ISSN: 0973-7510
E-ISSN: 2581-690X
To investigate the in vitro effects of interactions between ciprofloxacin (CP) and magnesium-aluminium hydroxide (MA) combined against selected Gram-positive and Gram-negative bacteria. The interaction between CP and MA was accessed by agar diffusion method. Comparing the susceptibility of the isolates to CP alone with those of Ciprofloxacin-Magnesium-aluminum hydroxide (CPMA) combined showed that there were significant antagonistic and synergistic interactions in vitro. Antibacterial activities of ciprofloxacin were increased with 4.5-6.0 µg/ml of the MA but were drastically decreased with concentrations lower and higher than 4.5-6.0 µg/ml while development of resistant colonies within these zones of inhibitions was recorded. The susceptibility of the isolated resistant colonies was lesser than those obtained from the original isolates. The combination of ciprofloxacin (CP) and magnesium-aluminum hydroxide (Maalox) (MA) against bacterial isolates could result in development of resistant colonies. The roles of aluminum and magnesium in resistance development at the molecular level require further studies.
Ciprofloxacin; Ciprofloxacin-magnesium-aluminum hydroxide; Antagonistic Interactions; Polyvalent Metallic Ions; Resistance Development
Ciprofloxacin or 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl]-3-quinoline carboxylic acid is a fluorinated quinolone antibacterial agent extensively used worldwide1 against both Gram-positive and Gram-negative microorganisms2. While ciprofloxacin is a fluorinated quinolone synthesized in late 1980s3, it is a quinolone carboxylic acid derivative with an extensive antibacterial spectrum4, 5 showing greater potency and lower toxicity than other fluoroquinolones6, 7. After oral administration, it has excellent tissue penetration, good bioavailability and it is relatively safe8,9. Though it is effective in the treatment of soft tissue infections10-13, Neuhauser et al14, Friedland et al15 and MacDougall et al16 reported that increasing incidence of ciprofloxacin resistance among Gram negative bacteria is associated with increased use of fluoroquinolones. While ciprofloxacin is well tolerated with low incidence of adverse effects17, Wolfson and Hooper18 and Petri19 indicated few clinical reactions including gastrointestinal disturbances, central nervous systems toxicity and rash occurring in about 5–12 % of patients.
Fluoroquinolones, generally, are known to have numerous interactions with agents with local activity in the gastrointestinal system such as antacids 20-23 leading to loss of antibiotic activity. Several studies24-26 reported that concomitant administration of aluminum, magnesium, zinc or iron with fluoroquinolones resulted in a significant decrease in the systemic availability of the drug indicating that widely used antacids markedly reduce the bioavailability of fluoroquinolone antimicrobial agents through chelation in the gastrointestinal tract27,28.
While several studies29-32 have reported that the bioavailability of ciprofloxacin was significantly impaired when orally co-administered with iron, aluminum- and magnesium- containing antacids, there is a dearth of information on the effect this combinations may have on the antimicrobial activities of ciprofloxacin against microorganisms in vitro. The study was, therefore, aimed at investigating in vitro interaction between ciprofloxacin and magnesium-aluminum hydroxide and the effects of this metallic ion on the antibacterial activity of ciprofloxacin against selected bacterial strains.
Preparation of drug stock solutions
Stock solution of ciprofloxacin was prepared according to the NCCLS guidelines or manufacturer’s recommendations33. Forty milligram of pure drug powder of ciprofloxacin, obtained from a pharmaceutical company in Lagos, Nigeria, dissolved in 3 ml of ethanol was made up to 100 ml in sterile distilled water to form the stock solution. Concentrations of 0.2 – 3.0 µg/ml of ciprofloxacin were prepared from stock solutions. Three antacid tablets, each containing magnesium trisilicate-250 mg and aluminum hydroxide-120 mg, were aseptically crushed into powdery form after obtaining the average weight. Ten milligram (10 mg) of the powdered antacid tablets was dissolved in 100 ml of sterile distilled water to form the initial stock solution. Concentrations ranging between 1.0 and 10.0 µg/ml of the magnesium-aluminum-hydroxide (Maalox) were prepared from the stock. Fresh drug solutions were daily prepared from stock solutions which were stored at -20oC for susceptibility studies.
Clinical isolates
Routine clinical Gram-negative and Gram-positive bacteria were used in this study. These bacterial isolates included Bacillus brevis, Escherichia coli, Salmonella typhi, Staphylococcus aureus, Staphylococcus epidermidis, Pseudomonas aeruginosa and Staphylococcus albus. The bacterial colonies were subjected to Gram staining, microscopic appearance, colony morphology and biochemical tests according to standard protocols for identification 34-36.
Antimicrobial susceptibility testing
The antibacterial activity was determined using agar diffusion assay technique according to the modified Kirby–Bauer diffusion technique35 by swabbing the Mueller-Hinton agar (MHA) (Oxoids UK) plates with the resultant overnight culture of each of the test isolates. Multi-discs (Abtek) containing different antibiotics including Ofloxacin (5 µg), Ciprofloxacin (5 µg), Pefloxacin (5 µg), Streptomycin (10 µg), Gentamicin (10 µg), Chloramphenicol (30 µg), Amoxicillin (25 µg), Cefuroxime (30 µg) and Erythromycin (10 µg) were aseptically placed on the inoculated agar plates and incubated at 37°C for 24 h.
To determine the drug-drug interactions, prepared Mueller Hinton agar plates were swabbed with the resultant saline suspension of each bacterial strain. Wells were then bored into the agar medium with heat sterilized 6 mm cork borer. The wells were aseptically filled with 100 µl of freshly prepared different concentrations of ciprofloxacin (CP) alone and its combination with magnesium-aluminum-hydroxide (Maalox) preparations (CPMA) taking care not to allow spillage of the solutions onto the surface of the agar. The plates were allowed to stand for at least 30 min before being incubated at 37°C for 24 h37. The determinations were done in duplicate. After 24 h of incubation, all the plates were examined for zones of inhibition38. The diameter of the inhibition zones produced by the different antibiotic discs, ciprofloxacin alone and those of its combination with the Maalox were measured and interpreted using the CLSI zone diameter interpretative standards39. Resistant colonies (Rc) were isolated from within inhibition zones of plates containing drug combinations and were further identified and subjected to susceptibility testing. Each bacterial isolate was classified as susceptible (S), intermediate (I) and resistant (R) to antibiotics according to the zone diameter interpretation standard recommended by the Clinical Laboratory Standards Institute40.
In this study, all the clinical strains were susceptible in vitro with the inhibition zones showing concentration-dependent activity of ciprofloxacin. The inhibition zones increases with increase in the concentration of ciprofloxacin as presented in Figures 1 – 7 showing antibacterial activity of ciprofloxacin (CP) alone and its combination with magnesium-aluminum hydroxide (CPMA) complex at different concentrations. Although, CP exhibited a significant activity against the different test strains of bacteria, the extent and activity observed for CPMA was significantly different from those of CP.
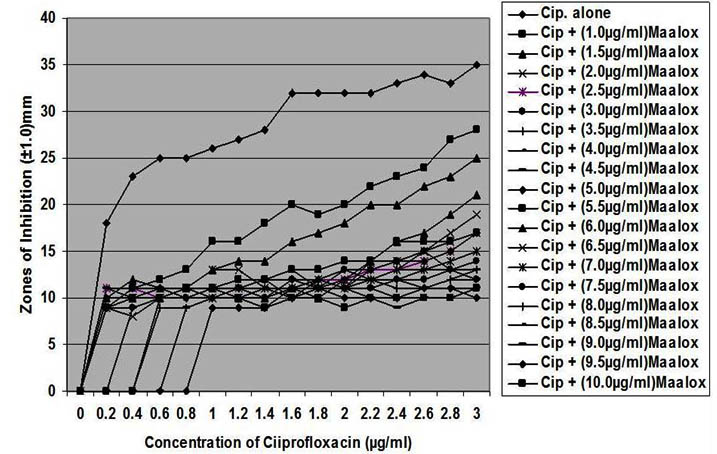
Fig. 1. In vitro susceptibility of Staphylococcus aureus to different concentrations of ciprofloxacin and its combination with magnesium/aluminum hydroxide
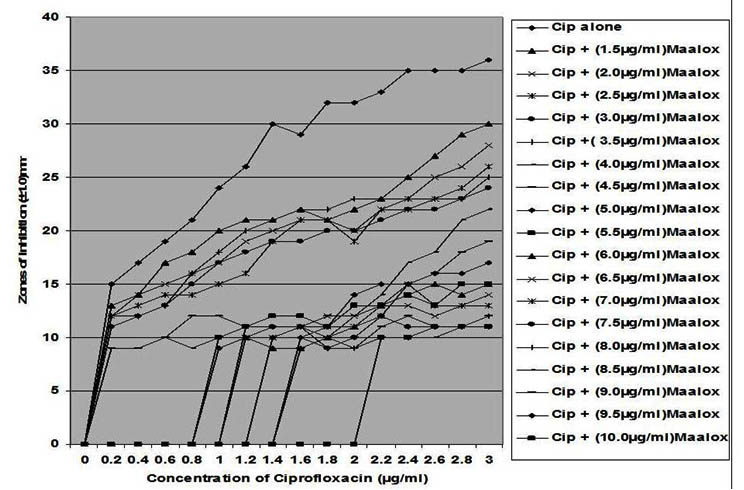
Fig. 2. In vitro susceptibility of Salmonella typhi to different concentrations of ciprof-loxacin and its combination with magnesium-aluminum hydroxide
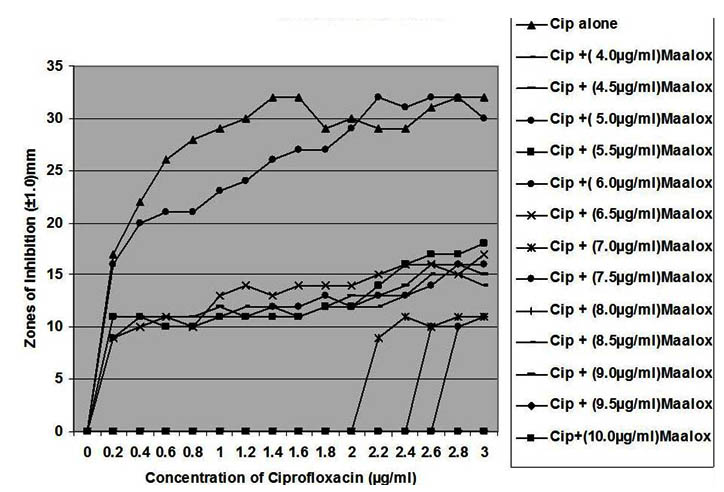
Fig. 3. In vitro susceptibility of Pseudomonas aeruginosa to different concentrations of ciprofloxacin and its combination with magnesium-aluminum hydroxide
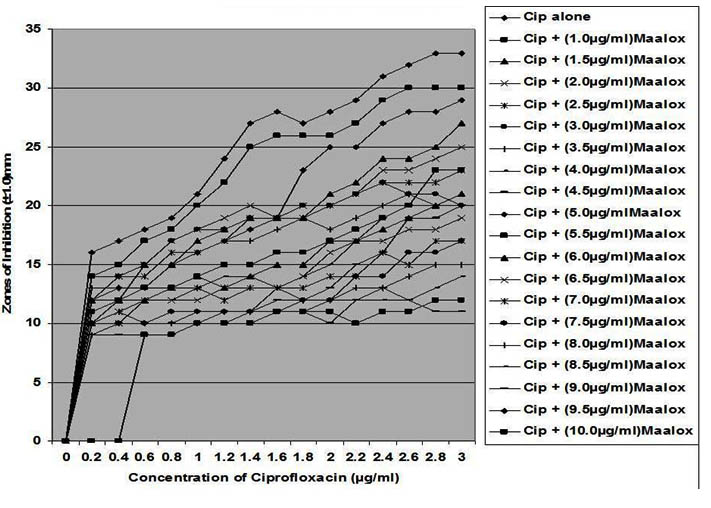
Fig. 4. In vitro susceptibility of Bacillus brevis to different concentrations of ciprofloxa-cin and its combination with magnesium-aluminum hydroxide
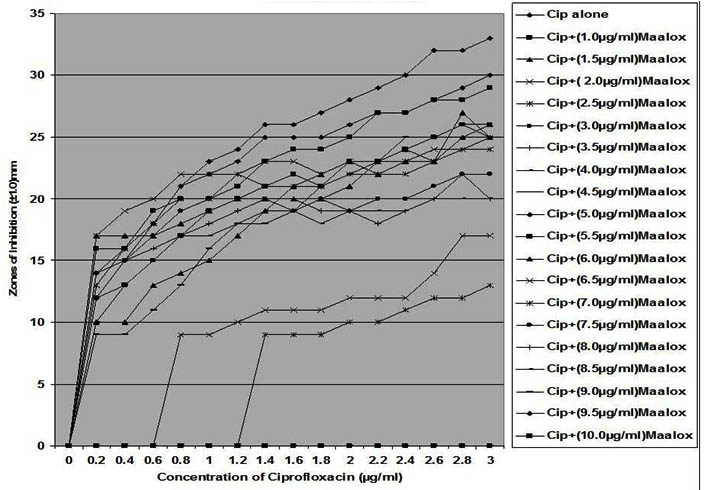
Fig. 5. In vitro susceptibility of Escherichia coli to different concentrations of ciproflox-acin and its combination with magnesium-aluminum hydroxide
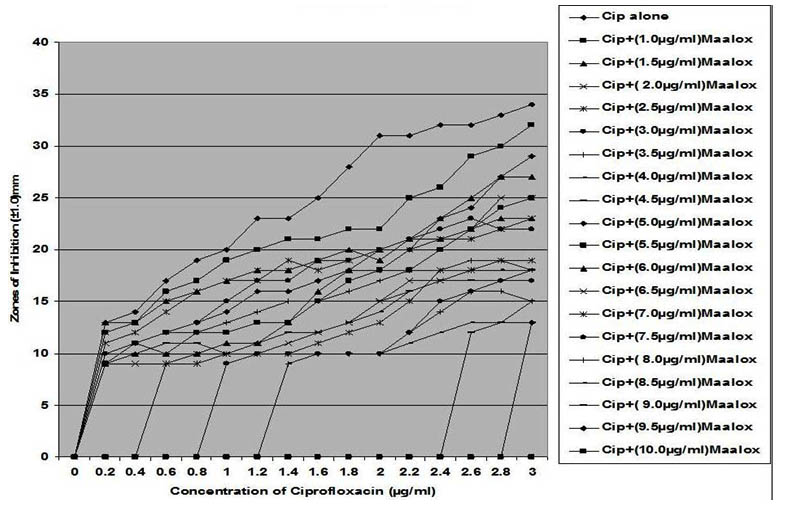
Fig. 6. In vitro susceptibility of Staphylococcus albus to different concentrations of ciprofloxacin and its combination with magnesium-aluminum hydroxide
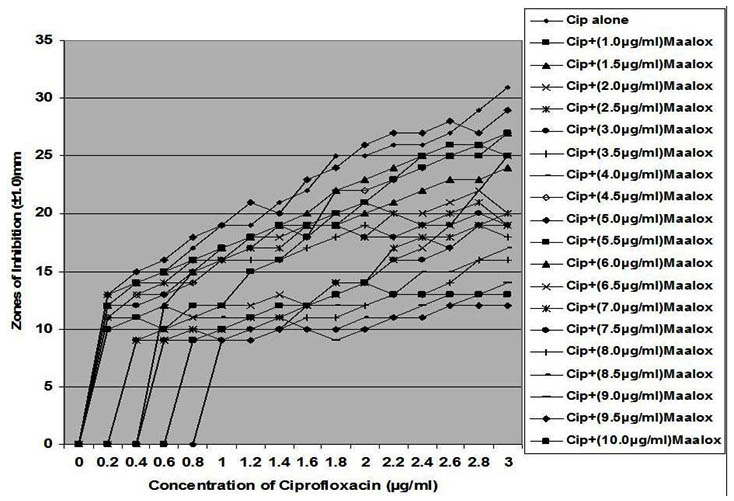
Fig. 7. In vitro susceptibility of Staphylococcus epidermidis to different concentrations of ciprofloxacin and its combination with magnesium/aluminum hydroxide
The interaction between CP and MA resulted in a complete lost or drastic reduction of antibacterial activities of CP as observed with the addition of 1.0 – 4.0 µg/ml and 8.0 – 10.0 µg/ml of the MA to the different concentrations of CP. However, there were increased antibacterial activities of CP in the presence of 4.5-6.0 µg/ml concentration of MA against the bacterial isolates. This increased antibacterial activities or synergy produced inhibition zones wider than those of CPMA at lower and higher concentration of the MA used. At lower concentrations (1 – 2 µg/ml) of MA combined with CP, more resistant colonies (Rc) were observed within the zones of inhibitions whereas at higher concentrations (8 – 10 µg/ml) of MA combined with CP, resistant colonies (Rc) within inhibition zones were drastically reduced or not observed within some inhibition zones. The resistant colonies (Rc) isolated from within the inhibition zones were characterized and further subjected to antibiotic susceptibility testing with standard antibiotic discs. These isolated resistant colonies (Rc) were less susceptible to the antibiotics when their inhibition zones were compared with those obtained from the original culture as shown in Table 1.
Table (1):
In vitro susceptibility of bacterial isolates and their mutants to different antibiotic discs
| Ofl (5 µg) | Cip (5 µg) | Pfx (5 µg) | Str (10 µg) | Gen (10 µg) | Chl (30 µg) | Amx (25 µg) | Cef (30 µg) | Ery (10 µg) | |
|---|---|---|---|---|---|---|---|---|---|
| Inhibition zones produced by original broth culture (± 1.00 mm) | |||||||||
| Staphylococcus aureus | 21 | 31 | 22 | 13 | 15 | 00 | 00 | 00 | 00 |
| Salmonella typhi | 21 | 33 | 20 | 00 | 00 | 00 | 00 | 00 | 00 |
| Pseudomonas aeruginosa | 32 | 30 | 16 | 00 | 00 | 00 | 00 | 00 | 00 |
| Escherichia coli | 30 | 32 | 32 | 16 | 00 | 00 | 00 | 00 | 00 |
| Bacillus brevis | 22 | 30 | 24 | 00 | 00 | 00 | 00 | 00 | 00 |
| Staphylococcus albus | 25 | 28 | 26 | 00 | 00 | 00 | 00 | 00 | 00 |
| Staphylococcus epidermidis | 25 | 32 | 30 | 00 | 00 | 00 | 00 | 00 | 00 |
| Ofl (5 µg) | Cip (5 µg) | Pfx (5 µg) | Str (10 µg) | Gen (10 µg) | Chl (30 µg) | Amx (25 µg) | Cef (30 µg) | Ery (10 µg) | |
| Inhibition zones produced by isolated mutant colonies (± 1.00 mm) | |||||||||
| Staphylococcus aureus | 16 | 20 | 19 | 00 | 00 | 00 | 00 | 00 | 00 |
| Salmonella typhi | 20 | 22 | 00 | 00 | 00 | 00 | 00 | 00 | 00 |
| Pseudomonas aeruginosa | 20 | 22 | 00 | 00 | 00 | 00 | 00 | 00 | 00 |
| Escherichia coli | 15 | 25 | 16 | 00 | 00 | 00 | 00 | 00 | 00 |
| Bacillus brevis | 20 | 22 | 22 | 00 | 00 | 00 | 00 | 00 | 00 |
| Staphylococcus albus | 18 | 26 | 20 | 00 | 00 | 00 | 00 | 00 | 00 |
| Staphylococcus epidermidis | 19 | 20 | 15 | 00 | 00 | 00 | 00 | 00 | 00 |
Key: Ofl – Ofloxacin; Cip – Ciprofloxacin; Pfx – Pefloxacin; Str – Streptomycin; Gen – Gentamicin; Chl – Chloramphenicol; Amx – Amoxicillin; Cef – Cefuroxime; Ery – Erythromycin
From this study, the susceptibility of bacteria to ciprofloxacin-magnesium-aluminum hydroxide (CPMA) indicated that synergistic and antagonistic interactions occurred in vitro. Synergy between CP and MA occur when concentrations of 4.5 – 6.0 µg/ml of MA was combined with CP and resulted in increase in the sizes of the inhibition zones as compared to those obtained from CP alone whereas interaction between CP and MA at other concentrations where there were resistant colonies within the inhibition zones indicated antagonism. This is contrary to previous study [41] that reported synergistic interaction at low concentrations of ciprofloxacin (0.78 to 1.85 µg/ml) and amphotericin B (0.24 to 0.39 µg/ml) and antagonistic interaction at higher concentrations of both ciprofloxacin (52.75 to 74.25 µg/ml) and amphotericin B (0.53 to 1.12 µg/ml). However, the synergy observed in vitro at lower concentrations is contrary to earlier in vivo reports that showed that aluminum-magnesium-containing antacids reduce the absorption of fluoroquinolone antibiotics42-44.
Although it has been reported that hydrophobic quinolones chelate outer membrane-bound magnesium to become more hydrophobic in order to diffuse across the exposed lipid domains of the outer membrane45, the combination of CP and MA may have prevented the CP from becoming more hydrophilic, thereby altering the outer membrane proteins and porin pathway to retard the accessibility of ciprofloxacin to their targets cells46. On the other hand, Palu et al47, Willmott and Maxwell48 and Bazile and Moreau49 suggested that quinolone-Mg2+ complex formed as a result of interaction between ciprofloxacin and magnesium-aluminum preparations interact with DNA and gyrase possibly by forming a Mg2+ bridge to phosphates in the DNA backbone 50 and not a direct interaction of free quinolones with DNA51. This showed that CPMA could have caused the selection of resistant strains and enhanced alteration of the antibacterial activities of CP at the target site of action resulting in the development of resistant colonies. While the in vitro interaction between CP and MA is consistent with several in vivo studies showing that magnesium-aluminum-containing antacids reduce the absorption of fluoroquinolone antibiotics42,43, this interaction is, possibly, due to chelation of the antibiotic by the metallic ions as reported in vivo by Höffken et al.52, Lode,53, and Randandt, et al.,24 with aluminum forming a very stable complex which is not easily soluble with quinolones54.
The biphasic activity observed with combinations of CP and MA, however, could not be explained with the simple complexes formed between CP and MA. While McCaffrey et al55, Kaatz et al56 and Piddock et al57 reported mechanism of resistance to include a spontaneous single-step chromosomal mutation resulting in alterations in the A subunits of DNA gyrase, an active efflux system that prevents net drug accumulation mediated by the norA gene and low-level resistance mediated by the cfx-ofx locs, the 4.5–6.0 µg/ml concentrations of the MA could have suppressed this spontaneous single-step chromosomal mutation which might not have been possible with the lower concentrations of 1.0 – 4.0 µg/ml and higher concentrations of 6.5 – 10.0 µg/ml. While increasing molecular mass, hydrophobicity and negative charge hinder penetration of antibiotics through porin channels, the increased antibacterial activity resulting from combining CP with 4.5 – 6.0 µg/ml concentration of MA could have decreased the hydrophobicity of CP to cause an effective concentration of this antibiotic to get to the target sites. Thus, combining CP with MA at these concentrations could enhance the ability of CP to penetrate the cell envelope and reach its target to produce antibacterial effects almost equal to what was obtained with CP alone in some cases.
Although Aldred et al58 indicated that quinolones convert gyrase and topoisemerase IV into toxic enzymes that fragment the bacterial chromosome, and mutations in gyrase or topoisomerase IV could lead to resistance59-61, combining CP with MA could have incited resistance mechanisms such as altered protein interactions, drug metabolism and uptake and/or efflux of CP from the cells62,63. The development of resistance and the isolation of resistant colonies could be responsible for therapeutic failure observed when there is co-administration of metallic ions such as magnesium-aluminum hydroxide with ciprofloxacin in vivo. This is of significant medical importance during chemotherapy of bacterial infections as Noyes and Polk64 had earlier reported failure of norfloxacin treatment resulting from concomitant treatment with an aluminum- and magnesium- containing antacid suspension while Preheim et al65 reported that antacids interfere with the efficacy of ciprofloxacin, particularly, in patients infected with Pseudomonas aeruginosa. Also, because currently used antibiotics are not bactericidal against most strains of bacteria at concentrations readily achievable in the serum, combination of drugs for synergistic bactericidal activity becomes necessary66. However, while these agents are generally safe in the medical setting, their use can result in interactions leading to adverse clinical outcomes including sub-minimal doses of antibiotics and serious morbidity in patients67. In addition to decrease in optimal plasma concentrations and urinary recoveries due to these interactions, there is also the potential for therapeutic failure, the development of antibiotic resistance and increases in health care costs26.
In conclusion, combining CP and MA could result in both synergistic and antagonistic interactions and the in vitro effect of the antagonistic interaction could lead to development of bacterial resistance. While ineffective actualization of treatment of bacterial infections may result in development of resistance among bacteria, pharmacokinetic data from previous studies and data from this study suggested that combination of CP and MA preparations in antimicrobial therapy should be discouraged not only due to production of sub-therapeutic doses but also because of development of bacterial resistance that may necessitate the use of more expensive and probably more toxic antibiotics. Patients should be encouraged to avoid taking the antacid within two hours after a fluoroquinolone dose or six hours prior to the next antimicrobial dose. However, the mechanisms of interactions resulting in both antagonistic and synergistic effects in vitro between ciprofloxacin and magnesium-aluminum hydroxide (Maalox) require further studies.
- Sultana, N., Arayne, MS., Hussain, F. In vitro monitoring of ciprofloxacin antacids interactions by UV & HPLC. Pak. J. Pharm. Sci., 2005, 18(4): 23-31.
- Arayne, M.S., Sultana, N., Hussain F. Interactions between ciprofloxacin and Antacids dissolution and adsorption studies. Drug Metab. Drug Interact., 2005, 21(2): 117-29.
- Stahlmann, R., Kuhner, S., Shakibaei, M., Schwabe, R., Flores, J., et al., Chondrotoxicity of ciprofloxacin in immature Beagle dogs: Immunohistochemistry, electron microscopy and drug plasma concentrations. Arch. Toxicol., 2000, 73: 564-572.
- Deborah, C.R., John, P.M., Allan, P., Paul, B., Peter, A.T., Allen, W. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic use. Drugs 1988, 35: 373-447.
- Oliphant, C.M., Green, G.M. Quinolones: a comprehensive review. Am. Fam. Physician 2002, 65: 455–464.
- Cornett, J.B., Wagner, R.B., Dobson, R.A., Wentland, M.P., Bailey, D.M. In vitro and in vivo antibacterial activities of the fluoroquinolone WIN 49375 (amifloxacin). Antimicrob. Agents Chemother. 1985, 27(1): 4–10.
- Hooper, D.C., Wolfson, J.S. Fluoroquinolone antimicrobial agents. N. Engl. J. Med., 1991, 423: 384-394.
- Ball, P., Tillotson, G. Tolerability of fluoroquinolone antibiotics. Past, present and future. Drug Saf. 1995, 13: 345-358.
- Papich, M. Antibacterial drug therapy. Focus on new drugs. Vet Clin. Pharm. Ther., 1998, 28: 215-231.
- Pfau, A., Sacks, T.G. Single dose quinolone treatment in acute uncomplicated urinary tract infection in women. J. Urol., 1993, 149: 532-534.
- Ridgway, G.L. Quinolones in sexually transmitted diseases. Drugs 1993, 45(Suppl. 3): 134-138.
- Moi, H., Morel, P., Gianotti, B., Barlow, D., Phillips, J.I. Comparative efficacy and safety of single oral doses of sparfloxacin versus ciprofloxacin in the treatment of acute gonococcal urethritis in men. J. Antimicrob. Chemother., 1996, 37(Suppl. A): 115-122.
- Dryden, M.S., Gabb, R.J.E., Wright, S.K. Empirical treatment of severe acute community acquired gastroenteritis with ciprofloxacin. Clin. Infect. Dis., 1996, 22: 1019-1025.
- Neuhauser, M.M., Weinstein, R.A., Rydman, R., Danziger, L.H., Karam, G., Quinn, J.P. Antibiotic resistance among Gram-negative bacilli in US intensive care units: implications for fluoroquinolone use. JAMA 2003, 289(7): 885–8.
- Friedland, I., Gallagher, G., King, T., Woods, G.L. Antimicrobial susceptibility patterns in Pseudomonas aeruginosa: data from a multicenter Intensive Care Unit Surveillance Study (ISS) in the United States. J. Chemother., 2004, 16(5): 437–41.
- MacDougall, C., Powell, J.P., Johnson CK, Edmond MB, Polk RE. Hospital and community fluoroquinolone use and resistance in Staphylococcus aureus and Escherichia coli in 17 US hospitals. Clin. Infect. Dis., 2005, 41: 435–40.
- Ball, P. Adverse reactions and interactions of Fluoroquinolones. Clin. Invest. Med., 1989, 12(1): 28-24.
- Wolfson, J.S., Hooper, D.C. Overview of Fluoroquinolones safety. Am. J. Med., 1991, 91(6A): 575-605.
- Petri, W.A. Sulfonamides, Trimethroprim-Sulforne Thoxazole, Quinolones and agents of urinary tract infections. In: Goodman Gilman’s the Pharmacological Buns of Therapeutics, Brunron L.L., (Ed.)., The MC Graw Hill Companies Inc., New York, pp: 2001, 1463-1476.
- Jaehde, U., Sörgel, F., Stephan, U., Schunack, W. Effect of an antacid containing magnesium and aluminum on absorption, metabolism and mechanism of renal elimination of pefloxacin in humans. Antimicrob. Agents. Chemother., 1994, 38(5): 1129-33.
- Stein, G. Pharmacokinetics and parmacodynamics of newer fluoroquinolones. Clin. Infect. Dis., 1996, 23(Suppl 1): S19-24.
- Zupancic, M., Bukovec, P. Complexation of magnesium (II) with ciprofloxacin and norfloxacin. Acta Pharm., 1996, 46: 221-8.
- Ma, H.H., Chiu, F.C., Li, R.C. Mechanistic investigation of the reduction in antimicrobial activity of ciprofloxacin by metal cations. Pharm. Res., 1997, 14(3): 366-70.
- Randandt, J.M., Marchbanks, C.R., Dudley, M.N. Interactions of fluoroquinolones with other drugs: mechanisms, variability, clinical significance and management. Clin, Infect. Dis., 1992, 14: 272-84.
- Shimada, J., Shiba, K., Oguma, T., Miwa, H., Yoshimura, Y., Nishikawa, T., Okabayashi, Y., Kitagawa, T., Yamamoto, S. Effect of antacid on absorption of the quinolone lomefloxacin. Antimicrob. Agents Chemother., 1992, 36: 1219-1224.
- O’Donnell, J., Gelone, S. Fluoroquinolones. Inf. Dis. Clin. North Am., 2000, 14(2): 489-504.
- Lubowski, T.J., Hightingale, C.H., Sweeney, K., Quintiliani, R. Effect of sucralfate on pharmacokinetics of fleroxacin in healthy volunteers. Antimicrob. Agents Chemother., 1992, 36: 2758-2760.
- Lehto, P., Kivisto, K.T. Effect of sucralfate on abosorption of norfloxacin and ofloxacin. Antimicrob. Agents Chemother., 1994, 38: 248-251.
- Campbell, N.R., Hasinoff, B.B. Iron supplements: A common cause of drug interactions. Brit. J. Clin. Pharmacol., 1991, 31(3): 251-255.
- Brouwers, J.R. Drug interactions with quinolones antibacterials. Drug Saf., 1992, 7(4): 268-81.
- Marchbank, C.R. Drug-drug interactions with fluoroquinolones. Pharmacother., 1993, 13(2 Pt 2): 23S-28S.
- Lehto, P., Kivisto, K.T., Neuvonen, P.J. The effect of ferrous sulphate on the absorption of norfloxacin, ciprofloxacin and ofloxacin. Br. J. Clin. Pharmacol., 1994, 37(1): 82-85.
- National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed. Approved standard M7–A3. National Committee for Clinical Laboratory Standards, Villanova, Pa, 1997.
- Holt, J.G., Krieg, N.R., Sneath, P.H.A., Williams, S.T. Staphylococcus spp. In: Bergey’s manual of determinative bacteriology, 9th ed. Baltimore, MD: Williams & Wilkiins; 1994, p. 544-51.
- Cheesbrough, M. Medical Laboratory Manual for Tropical Countries, ELBS ed; Tropical health technology publications and Butterworth–Heinemann Ltd: Cambridge, UK, 2002, 2: 2-392.
- Cheesbrough, M. District Laboratory Practice in Tropical Countries. Part 2: Cambridge University press, Cambridge, 2009, 62-69.
- BSAC, British Society of Antimicrobial Chemotherapy. Disc diffusion method for antimicrobial susceptibility testing. Brit. Soc. Antimicrob. Chemother., 2002, 2: 1-46.
- Bauer, A.W., Kirby, W.M., Sherris, J.C., Truck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol., 1966, 45: 493-496.
- Clinical and Laboratory Standard Institute (CLSI). Performance standards for Antimicrobial Susceptibility Testing Eighteenth informational supplement. M100 S18, 2008, 28(1): 46-52.
- Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing.19th Informational Supplement. (M100-S19), Wayne, Pa, USA, 2009.
- Stergiopoulou, T., Meletiadis, J., Sein, T., Papioannidou, P., Tsiouris, I., Roilides, E., Walsh, T.J. Isobolograpic analysis of pharmacodynamic interactions between antifungal agents and ciprofloxacin against Candida albicans and Aspergillus fumigatus Antimicrob. Agents Chemother., 2008, 52(6): 2196-2204.
- Nix, D.E., Watson, W.A., Handy, L., Frost, R.W., Rescott, D.L., Goldstein, H.R. The effects of sucralfate pretreatment on the pharmacokinetics of ciprofloxacin. 1989, 9: 377-380.
- Flor, S., Guay, D.R.P., Opsahl, J.A., Tack, K., Matzke, G.R. Effects of magnesium aluminum hydroxide and calcium carbonate antacids on bioavailability of ofloxacin. Antimicrob. Agents Chemother., 1990, 34: 2436-2438.
- Yamanaka-Yuen N.A., Gantu T.G. Fluoroquinolone drug interactions. Am. J. Hosp. Pharm., 1990, 47: 1270.
- Bazile, S., Moreau, N., Bouzard, D., Essiz, M. Relationships among antibacterial activity, inhibition of DNA gyrase, and intracellular accumulation of 11 fluoroquinolones. Antimicrob. Agents Chemother., 1992, 36: 2622-2627.
- Hirai, K., Suzue, S., Irikura, T., Iyobe, S., Mitsuhashi, S. Mutation producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob. Agents Chemother., 1987, 31: 582-586.
- Palu, G., Valisena, S., Ciarrochi, G., Gatto, B., Palumbo, P. Quinolone binding to DNA is mediated by magnesium ions. Proc. Natl. Acad. Sci., USA, 1992, 89: 9671-9675.
- Willmot, C.J.R., Maxwell, A. A single point mutation in the DNA gyrase a protein greatly reduces binding of fluoroquinolones to the gyrase-DNA complex. Antimicrob. Agents Chemother., 1993, 37: 126-127
- Bazile, S., Moreau, N.J. Interactions between fluoroquinolones, Mg2+, DNA and DNA gyrase, studied by phase partitioning in an aqueous two-phase system and by affinity chromatography. J. Chromatogr., 1994, 668: 241-247.
- Palumbo, M., Gatto, B., Zagotto, Z., Palu, G. On the mechanism of action of quinolone drugs. Trends Microbiol., 1993, 1: 232-235.
- Shen, L.L., Pernet, A.G. Mechanism of inhibition of DNA gyrase by analogues of nalidixic acid: the target of the drugs is DNA. Proc. Nat’l Acad. Sci., USA. 1985, 82: 307-311.
- Höffken, G., Lode, H., Wiley, R., Glatzel, R.D., Sievers, D., Olschewski, T., Borner, K., Koeppe. Pharmacokinetics and bioavailability of ciprofloxacin and ofloxacin: effect of food and antacid intake. Rev. Infect. Dis., 1988, 10(Suppl. 1): S138-S139.
- Lode, H. Drug interactions with quinolones. Rev. Infect. Dis., 1988; 10(Suppl. 1): 132-136.
- TimmersI, K., Sternglanz, R. Ionization and divalent cation dissociation constants of nalidixic and oxolinic acids. Bioinorg. Chem., 1978, 9: 145-155.
- McCaffrey, C., Bertasso, A., Pace, J., Georgopapadakou, N.H. Quinolone accumulation in Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Antimicrob. Agents Chemother., 1992, 36: 1601-5.
- Kaatz, G.W., Seo, S.M., Buble, C.A. Efflux-mediated fluoroquinolones resistance in Staphylococcus aureus. Antimicrob. Agents Chemother., 1993, 37: 1086-10.
- Piddock, L.J.V., Jin, Y.F., Griggs, D.J. Effect of hydrophobicity and molecular mass on the accumulation of fluoroquinolones by Staphylococcus aureus. J. Antimicrob. Chemother., 2001, 47(3): 261-270.
- Aldred, K.J., Kerns, R.J., Osheroff, N. Mechanism of quinolone action and resistance. Biochem., 2014, 53: 1565-1574.
- Wohlkonig, A., Chan, P.F., Fosberry, A.P., Homes, P., Huang, J., Kranz, M., Leydon, V.R., Miles, T.J., Pearson, N.D., Perera, R.L., Shillings, A.J., Gwynn, M.N., Bax, B.D. Structural basis of quinolone inhibition of type IIA topoisomerases and target-mediated resistance. Nat. Struct. Mol. Biol., 2010, 17: 1152″1153.
- Aldred, K.J., McPherson, S.A., Wang, P., Kerns, R.J., Graves, D.E., Turnbough, C.L., Jr., Osheroff, N. Drug interactions with Bacillus anthracis topoisomerase IV: Biochemical basis for quinolone action and resistance. Biochem., 2012, 51: 370″381.
- Aldred, K.J., McPherson, S.A., Turnbough, C.L., Jr., Kerns, R.J., Osheroff, N. Topoisomerase IV-quinolone interactions are mediated through a water-metal ion bridge: Mechanistic basis of quinolone resistance. Nucleic Acids Res. 2013, 41: 4628″4639.
- Hooper, D.C. Emerging mechanisms of fluoroquinolone resistance. Emerg. Infect. Dis., 2001, 7: 337″341.
- Guan, X., Xue, X., Liu, Y., Wang, J., Wang, Y., Wang, K., Jiang, H., Zhang, L., Yang, B., Wang, N., and Pan, L. Plasmidmediated quinolone resistance-current knowledge and future perspectives. J. Int. Med. Res. 2013, 41: 20″30.
- Noyes, M., Polk, R.E. Norfloxacin and absorption of magnesium-aluminium. Ann. Intern. Med., 1988, 109(2): 168-9.
- Preheim, I.C., Cuevas, T.A., Roccaforte, J.S., Mellencamp, M.A., Bittner, M.J. Ciprofloxacin and antacids. Lancet 1985, i: 48. (Letter).
- Shen, M.A., Scheld, W.M. Combination antibiotic therapy of bacterial endocarditis. Ann. Intern. Med., 1980; 92: 390-395.
- Hersh, E.V. Adverse drug interactions in dental practice: interactions involving antibiotics. Part II of a series. J. Am. Dent. Assoc., 1999, 130(2): 236-51.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


