ISSN: 0973-7510
E-ISSN: 2581-690X
Some microorganisms known as endophytes live in symbiotic relationships in the living tissues of plants without posing a health risk. As a result, they synthesize many metabolites which are helpful for the plants in many ways. So, these metabolites are known to exhibit many biological properties like antioxidant, antidiabetic anti-inflammatory, etc. Currently, many drugs are used to control inflammatory diseases like arthritis and, irritable bowel disease; however, they pose a lot of side effects. The present study was taken up to explore the anti-inflammatory properties along with the phytochemicals present, its quantification, and other in vitro biological activities of the less reported Aspergillus melleus, an endophytic fungus, isolated from Premna serratifolia L., a medicinal plant. The results of the investigation demonstrated the presence of alkaloids, phenols, terpenoids, flavonoids, tannins, cardiac glycosides and amino acids in the methanolic extract of endophytic fungus. It yielded 25.28 µg GAE/g and 19.465 µg GAE/g of total phenolic and flavonoid content, respectively. The results of anti-inflammatory activity showed 84.69+0.82% protein inhibition by BSA and also showed IC50 values of 68.53 µg/mL and 43.34 µg/mL for COX1 and COX 2, respectively. It exhibited 63.91+0.08% of radical scavenging activity by DPPH. The IC50 values of 181.41 µg/mL and 190.62 µg/mL were found for the in vitro antidiabetic activity. This study shows that the endophytic fungus A. melleus has exhibited considerably good results with respect to its in vitro biological activities. Yet, there is a scope for future researchers to isolate the bioactive metabolites to explore for future needs.
Aspergillus melleus, Anti-inflammatory Activity, COX-1, COX-2, Antidiabetic Activity
Inflammation is a bodily response to external agents such as wounds, infection and other irritants like chemicals. During this process, many inflammatory responses are incited which are mediated by proinflammatory mediators that release certain proteins, prostaglandins, nitric oxide, and oxygen free radicals.1 Also, these pro-inflammatory mediators like interleukin 1b, interleukin-6 and TNF-a initiate the preliminary/ primary inflammatory responses.2 Along with enzymes like cyclooxygenases, they help in the release of prostaglandins, which are responsible for inflammatory responses.3 The release of free radicals like nitric oxide and oxygen enhances oxidative stress and inflammatory responses.4 Inflammatory diseases like arthritis, lupus or vasculitis are usually treated with steroidal anti-inflammatory and non-steroidal anti-inflammatory drugs. The overuse of these drugs leads to serious health concerns like cardiovascular or renal failures in addition to gastrointestinal disorders and bleeding.5 Hence, there is a need to search for safe and effective anti-inflammatory molecules.
In recent years, there has been an increase demand for exploring natural and safe products with less side effects. As a result, pharma companies and agriculture industries are continuously screening for microorganisms and microbial products.6 Furthermore, there is also more inclination towards the products from unexplored sources.7 Endophytes are the microorganisms residing in the tissues of plants without disease conditions. They are in symbiotic association with the host plant.8 They are known to produce metabolites which protect the host. These secondary metabolites possess antimicrobial, anti-inflammatory and antiviral properties.9,10
For the present study, the leaves of the medicinal plant Premna serratifolia L. were selected. It belongs to the family Lamiaceae. The tree has various ethnomedicinal values, hence, it is used by traditional healers. It is mainly used to treat rheumatism, diabetes, hypoglycaemia gout, liver problems, cardiac troubles, etc.11 The current study was aimed to explore the anti-inflammatory activities in addition to studying the in vitro biological activities of less explored/reported fungal endophyte Aspergillus melleus, isolated from the medicinal plant Premna serratifolia L.
Isolation, molecular identification and biomass cultivation
The leaves from the medicinal plant Premna serratifolia L. were collected and washed in gently running tap water to remove the soil particles attached to them. The leaves were surface sterilized using 4% sodium hypochlorite solution. The cut leaves were placed on the solid media for 2-5 days12 and a pure culture of the fungus was obtained, which was sequenced using 18S rRNA sequencing technique. The obtained pure culture of endophytic fungus was identified by comparing the query sequence in NCBI-BLAST and the phylogenetic tree was constructed with the help of MEGA X software.13
Mass culturing of the endophytic fungus was done by growing the fungus for 21 days in a liquid medium. The methanolic extract of endophytic fungus was obtained by maceration method using the solvent methanol. The extract was concentrated using the Rota evaporator at 40°C at 60 rpm, and later which was stored for further activities.
Phytochemical analysis
The presence of secondary metabolites in the methanolic extract of the endophytic fungus was tested for phenolics, flavonoids, alkaloids, saponins, tannins, terpenoids, carbohydrates, amino acids, coumarins and cardiac glycosides.14
Total phenolic content
The total phenolic content of the methanolic extract of endophytic fungus was found by using FCR reagent and gallic acid as the standard.15 To 2.5 mL of FCR reagent, 0.5 mL of endophytic fungal extract was added and was incubated in the dark at room temperature for 30 minutes. Later the absorbance of this mixture was read at 765 nm. The phenolic content was expressed in terms of µg GAE/g.
Total flavonoid content
Methanolic extract of endophytic fungus was subjected for total flavonoid content using quercetin as the standard.16 0.5mL of the fungal extract was added to the mixture of 10% aluminium chloride, sodium acetate, and methanol. The reaction was maintained at room temperature for 30 min and the absorbance was read at 415 nm. The flavonoid content was expressed as µg QE/g.
In vitro antioxidant activities
In vitro antioxidant activities of the methanolic extract of endophytic fungus were carried out by phosphomolybdenum assay, ferric reducing activity assay and radical scavenging assay using DPPH.
PM (Phosphomolybdenum) assay
The total antioxidant power of the fungal extract was found out using a mixture of ammonium molybdate and sodium phosphate. Various concentrations of the extract were treated with equal volumes of the reagent. It is incubated at 95°C for 90 mins. On cooling, the absorbance was read at 695 nm.17
FRAP (Ferric Reducing Antioxidant Power) assay
Using the standard procedure,18 the total reducing power of the endophytic fungus methanolic extract was determined. Herein, the antioxidants included in the endophytic fungal extract undergo the reaction such as reduction of Fe+3 to Fe+2. The reaction mixture consisted of 1% potassium ferricyanide, 2.5 mL of phosphate buffer, and various quantities of fungal extracts were combined and incubated for 30 minutes. 10% trichloroacetic acid was then added, and centrifugation was run for 10 minutes at 3000 rpm. To the collected supernatant, 2.5 mL of distilled water and 0.5 mL of ferric chloride were added. At 700 nm, the absorbance was recorded and the standard reference used was ascorbic acid.
DPPH (Radical Scavenging) assay
The endophytic fungal extract’s capacity for radical scavenging was determined using DPPH assay.19 An equal volume of 0.1 mM DPPH was combined endophytic fungus’s methanolic extract. After 30 minutes of dark incubation at room temperature, the absorbance of this mixture was measured at 517 nm. Inhibition percentage of DPPH was calculated using the formula, % of inhibition= [(Ac-As/Ac)] × 100, where Ac is the absorbance of the control and As is the absorbance of the sample.
In vitro antidiabetic assay
α-amylase inhibition assay
Pancreatic amylase was used for this experiment. 0.1 mL of phosphate buffer and 0.2 mL of saline were added to varying amounts of methanolic extract of endophytic fungus. The mixture was then incubated at 25°C for 10 minutes, after which 0.4 mL of DNS solution was added. This was further incubated at 90°C for 5 minutes. The absorbance was read at 500 nm after cooling. % Of inhibition = (1-As/Ac) × 100 where Ac is the absorbance of the control and As is the absorbance of the sample, was the formula used to determine the percentage of inhibition.20
α-glucosidase inhibition assay
Various concentrations of endophytic fungal extract were added to the mixture of phosphate buffer and a-glucosidase and incubated for 5 minutes at 37°C. 200 µL of p-nitrophenyl-alpha-D-glucopyranoside was added and incubated at 37°C for 30 minutes which was followed by the addition of 500 µL of Na2CO3. The absorbance of the end product was read at 405 nm and the percentage inhibition was calculated using the formula, % inhibition = (1-As/Ac) × 100, where As is the absorbance of the sample and Ac is the absorbance of the control.21
In vitro anti-inflammatory assay
Protein denaturation assay
Using BSA, the anti-inflammatory properties of the endophytic fungus’s methanolic extract were investigated. Different amounts of the endophytic fungal extract were added to 1.8 mL of 1% BSA solution, and the pH was adjusted to 6.5. This mixture was heated for 15 minutes at 60°C after being incubated for 20 minutes at room temperature. The absorbance was measured at 660 nm after cooling. The equation used to compute the percentage inhibition of protein denaturation, is % inhibition= (Ac-As/Ac) × 100, As represents the sample absorbance and Ac represents the control absorbance.22
In vitro cyclooxygenase inhibitory activity
Varying concentrations of the endophytic fungal extract were treated with equal concentrations of enzyme solution. Arachidonic acid was added to start the reaction. This mixture was incubated at 37°C for ten minutes. The extract’s IC50 value was determined to determine its strength. The formula used to compute the percentage inhibition of the activity was, % inhibition=(1-As/Ac) × 100, where As is the sample’s absorbance and Ac is the control absorbance.23,24
Statistical analysis
The readings in the graph are given as the mean ± standard error, and the experiments were carried out in triplicate. One-way ANOVA was used to obtain the p-value, and the p-value of less than 0.05 was deemed statistically significant.
Isolation, characterization and fermentation
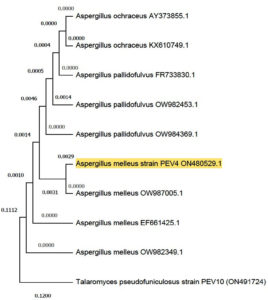
The results upon subjecting for isolation indicate that Premna serratifolia L. represented a good source of endophytes. The obtained pure culture of endophytic fungus was morphologically identified using Barnet and Hunter. The sequence of phylogenetically identified endophytic fungus Aspergillus melleus was deposited in GenBank with accession ID ON480529. With the help of 18S rRNA data, the phylogenetic tree was constructed by the neighbour joining method with boot strap values as shown in Figure 1. The concentrated endophytic fungal extract extracted by maceration was used for future experiments.
Figure 1. Phylogenetic tree of Aspergillus melleus (Accession number ON480529) constructed by neighbour joining method
Phytochemical analysis
The primary and the secondary phytoconstituents such as the flavonoids, alkaloids, phenolics, tannins, terpenoids, and cardiac glycosides were present in the methanolic extract of the endophytic fungus, Aspergillus melleus. However, the secondary metabolites such as saponins, amino acids and coumarin were not detected, (Table 1).
Table (1):
Phytochemical analysis of endophytic fungus extract
Phytochemicals |
Endophytic fungus extract |
|---|---|
Alkaloids |
+ |
Flavonoids |
+ |
Phenols |
+ |
Terpenoids |
+ |
Tannins |
+ |
Saponins |
– |
Cardiac glycosides |
+ |
Amino acids |
– |
Carbohydrates |
+ |
Coumarin |
– |
Total phenolic and flavonoid content (TPC and TFC)
The methanolic extract of the endophytic fungus on determination of the phenolic content yielded 25.28 µg GAE/g, while its total flavonoid was found to be 19.465 µg QE/g, suggesting that flavonoid content was slightly lower than the phenolic content (Table 2).
Table (2):
Total phenolic and flavonoid content
Test |
Aspergillus melleus |
|---|---|
Total Phenolic Content (µg GAE/g) |
25.28 |
Total Flavonoid Content (µg GAE/g) |
19.465 |
In vitro antioxidant activities
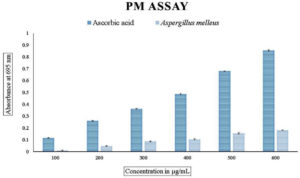
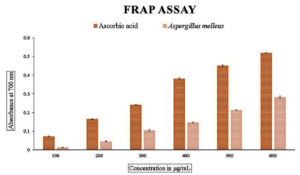
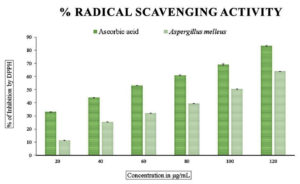
The total antioxidant power assay carried out using phosphomolybdate showed that the values ranged from 0.009+0.17 to 0.181+0.20 compared to the standard ascorbic acid as seen in Figure 2. A similar pattern of values could be seen in the total reducing power of the endophytic fungal extract carried out using the ferricyanide reagent. The values ranged from 0.013+0.17 to 0.283+0.53, indicating that the values increased with the increase in the concentration of the endophytic fungal extract as shown in Figure 3. The radical scavenging method, the reduction of DPPH, was also used to find out the antioxidant power of the methanolic extract of the endophytic fungus. The percentage scavenging ranged from 11.40% to 63% with IC50 value of 96.14 µg/mL as compared to the standard ascorbic acid which ranged from 33% to 83% which showed the IC50 value of 54.88 µg/mL (Figure 4 and Table 3).
Table (3):
IC50 values of samples
| IC50 values µg/mL | ||||||
|---|---|---|---|---|---|---|
| Samples | DPPH | Alpha amylase | Alpha glucosidase | Anti-inflammatory | COX 1 | COX 2 |
| Aspergillus melleus | 96.14 | 181.41 | 190.62 | 118.20 | 68.53 | 43.34 |
| Metformin | – | 79.71 | – | – | – | – |
| Acarbose | – | – | 47.68 | – | – | – |
| Diclofenac sodium | – | – | – | 60.72 | – | – |
| Ascorbic acid | 54.88 | – | – | – | – | – |
| STD | – | – | – | – | 49.41 | – |
| STD | – | – | – | – | – | 25.61 |
In vitro antidiabetic assay
α-amylase inhibition assay
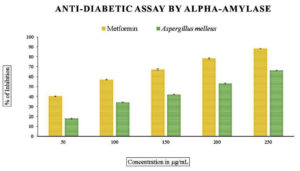
When compared to standard metformin, which ranged from 27% to 85% and an IC50 value of 79.71 µg/mL, the methanolic extract of endophytic fungus showed the inhibition of enzyme a-amylase, the IC50 value of 181.41 µg/mL after subjecting it to an in vitro antidiabetic analysis (Figure 5 and Table 3).
Figure 5. In vitro anti-diabetic assay by α-amylase inhibition activity. Data were expressed as mean±SE
α-glucosidase inhibition assay
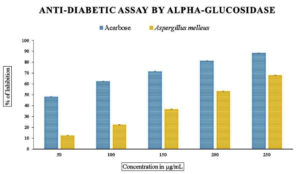
The in vitro antidiabetic assay using a-glucosidase enzyme with methanolic extract of endophytic fungus revealed variation in the percentage. Figure 6 and table 3 illustrated the range of inhibition, which was 12% to 64% with an IC50 value of 190.62 µg/mL for fungal extract, in contrast to the typical standard acarbose, which was from 40 % to 88% and an IC50 value of 47.68 µg/mL.
Figure 6. In vitro anti-diabetic assay by α-glucosidase inhibition activity. Data were expressed as mean±SE
In vitro anti-inflammatory assay
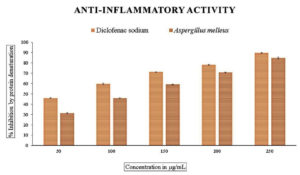
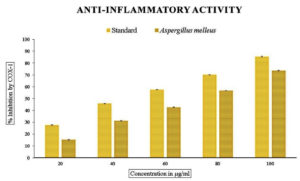
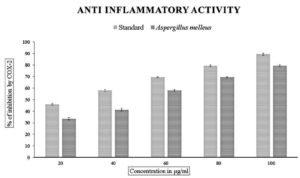
The methanolic extract subjected to in vitro anti-inflammatory activity was done by finding out the percentage of denaturation using bovine serum albumin with diclofenac sodium as the standard, which showed the inhibition percentage ranging from 45% to 90% with an IC50 value of 60.72 µg/mL. The cyclooxygenase inhibitory activity with respect to COX-1 ranged from 15-73% and 33-79% for COX-2 with IC50 values of 68.53 and 43.34 for COX-1 and COX-2, respectively (Figures 7, 8, and 9 and Table 3).
Figure 7. In vitro anti-inflammatory activity by protein denaturation method. Data were expressed as mean±SE
Endophytic fungi are the organisms which inhabit the plant by spending their life cycle or part of their life cycle. They are the source of many novel compounds with many therapeutic applications. Thus, the secondary metabolite synthesized by endophytic fungi are used in agricultural and pharmaceutical industries.25 These endophytic fungi produce metabolites which are very much similar to the host plant. Premna serratifolia L. is an important medicinal plant with medicinal properties like anti-inflammatory,26 antidiabetic,27 antioxidant,28 and anti-parasitic.29 These properties indicate that the plant possesses the various phytoconstituents which impart them the above-mentioned qualities.
The presence of almost all the phytochemicals can be seen in the crude methanolic extract of the endophytic fungus Aspergillus melleus. The quality and the quantity of bioactive substances reduced by the endophytic fungus depends on the type of media used and also the environmental interaction.30 In the current study, a crude extract of the endophytic fungus confirmed the presence of almost all the phytochemicals like phenolic, flavonoid, alkaloids, tannins, terpenoids, cardiac glycosides and carbohydrates. Previous studies report that there is a direct correlation between phenolic content and the antioxidant property.31
Synthesis of harmful free radicals has shown to have a deleterious effect on human health and hence, to combat the effects, they need to be supplied with therapeutic bioactive molecules. The bioactive molecules from endophytic fungi have been proven to be safer alternatives in the drug formulations.32 The present work suggests that the total phenolic content was higher than the flavonoid content suggesting that it may exhibit higher antioxidant properties. Our results show that the organism A. melleus exhibited a dose-dependent increase in the antioxidant properties concerning total reducing power assay and total antioxidant assay. In the case of radical scavenging by DPPH, the fungus exhibited 63.91+0.08 per cent of inhibition with an IC50 value of 96.14 µg/mL. Our findings are consistent with the results on other endophytic fungi. Huang and his coworkers reported moderate antioxidant activity in most of the endophytic fungi isolated from Nerium oleander.33 The endophytic fungi Colletotrichum tropicale, Colletotrichum gloeosporioides and Fusarium solani isolated from Justicia gendarussa showed moderate activity.34
The alpha-amylase inhibition assay revealed that our organism was exhibited 66.24+0.23%. This result was consistent with the results obtained in the study. Aspergillus sp isolated from Ficus religiosa showed 91± 0.06% inhibition.35 In another study, Curvularia lunata isolated from Ficus religiosa exhibited the IC50 value of 0.35± 0.46 mg/mL.36
Our study shows that the organism A. melleus exhibited good anti-inflammatory activity with respect to the protein denaturation method by BSA and also with respect to the enzymes COX-1 and COX-2 which are released in response to the body’s reaction during the inflammation process. Our results show that the organism, Aspergillus melleus exhibited inhibition ranging from 31% to 85% in comparison with standard diclofenac sodium. Interestingly, much better activity was exhibited when subjected to cyclooxygenases with IC50 values of 68.53 µg/mL and 43.34 µg/mL for COX-1 and COX-2 respectively. This result shows that our organism fared better in comparison with the endophytic fungi Aspergillus, Penicillium, and Alternaria exhibited around 78+1% of anti-inflammatory activity.22,37 This activity with respect to other fungi are less reported.
The medicinal plant Premna serratifolia L. is a host to many endophytic fungi. The organism, Aspergillus melleus exhibited considerable phytoconstituents as evidenced by its the phytochemical analysis, quantitative and flavonoid evaluations which was revealed in its phenolic content and also its antioxidant activities. In addition to these, the organism exhibited good anti-inflammatory activities and moderate antidiabetic activities. However, there is a need for further research to explore for bioactive compounds present in the organism which could be exploited for pharmaceutical and agricultural needs for future.
ACKNOWLEDGMENTS
The authors are thankful to the Department of Biotechnology and Microbiology, Karnatak University, Dharwad, Karnataka, for providing the necessary facilities for conducting the research experiments.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Sychrova A, Kolarikova I, Zemlieka M, Smejkal K. Natural compounds with dual antimicrobial and anti-inflammatory effects. Phytochem Rev. 2020;1471-502.
Crossref - Kaneko N, Kurata M, Yamamoto T, Morikawa S, Masumoto J. The role of interleukin-1 in general pathology. Inflamm Regen. 2019;39:1-16.
Crossref - Spina R, Ropars A, Bouazzi S, et al. Screening of Anti-Inflammatory Activity and Metabolomics Analysis of Endophytic Fungal Extracts; Identification and Characterization of Perylenequinones and Terpenoids from the Interesting Active Alternaria Endophyte. Molecules. 2023;28(18):6531.
Crossref - Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30:11-26.
Crossref - Saleh A, Negm WA, El-Masry TA, et al. Anti-inflammatory potential of Penicillium brefeldianum endophytic fungus supported with phytochemical profiling. Microbial Cell Factories. 2023;22(1):83.
Crossref - Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629-661.
Crossref - Niu G, Li W. Next-generation drug discovery to combat antimicrobial resistance. Trends Biochem Sci. 2019;44(11):961-972.
Crossref - Nair DN, Padmavathy SJ. Impact of endophytic microorganisms on plants, environment and humans. Sci. World J. 2014.
Crossref - Hassane AM, Hussien SM, Abouelela ME, et al. In vitro and in silico antioxidant efficiency of bio-potent secondary metabolites from different taxa of black seed-producing plants and their derived mycoendophytes. Front Bioeng Biotechnol. 2022;10:930161.
Crossref - Deshmukh SK, Gupta MK, Prakash V, Saxena S. Endophytic fungi: a source of potential antifungal compounds. J Fungi. 2018;4(3):77.
Crossref - Quattrocchi U. CRC world dictionary of medicinal and poisonous plants: common names, scientific names, eponyms, synonyms, and etymology (5 Volume Set). CRC press.2012.
- Singh P, Sharma A, Bordoloi M, Nandi SP. Molecular identification of endophytic fungi isolated from medicinal plant. Biointerface Research in Applied Chemistry. 2020;10(5):6436-6443.
Crossref - Talukdar R, Padhi S, Rai AK, et al. Isolation and characterization of an endophytic fungus Colletotrichum coccodes producing tyrosol from Houttuynia cordata Thunb. using ITS2 RNA secondary structure and molecular docking study. Front Bioeng Biotechnol. 2021;9:650247.
Crossref - Kumar V, Prasher IB. Phytochemical Analysis and Antioxidant Activity of Endophytic Fungi Isolated from Dillenia indica Linn. Appl Biochem Biotechnol. 2024;196(1):332-349.
Crossref - Marsola SJ, Jorge LF, Meniqueti AB, et al. Endophytic fungi of Brunfelsia uniflora: Isolation, cryopreservation, and determination of enzymatic and antioxidant activity. World J Microbiol Biotechnol. 2022;38(6):94.
Crossref - Santos TF, dos Santos Carvalho C, de Almeida MA, Delforno TP, Duarte IC. Endophytic fungi isolated from Brazilian medicinal plants as potential producers of antioxidants and their relations with anti-inflammatory activity. 3 Biotech. 2020;10(5):223.
Crossref - Chutulo EC, Chalannavar RK. Daldinia eschscholtzii: an endophytic fungus isolated from Psidium guajava as an alternative source of bioactive secondary metabolites. Asian J Mycol. 2020;3(1):376-398.
Crossref - Gan PT, Lim YY, Ting AS. Inducing antioxidant and antimicrobial activities in endophytic and endolichenic fungi by the use of light spectra treatments. Arch Microbiol. 2023;205(9):304.
Crossref - Gautam VS, Singh A, Kumari P, et al. Phenolic and flavonoid contents and antioxidant activity of an endophytic fungus Nigrospora sphaerica (EHL2), inhabiting the medicinal plant Euphorbia hirta (dudhi) L. Arch Microbiol. 2022;204(2):140.
Crossref - Mukhammedov II, Ruzieva DM, Gulyamova TG. Comparative Evaluation of Antidiabetic and Antioxidant Activities of Methanol Fractions of Penicillium brevicaule alba CC200 and Aspergillus egypticus HT166S. Moscow University Biological Sciences Bulletin. 2022;77(4):245-50.
Crossref - Sharma A, Kaur R, Kaur J, Garg S, Bhatti R, Kaur A. An endophytic Schizophyllum commune Fr. exhibits in-vitro and in-vivo antidiabetic activity in streptozotocin induced diabetic rats. AMB Express. 2021;11:1-1.
Crossref - Govindappa M, Channabasava R, Sowmya DV, et al. Phytochemical screening, antimicrobial and in vitro anti-inflammatory activity of endophytic extracts from Loranthus sp. Pharmacognosy Journal. 2011;3(25):82-90.
Crossref - Pham VC, Shin JS, Choi MJ, et al. Biological evaluation and molecular docking study of 3-(4-sulfamoylphenyl)-4-phenyl-1H-pyrrole-2, 5-dione as COX-2 inhibitor. Bulletin of the Korean Chemical Society. 2012;33(2):721-724.
Crossref - Oniga SD, Pacureanu L, Stoica CI, et al. COX inhibition profile and molecular docking studies of some 2-(trimethoxyphenyl)-thiazoles. Molecules. 2017;22(9):1507.
Crossref - Bisht R, Sharma D, Agrawal PK. Antagonistic and antibacterial activity of endophytic fungi isolated from needle of Cupressus torulosa D. Don. Asian J Pharm Clin Res. 2016;9(3):282-288.
- Simamora A, Santoso AW, Timotius KH, et al. Antioxidant activity, enzyme inhibition potentials, and phytochemical profiling of Premna serratifolia L. leaf extracts. Int. J. Food Sci., 2020.
Crossref - Dash GK, Patro CP, Maiti AK. A study on the anti-hyperglycaemic effect of Premna corymbosa Rottl. roots. Journal of Natural Remedies. 2005(1):31-34.
- Rajendran R, Basha NS, Ruby S. Evaluation of In vitro Antioxidant Activity of stem-bark and stem-wood of Premna serratifolia Lin.,(Verbenaceae). Research Journal of Pharmacognosy and Phytochemistry. 2009;1(1):11-14.
- Desrivot J, Waikedre J, Cabalion P, et al. Antiparasitic activity of some New Caledonian medicinal plants. J Ethnopharmacol. 2007;112(1):7-12.
Crossref - Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev. 2003;67(4):491-502.
Crossref - Kefayati Z, Motamed SM, Shojaii A, Noori M, Ghods R. Antioxidant activity and phenolic and flavonoid contents of the extract and subfractions of Euphorbia splendida Mobayen. Pharmacognosy Research. 2017;9(4):362-365.
Crossref - Gouda S, Das G, Sen SK, et al. Endophytes: a treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016; 7:1538.
Crossref - Huang WY, Cai YZ, Hyde KD, Corke H, Sun M. Endophytic fungi from Nerium oleander L (Apocynaceae): main constituents and antioxidant activity. World J Microbiol Biotechnol. 2007;23:1253-1263.
Crossref - Mahmud SN, Sohrab MH, Begum MN, et al. Cytotoxicity, antioxidant, antimicrobial studies and phytochemical screening of endophytic fungi isolated from Justicia gendarussa. Ann Agric Sci. 2020;65(2):225-232.
Crossref - Tiwari P, Nathiya R, Mahalingam G. Antidiabetic activity of endophytic fungi isolated from Ficus religiosa. Asian J Pharm Clin Res. 2017;59-61.
Crossref - Jayant KK, Vijayakumar BS. In-Vitro anti-oxidant and anti-diabetic potential of endophytic fungi associated with Ficus religiosa. Ital J Mycol. 2021;50:10-20.
- Moharram AM, Zohri AA, Omar HM, Abd El-Ghani OA. In vitro assessment of antimicrobial and anti-inflammatory potential of endophytic fungal metabolites extracts. Eur J Biol Res. 2017;7(3):234-44.
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.