomato illustrated the existence of quercetin and kaempferol, as depicted in (Figures 9-11). It could be noticed that the two polyphenols were presented as major components in the control sample, and other derivatives could be seen upon treatment with either TiNPs or SeNPs, which might reflect their roles in the eradication of fungal infection. A research group of Wach et al.31 reported that quercetin has anti-histamine, anticancer, and anti-inflammatory properties Kaempferol, a flavanol present in many edible plants and one of the natural polyphenolics, is said to have powerful pharmacological and nutraceutical properties. Kaempferol is used in cancer treatment and has some pharmacological effects, including antibacterial, anti-inflammatory, neuroprotective, and antidiabetic activity. According to other study organizations, the antioxidant qualities are what are recognized to be responsible for these health advantages.32, 33
Figure 9. High performance liquid chromatography chromatogram of tomato fruit components of the control experiment
Figure 10. High performance liquid chromatography chromatogram of tomato fruit components treated with TiNPs
Figure 11. High performance liquid chromatography chromatogram of tomato fruit components treated with SeNPs
This research work was designed to test the impact of biosynthesized titanium and selenium nanoparticles on the control of Fusarium wilt disease in tomato plants. The results indicate that all tested levels of treatment drastically decreased tomato plant wilt disease. The most effective treatments are TiNPs at a high concentration of 150 ppm, which reduced the disease severity by 20.4 %, followed by SeNPs at 41.5%. The antimicrobial activity of the biosynthesized TiNPs and SeNPs showed a moderate impact against pathogenic microbes of bacteria and fungi compared to the commercial antibiotic drugs, with a negligible effect on the investigated human and animal microflora.
The result suggests that biosynthesized TiNPs and SeNPs can effectively control the plant pathogen F. oxysporum with safety for human and animal microflora. This is the first demonstration of the nanocidal effect of biological TiNPs and SeNPs against the tomato wilt disease causative pathogen F. oxysporum under greenhouse and open-field conditions.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This work was supported by Research supporting project, King Saud University, Riyadh, Saudi Arabia [grant number RSP-2023R439].
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Giovannucci E. Tomatoes tomato-based products, lycopene and cancer: Review of the epidemiologic literature. J Nat Cancer Inst. 1999;91(4):317-331.
Crossref - Mohd KK, Mahamad MMH, Abdul Rahim N, Hashim YZH, Abdullah SMS, Azizan KA. Bacterial Metabolomics: Sample Preparation Methods.Biochem Res Int.;2022:9186536.
Crossref - Conti V, Parrotta L, Romi M, Del Duca S, Cai G. Tomato Biodiversity and Drought Tolerance: A Multilevel Review. Int J Mol Sci. 2023;24(12):10044.
Crossref - Ajayi-Oyetunde OO, Bradley CA. Identification and Characterization of Rhizoctonia Species Associated with Soybean Seedling Disease. Plant Dis. 2017;101(4):520-533.
Crossref - Coque JJR, Álvarez-Pérez JM, Cobos R, González-García S, Ibáñez AM, Diez Galán A, Calvo-Peña C. Advances in the control of phytopathogenic fungi that infect crops through their root system. Adv Appl Microbiol. 2020;111:123-170.
Crossref - Andolfo G, Ferriello F, Tardella L, Ferrarini A, Sigillo L, Frusciante L, Ercolano MR. Tomato genome-wide transcriptional responses to Fusarium wilt and Tomato Mosaic Virus. PLoS One. 2014;9(5):e94963.
Crossref - Elshawy O, Helmy E, Rashed L. Preparation, Characterization and in Vitro Evaluation of the Antitumor Activity of the Biologically Synthesized Silver Nanoparticles. Advances in Nanoparticles. 2016;5(2):149-166.
Crossref - Khan MR, Rizvi TF. Nanotechnology: scope and application in plant disease management. Plant Pathol J. 2014;13(3):214-231.
Crossref - Lin G, Tian M, Lu YL, Zhang XJ, Zhang LQ. Morphology, Antimicrobial and Mechanical Properties of Nano-TiO2/Rubber Composites Prepared by Direct Blending. Polymer Journal. 2006;38(5):498-502.
Crossref - Gandin V, Khalkar P, Braude J, Fernandes AP. Organic selenium compounds as potential chemotherapeutic agents for improved cancer treatment. Free Radic Biol Med. 2018;127:80-97.
Crossref - Zhang H, Zhou H, Bai J, Li Y, Yang J, Ma Q, Qu Y. Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ and their characterization. Colloids Surf. A: Physicochem Eng Asp. 2019;571: 9-16.
Crossref - Kumar A, Prasad KS. Role of nano-selenium in health and environment. J Biotechnol. 2021;325:152-163.
Crossref - Menon S, Shrudhi SD, Agarwal H, Shanmugam VK. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloid Interface Sci Commun. 2019;29:1-8.
Crossref - Ramesh R, Tarafdar JC. Biosynthesis and characterization of zinc, magnesium and titanium nanoparticles: an eco-friendly approach. Int Nano Lett. 2014;4:93-103.
Crossref - Assadawy M, Helmy E. Porphyromonas gingivalis Biofilm Formation on Implant Surface Treated with Nanoselenium. Int J Dent Oral Health. 2015;7(5):
Crossref - Tripathy AM, Raichur N, Chandrasekaran TC, Prathna A, Mukherjee K. Process variables in biomimetic synthesis of silver nanoparticles by aqueous extract of Azadirachta indica (Neem) leaves. J Nanopart Res. 2010;12(1):237-246.
Crossref - Dicastillo CL, Patiסo C, Galotto MJ, et al. Novel hollow titanium dioxide nanospheres with antimicrobial activity against resistant bacteria. Beilstein J Nanotechnol. 2019;10:1716-1725.
Crossref - Leslie JF, Summerell BA. The Fusarium laboratory manual. 1st ed. Blackwell Publishing Ltd; Oxford, London, 2006:388.
Crossref - Nelson PE, Toussoun TA, Marasas WFO. Fusarium Species: An Illustrated Manual for Identification. Pennsylvania State University Press; University Park, Pennsylvania, USA. 1983:138.
- Silva JC, Bettiol W. Potential of non-pathogenic Fusarium oxysporum isolates for control of Fusarium wilt of tomato. Fitopatologia Brasileira. 2005;30:409-412.
Crossref - Zia M, Gul S, Akhtar J, et al. Green synthesis of silver nanoparticles from grape and tomato juices and evaluation of biological activities. IET Nanobiotechnol. 2017;11(2):193-199.
Crossref - Khairy AM, Tohamy MRA, Zayed MA, et al. Eco-friendly application of nano-chitosan for controlling potato and tomato bacterial wilt. Saudi J Biol Sci. 2022;29(4):2199-2209.
Crossref - Biswas AK, Islam MR, Choudhury ZS, Asif Mostafa A, Kadir MF. Nanotechnology based approaches in cancer therapeutics. Adv Nat Sci: Nanosci. Nanotechnol. 2014;5(45):043001.
Crossref - Dubois M, Gilles KA, Hamilton JK, Rebers PT, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28(3):350-356.
Crossref - Mousa AA, Hassan A, Mahindra R, Ernest S, Kamel AA. Myconanoparticles: synthesis and their role in phytopathogens management. Biotechnol Biotechnol Equip. 2015;29(2):221-236.
Crossref - Jain A, Singh A, Singh S, Singh HB. Comparative proteomics analysis in pea treated with microbial consortium of beneficial microbes reveals changes in protein network to enhance resistance against Sclerotinia sclerotiorum. J Plant Physiol. 2015;182:79-94.
Crossref - Mekawey AI, Helmy EA. Elucidative Physiological Optimization of Silver Nanospheres Biogenesis by Molds. International Journal of Nanotechnology and Allied Sciences. 2017;1(1):30-44.
- Ahmad B, Mukherjee S, Sastry M. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B: Biointerfaces. 2003;28(4):313-318.
Crossref - Raliya R, Rathore I, Tarafdar JC. Development of Microbial Nanofactory for Zinc, Magnesium, and Titanium Nanoparticles Production Using Soil Fungi. J Bionanosci. 2013;7(5):590-596.
Crossref - Vijay AK, Nalini SPK. Biotemplates in the green synthesis of silver nanoparticles. Biotech J. 2010;5(10):1098-1110.
Crossref - Wach A, Pyrzyסska K, Biesaga M. Quercetin content in some food and herbal samples. Food Chemistry, 2007;100(2):699-704.
Crossref - Imran A, Arshad MU, Arshad MS, Imran M, Saeed F, Sohaib M. Lipid peroxidation diminishing perspective of isolated theaflavins and thearubigins from black tea in arginine induced renal malfunctional rats. Lipid Health Dis. 2018;17:157.
Crossref - Al Aboody MS, Mickymaray S. Anti-Fungal efficacy and mechanisms of flavonoids. Antibiotics. 2020;9(2):45.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.