ISSN: 0973-7510
E-ISSN: 2581-690X
Many infectious diseases can be treated using herbal medicines. Therefore, plant materials play a major role in therapeutic medicine and are widely used in many developing countries. In this study, we analyzed the potential of Avicennia marina and Suaeda monoica leaf extracts as antidermatophytic agents. Molecular identification of the plant samples was performed via DNA sequencing of the internal transcribed spacer region using the primers ITS-u1 and ITS-u4. Leaf extracts of A. marina and S. monoica were prepared in cold and hot distilled water. Their antidermatophytic activities were evaluated against Trichophyton mentagrophytes, T. verrucosum, Microsporum gallinae, M. gypseum, M. canis, Epidermophyton floccosum, Candida albicans, and C. tropicalis using the dry weight method. E. floccosum was the most sensitive to both cold extracts of A. marina and S. monoica, whereas T. verrucosum was the most sensitive to the hot extract of A. marina. The minimum inhibitory concentrations of the hot extracts were determined. They ranged from 10 to 30 mg/ml, defining the anti-scavenging activity and total phenolic content of both plants. The hot extract of A. marina possessed the highest anti-scavenging activity (76%), whereas the cold extract of A. marina contained the highest phenolic content (40.06 mg/g dry weight). In addition, high-performance liquid chromatography was used to separate and estimate some of the bioactive compounds present in the plant extracts.
Avicennia Marina, Suaeda Monoica, dermatophytosis, anti-scavenging, total phenolic content, HPLC
Fungi, which are the major causative microorganisms of superficial mycosis, are clinically classified as dermatophytes, and can cause infections (mycoses).1 The infection causes dermatophytosis, generally referred to as ringworm, or filamentous keratinophilic disease, in which keratinized tissue (skin, hair, and nails) of the hosts is utilized as the supplement source.2,3 The mycoses formed by yeasts have the ability to parasitize keratin-rich tissues, prompting a dermal provocative reaction resulting in unusual itchiness and a corrective appearance.4
The efficacy of medicinal plants against pathogenic microorganisms is well known, their potential as novel antimicrobial compounds.5,6 Medicinal plants can serve therapeutic purposes without exerting any adverse effects that are often associated with synthetic antimicrobials.7 The marine environment is the richest of all the global resources and contains biologically active metabolic compounds.8 Many chemically unique compounds with different biological activities of marine origin have been isolated and are under investigation or development. This represents a great challenge that requires input from various scientific areas to realize the full therapeutic potential of marine compounds.9 Avicennia marina is a species of mangrove tree that belongs to the plant family Acanthaceae.10 Suaeda monoica is an annual spice plant that is adjusted to saline soil and lives in salt swamps or parched saline soil, and is classified in the plant family Amaranthaceae. Both plants are rich in steroids, triterpenes, saponins, flavonoids, alkaloids, tannins, fatty acids, carboxylic acids, coumarins, and phenolic compounds.11-13
This study was conducted to evaluate the antidermatophytic activity of cold and hot aqueous extracts of the marine medicinal plants A. marina and S. monoica against various dermatophytes and yeasts.
Plant material
Fresh leaves of A. marina and S. monoica plants were collected from the marine coast of the Yanbu region of Saudi Arabia, which extends from 24°2.742 N to 38°6.840 E. The leaves were air-dried in the shade at 25-28°C.
Molecular identification of the plant material
We performed DNA sequencing of the ITS region using primers ITS-u1 and ITS-u4.14 Following PCR with ITS-u1 and ITS-u4 primers, samples were separated on 1% agarose gels to confirm the accuracy and specificity of the amplification process. Fragments of 750 bp representing the target area were purified for sequencing using ITS-u1 and ITS-u4. The obtained DNA sequences were analyzed using nucleotide BLAST alignment tools.
Fungal isolates
Six dermatophytes (T. mentagrophytes, T. verrucosum, M. gallinae, M. gypseum, M. canis, and E. floccosum) and two yeasts (C. albicans and C. tropicalis) were obtained from the King Fahad Hospital in Jeddah.
Extract preparation
Aqueous extracts of dried A. marina or S. monoica leaves were soaked in either cold or hot distilled water at a concentration of 25%. The mixture was placed in a sterile conical flask, plugged with sterile cotton, and maintained on a rotary shaker at 150 rpm at 25-27°C for 48 h. The solution was filtered through Whatman No. 1 filter paper,36 followed by membrane filtration using a 0.22-μm Millipore Express PLUS filter. The filtrate was stored at 4°C until use.
Antidermatophytic assay
Dry weight of dermatophytes and yeasts
To determine the effect of the plant extracts on fungal biomass, various concentrations of the extracts were added to sterilized Sabouraud-dextrose broth to a final volume of 100 ml in sterile 20 ml conical flasks. In addition to the control sample, the conical flasks were inoculated with 10-mm discs of the terminal growth of 10-day-old colonies and incubated at 28°C for 7 days for M. gallinae, M. gypseum, and M. canis; 2 weeks for T. mentagrophytes and T. verrucosum; and 3 weeks for E. floccosum. Dry weight (mg) was determined.37,38
Determination of MICs of plant extracts
Serial dilutions of 100, 50, 40, 30, 20, 10, 5, 3, 2, 1, 0.5, 0.3, 0.2, and 0.1 mg/ml of the most potent plant extracts (hot extract of A. marina and S. monoica) were added to sterilized plates containing fresh Sabouraud-dextrose agar prepared with a standard number of fungi cells to determine the MIC.39 The lowest concentration that did not show any visible growth of microorganisms was considered the minimum inhibitory concentration.
Biochemical assay
Anti-scavenging activity
The ability of the plant extracts to donate a hydrogen atom or electron was determined by measuring the amount of bleaching of a purple-colored methanol solution of DPPH.40 Briefly, 50 μl of the plant extract was added to 5 ml of a 0.004% ethanolic solution of DPPH. After 50 min of incubation in the dark, absorbance at 517 nm was measured against a blank. The % inhibition (I%) of free radical DPPH was calculated using Equation (1):
I% = (A blank − A sample/A blank) × 100 …(1)
where A blank is the absorbance of the blank (containing all reagents except the test compound) and A sample is the absorbance of the test sample.
Determination of TPC
TPC was determined using the Folin-Ciocalteu reagent.41 First, 1 ml of plant extract was added to 10 ml of deionized water and 2 ml of the Folin–Ciocalteu reagent. Thereafter, the blend was allowed to remain at room temperature for 5 min before adding 1.5 ml of 2% (w/v) Na2CO3. Subsequently, at that point, the response blend was left at room temperature for 2 h with discontinuous shaking. Finally, absorbance was measured at 765 nm. The convergence of absolute phenolic compounds in all concentrates, filtrates, and new items was communicated as mg of gallic corrosive counterparts per gram dry loads of tests, utilizing direct equation (3) obtained from equation (2), from a known comparable grouping of gallic acid standard.
Absorbance (at 765 nm) constant × gallic acid concentration = gallic acid equivalents … (2)
Gallic acid equivalents = (Absorbance at 765 nm) / 0.0508 …(3)
HPLC
High-performance liquid chromatography (HPLC) was performed using an Agilent 1260 series fluid chromatography system. Separation was performed using C18 columns (4.6 mm × 250 mm i.d., 5 μm). The mobile phase comprised of 2% acetic acid (A) and acetonitrile (B) at a flow rate of 0.8 ml/min. The flow rate was sequentially customized in a straight linear gradient as follows: 0 min (85% A), 0-15 min (50% A), 15-17 min (20% A), 17-19 min (85% A), and 19-25 min (85% A). A multiwavelength peak was observed at 280 nm. The injection volume was 20 μl for every sample solution, and the column temperature was maintained at 25°C.42
Statistical analysis
The results are presented as the mean of three or four replicates ± standard error. Statistical analyses were performed using the SPSS 22 software. The data obtained were statistically analyzed to determine the degree of significance using a one-way ANOVA. P values of ≤ 0.05 were considered significant.
Sequencing analysis of the internal transcribed spacer (ITS) regions of the plant samples identified them as S. monoica and A. marina. The ITS sequences were submitted to the GenBank database (S. monoica, MK027294, for strain P1 isolated from Yanbu; and A. marina, MK027295, for strain P2 isolated from Yanbu).
Table (1):
Anti-scavenging activity and total phenolic contents of aqueous plant extracts.
Plant extracts |
Inhibition (%) |
Total phenolic content (mg/gdw) |
|---|---|---|
Suaeda monoica (cold) |
47.50% |
33.58 |
Suaeda monoica (hot) |
60% |
24.52 |
Avicennia marina (cold) |
70% |
40.06 |
Avicennia marina (hot) |
76% |
36.74 |
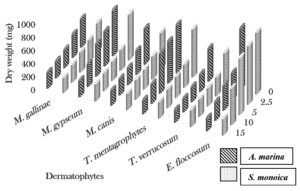
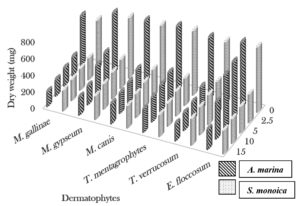
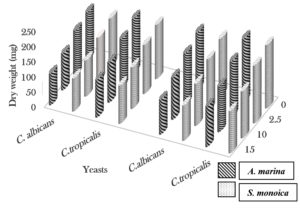
The cold extract of A. marina (15 ml) exhibited activity against Epidermophyton floccosum and Candida albicans, which was inhibited the microbial growth by 79.2% and 34.3%, respectively. At the same concentration (15 ml), the cold extract of S. monoica significantly reduced the dry weights of E. floccosum and C. albicans by 73.3% and 31.2%, respectively. Trichophyton verrucosum was the most sensitive to 15 ml of the hot extract of A. marina and showed 71.9% inhibition, whereas C. albicans showed 38.9% inhibition. Trichophyton mentagrophytes showed the highest sensitivity to the hot extract of S. monoica (15 ml), with 65.9% inhibition, whereas C. albicans was inhibited by only 37.8% (Fig. 1, 2 and 3).
Fig. 1. Effect of different concentrations of cold aqueous plant extracts (ml) on dry weight (mg) of the dermatophytes
Fig. 2. Effect of different concentrations of hot aqueous plant (ml) on dry weight (mg) of the dermatophytes
Fig. 3. Effect of different concentrations of cold and hot aqueous plant extracts (ml) on dry weight (mg) of the yeasts
The hot aqueous extracts of marine plants were more potent than the cold aqueous extracts against the tested fungi and yeasts. Minimum inhibitory concentration (MIC) values ranged from 10 to 30 mg/ml. Candida tropicalis was inhibited by 30 mg/ml extract of A. marina, whereas the 10 mg/ml extract was sufficient to inhibit M. gallinae, T. mentagrophytes, and E. floccosum. The extract of S. monoica at a concentration of 30 mg/ml inhibited C. tropicalis, while the 15 mg/ml extract could inhibit M. canis, T. mentagrophytes, T. verrucosum, and E. floccosum (Table 2).
Table (2):
Minimum Inhibitory Concentrations (MICs) of hot aqueous plant extracts.
Fungi |
Avicennia marina |
Suaeda monoica |
|---|---|---|
Microsporum gallinae |
10 |
20 |
Microsporum gypseum |
20 |
20 |
Microsporum canis |
15 |
15 |
Trichophyton mentagrophytes |
10 |
15 |
Trichophyton. verrucosum |
15 |
15 |
Epidermophyton. floccosum |
10 |
15 |
Candida albicans |
20 |
20 |
Candida tropicalis |
30 |
30 |
As indicated by the diphenylpicrylhydrazyl (DPPH) assay, the lowest antioxidant activity was found in the cold aqueous extract of S. monoica (47.5%). Hot aqueous extract of A. marina exhibited the highest antioxidant activity (76%).
The cold aqueous extract of A. marina possessed the highest total phenolic content (TPC) (40.06 mg/g dry weight [dw]). The lowest TPC was identified for the hot aqueous extract of S. monoica (24.52 mg/gdw) (Table 1).
Analysis of the aqueous extracts using high-performance liquid chromatography (HPLC) revealed that the cold aqueous extract of A. marina contained high quantities of gallic acid, rutin, caffeic acid, syringic acid, catechin, cinnamic acid, vanillin, and quercetin (65.1, 108, 23.8, 42, 431.8, 68.3, 60.2, and 120.8 mg/100 gdw, respectively). The hot aqueous extract of A. marina contained a high concentration of cinnamic acid (27.5 mg/100 gdw). The cold aqueous extract of S. monoica contained high concentrations of gallic acid, catechin, caffeic acid, syringic acid, coumaric acid, vanillin, quercetin, cinnamic acid, and rutin (197.2, 133.3, 16.6, 43.1, 5.5, 139.5, 9.5, 2.0, and 39.3 mg/100 gdw, respectively).
The selected mangrove plants were reported to contain heterogeneous mixtures of substances that were biochemically unique and might act in a synergistic or antagonistic manner to produce a wide array of novel natural products and therapeutic compounds.15,11 The cold aqueous extract of A. marina was highly active against E. floccosum; its growth was inhibited by 79.2In contrast, C. tropicalis was least susceptible to the extract (30.5%). These results are in agreement with those of Behbahani et al. (2014, 2016),16,17 who discovered that an aqueous extract of the mangrove plant A. marina exerted an inhibitory effect on Penicillium digitatum and P. italicum at 20, 40, 60, and 80 mg/ml concentrations. They also found that aqueous extracts of Alternaria citri and Aspergillus flavus inhibited P. digitatum and P. italicum at concentrations of 40, 60, and 80 mg/ml. In contrast, Saravanan and Radhakrishnan18 demonstrated that aqueous extracts prepared from the leaves of mangrove plants, such as Avicennia sp., Brugaria sp., Ceriops decandra, and Rhizophora sp., showed antimicrobial activity against medication-safe microbes (Escherichia coli, Staphylococcus aureus, Klebsiella sp., and Pseudomonas aeruginosa). They inferred that mangrove plants possess compounds that can potentially be used for creating novel bioactive mixtures against drug-safe microorganisms.
Furthermore, Ravikumar et al.19 studied the antibacterial effects of A. marina and revealed that aqueous extracts had the highest antibacterial action. The leaves are a rich source of an alternate class of terpenoids and stilbenes, which are effective against Bacillus sp. Moreover, xanthone is recognized as a functional substance in these plants. These mixtures have toxicological qualities, such as antitumor and antifungal activities.20 Unsaturated fats are generally found in regular fats and dietary oils and have antibacterial and antifungal properties.21 Phytochemicals, such as tannins, terpenoids, alkaloids, flavonoids, and antimicrobial peptides, have also been found to have antimicrobial activities.22
Trichophyton verrucosum was most sensitive to the hot aqueous extract of A. marina (71.9% inhibition). The growth inhibition by this extract against other pathogenic fungi ranged from 38.9% to 69.7%. However, the extract was less effective against C. tropicalis (35.7%). These data are in contrast to those of Al Maqtari and Nagi,23 who reported that an aqueous extract of A. marina leaves demonstrated antimicrobial activity against E. coli, S. aureus, and Bacillus subtilis, as well as antifungal activity against the growth of C. albicans and A. niger. Moreover, Moteriya et al.24 demonstrated that aqueous extracts of A. marina leaves and stems had high antimicrobial activity against Corynebacterium rubrum, B. cereus, B. subtilis, and S. aureus.
The aqueous extracts of the two plants investigated in this study contained higher amounts of TPC, phenols, and flavonoids, which are biosynthesized in different parts of the plant and are known for their antioxidant properties25 based on their ability to scavenge free radicals and reactive oxygen species.26 Similarly, aqueous extracts of A. marina and Rhizophora stylosa leaves showed inhibitory activity against P. digitatum and Fusarium oxysporum. On the other hand there are no inhibitory activity against C. albicans was observed. Furthermore, this extract also inhibited the growth of the pathogenic bacteria E. coli, S. aureus, and B. subtilis. Avicennia marina and R. stylosa leaves contained most of the primary compounds. Therefore, the beneficial medicinal effects of plant materials might result from the combination of antimicrobials, antioxidants, and other secondary products present in the plant, such as phytochemicals.27,28
A cold aqueous extract of S. monoica significantly inhibited the growth of E. floccosum by 73.3% and other pathogenic fungi by 31.2-65.7%. Candida tropicalis was less sensitive to the extract (29.4%). These results were similar to those of Lakshmi and Rao,13 who found that the aqueous extracts of the leaves and shoots of S. monoica exhibited the highest level of antifungal activity against Rhizopus stolonifer, Mucor recemosus, Saccharomyces cerevisiae, Rhizoctonia solani, and C. albicans. A significantly high level of antimicrobial activity was observed against B. subtilis, K. pneumonia, B. megaterium, Lactobacillus acidophilus, E. coli, Enterobacter cloacae, and E. aerogenes. Agoramoorthy et al.29 and Neves et al.7 suggested that polyphenols from Spartina maritima and S. monoica plants, which are employed as important sources of traditional medications, could cure hepatitis Due to their antiviral activity.
Trichophyton mentagrophytes showed the highest sensitivity (65.9%) to the hot aqueous extract of S. monoica. The growth inhibition of the other fungi by this extract ranged from 63.8% to 37.8%. The lowest activity was observed against C. tropicalis (33.3%). This result was similar to that of Arivuselvan et al.,30 who showed that the aqueous extract of the mangrove plant Pempis acidula and Ceriops tagal leaves and bark inhibited the growth of the tested pathogenic bacteria (P. aeruginosa, K. pneumonia, Vibrio parahaemolyticus, S. aureus, and V. cholera). To tolerate stressful conditions, mangrove plants might produce unique chemicals with unique biological activities.
Atindehou et al.31 reported the presence of galloyl, flavonol, and glycosides in P. acidula, and mixtures of the active constituents showed a broad spectrum of biological and pharmacological activities. Furthermore, Sahoo et al.32 demonstrated that the aqueous extract of Sonneratia alba leaves exerted antimicrobial activity against Proteus vulgaris. The aqueous extract of Excoecaria agallocha leaves showed antimicrobial activity against P. mirabilis, which might have been due to the presence of some unknown inorganic compounds. In contrast, Lakshmanan et al.33 reported that S. monoica and S. portulacastrum leaves possess essential and auxiliary metabolites, such as proteins, risen, tannins, glycosides, cardiovascular glycosides, terpenoids, phenols, flavonoids, and acidic mixtures. Our results indicated that the hot extracts of A. marina and S. monoica were effective against the tested dermatophytes and yeasts, showing the highest antioxidant activity (76% and 60%, respectively) and TPCs (36.74 and 24.52 mg/gdw, respectively).
The MIC values of the hot aqueous extracts of A. marina and S. monoica ranged from 10 to 30 mg/ml. This result is in contrast with that of Yazdi et al.,34 who showed that the MIC values of an aqueous extract of Mespilus germanica against Streptococcus pyogenes, Listeria innocua, E. aerogenes, and K. pneumonia were 4, 8, 64, and 32 mg/ml, respectively. In addition, Sharma et al.35 demonstrated that the MIC values for Solanum melongena and Justicia gendarussa were >12.5 mg/ml with an aqueous extract against the tested dermatophyte samples (T. mentagrophytes, T. rubrum, Microsporum gypseum, and Manniophyton fulvum), indicating that the antifungal agents present in the tested plants were found effective in inhibiting the growth of both Trichophyton sp. and Microsporum sp.
This study screened and characterized the phytochemical components of mangrove plants A. marina and S. monoica and determined their antidermatophytic efficiency. Further investigation of the purification and formulation of the extracts is necessary to elucidate the mechanisms underlying the antidermatophytic effects of these plant extracts.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
- Bitew A. Dermatophytosis: prevalence of dermatophytes and non-dermatophyte fungi from patients attending Arsho advanced medical laboratory, Addis Ababa, Ethiopia. Dermatol Res Pract. 2018;2018:8164757.
Crossref - Gnat S, Lagowski D, Nowakiewicz A. Major challenges and perspectives in the diagnostics and treatment of dermatophyte infections. J Appl Microbiol. 2020;129(2):212-232.
Crossref - Brescini L, Fioriti S, Morroni G, Barchiesi F. Antifungal combinations in dermatophytes. Journal of Fungi. 2021;7(9):727.
Crossref - Grover S, Roy P. Clinico-mycological profile of superficial mycosis in a hospital in northeast India. Med J Armed Forces India. 2003;59(2):114-116.
Crossref - Hammer KA, Carson CF, Riley TV. Antimicrobial activity of essential oils and other plant extracts. J Appl Microbiol. 1999;86(6):985-990.
Crossref - Natarajan V, Venugopal PV, Menon T. Effect of Azadirachta indica (Neem) on the growth pattern of dermatophytes. Indian J Med Microbiol. 2003;21(2):98-101.
Crossref - Joshi B, Panda SK, Jouneghani RS, et al. Antibacterial, antifungal, antiviral, and anthelmintic activities of medicinal plants of Nepal selected based on ethnobotanical evidence. Evid Based Complement Alternat Med. 2020;2020:1043471.
Crossref - Karpinski TM. Marine macrolides with antibacterial and/or antifungal activity. Marine Drugs. 2019;17(4):241.
Crossref - Havilah K, Jeyasanta KI, Edward JKP. Antibacterial activity from marine halophyte salt marsh plant (Salicornia brachiata) against bacterial pathogens. Middle-East J Sci Res. 2015;23:1262-1269.
Crossref - Okla MK, Alatar AA, Al-Amri SS, Soufan WH, Ahmad A, Abdel-Maksoud MA. Antibacterial and antifungal activity of the extracts of different parts of Avicennia marina (Forssk.) Vierh. Plants. 2021;10(2): 252.
Crossref - Roy M, Dutta TK. Evaluation of Phytochemicals and Bioactive Properties in Mangrove Associate Suaeda monoica Forssk. ex JF Gmel. of Indian Sundarbans. Front Pharmacol. 2021;12:584019.
Crossref - Parvez MK, Al-Dosari MS, Rehman MT, Alajmi MF, Alqahtani AS, AlSaid MS. New terpenic and phenolic compounds from Suaeda monoica reverse oxidative and apoptotic damages in human endothelial cells. Saudi Pharm J. 2021;29(10):1102-1111.
Crossref - Khattaba RA, Temraz TA. Mangrove Avicennia marina of Yanbu, Saudi Arabia: GC-MS constituents and mosquito repellent activities. Egyptian Journal of Aquatic Biology and Fisheries. 2017;1(3):45-54.
Crossref - Cheng T, Xu C, Lei L, Li C, Zhang Y, Zhou S. Barcoding the kingdom plantae: new PCR primers for ITS regions of plants with improved universality and specificity. Mol Ecol Resour. 2016;16(1):138-149.
Crossref - Robinson T. The organic constituents of higher plants, their chemistry and interrelationship. North Amherst: Burgess Publishing Company; 1967.
- Behbahani BA, Yazdi FT, Riazi F, Shahidi F, Noorbakhsh H, Yazdi FT. Antifungal potential of mangrove extracts against Aspergillus flavus and Penicillium italicum.
Archives of Advances in Biosciences. 2014;5(4):32-38. - Behbahani BA, Yazdi FT, Shahidi F, Riazi F. Antifungal effect of the aqueous and ethanolic Avicennia marina extracts on Alternaria citri and Penicillium digitatum. Zahedan J Res Med Sci. 2016;18(2):e5992.
- Saravanan D, Radhakrishnan M. Antimicrobial activity of mangrove leaves against drug resistant pathogens. Int J Pharmtech Res. 2016;9(1):141-146.
- Ravikumar S, Gnanadesigan M, Suganthi P, Ramalakshmi A. Antibacterial potential of chosen mangrove plants against isolated urinary tract infectious bacterial pathogens. International Journal of Medicine and Medical Sciences 2010;2(3):94-99.
- Duke NC. Phenological trends with latitude in the mangrove tree Avicennia marina. J Ecol. 1990;78(1):113-133.
Crossref - Shelar P, Reddy SVK, Shelar SGS, Reddy GVS. Medicinal value of mangroves and its antimicrobial properties- a review. Continental Journal of Fisheries and Aquatic Sciences. 2012;6:26-37.
- Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4(7):685-688.
Crossref - Al-Maqtari MA, Nagi, HM. Screening of salt-stress, antioxidant enzyme, and antimicrobial activity of leave extracts of mangroves Avicennia marina L. from Hodaidah, Yemen. Journal of Stress Physiology and Biochemistry. 2014;10(2):190-199.
- Moteriya P, Dalsaniya A, Chanda S. Antioxidant and antimicrobial activity of a mangrove plant Avicennia marina (forsk.). Journal of Coastal Life Medicine. 2015;3:713-717.
- Kaneria M, Chanda S. Evaluation of antioxidant and antimicrobial capacity of Syzygium cumini L. leaves extracted sequentially in different solvents. J Food Biochem. 2013;37(2):168-176.
Crossref - Arun KB, Chandran J, Dhanya R, Krishna P, Jayamurthy P, Nisha P. A comparative evaluation of antioxidant and antidiabetic potential of peel from young and matured potato. Food Biosci. 2015;9:36-46.
Crossref - Jadhav BL, Mukhtar QF, Pagare BG. Evaluation of antioxidant properties and phytochemical analysis in the stem and leaves of Ceriops tagal mangroves. Res J Biotechnol. 2013;8(9):28-31.
- Mouafi FE, Abdel-Aziz SM, Bashir AA, Fyiad AA. Phytochemical analysis and antimicrobial activity of mangrove leaves (Avicenna marina and Rhizophora stylosa) against some pathogens. World Appl Sci J. 2014;29:547-554.
- Agoramoorthy G, Chen FA, Venkatesalu V, Kuo DH, Shea PC. Evaluation of antioxidant polyphenols from selected mangrove plants of India. Asian Journal of Chemistry. 2008;20(2):1311-1322.
- Arivuselvan N, Silambarasan D, Govindan T, Kathiresan, K. Antibacterial activity of mangrove leaf and bark extracts against human pathogens. Adv Biol Res. 2011;5(5):251-254.
- Atindehou KK, Kone M, Terreaux C, Traore D, Hostettmann K, Dosso M. Evaluation of the antimicrobial potential of medicinal plants from the Lvory coast. Phytother Res. 2002;16(5):497-502.
Crossref - Sahoo G, Mulla NSS, Ansari ZA, Mohandass C. Antibacterial activity of mangrove leaf extracts against human pathogens. Ind J Pharm Sci. 2012;74(4):348-351.
Crossref - Lakshmanan G, Rajeshkannan C, Kavitha A, Mekala B, Kamaladevi N. Preliminary screening of biologically active constituents of Suaeda monoica and Sesuvium portulacastrum from Palayakayal mangrove forest of Tamilnadu. J Pharmacogn Phytochem. 2013;2(3):149-152.
- Yazdi FT, Behbahani BA, Zanganeh H. The comparison among antibacterial activity of Mespilus germanica extracts and number of common therapeutic antibiotics “in vitro“. Zahedan J Res Med Sci. 2015;17:e5190.
- Sharma KK, Saikia R, Kotoky J, Kalita JC, Devi R. Antifungal activity of Solanum melongena L, Lawsonia inermis L. and Justicia gendarussa B. against dermatophytes. Inter J Pharmtech Res. 2011;3(3):1635-1640.
- Zamin I, Shah JA, Khan I, et al. In vitro efficacy of crude extract of Zizipus Jujuba against selected bacterial strains. International Journal of Scientific and Research Publications 2014;4(2):1-5.
- Zaki D, El-Badrawy S, Naguib M, El-Din ME. Effect of Different Hydrocarbons on the Growth and Metabolism of Mycobacterium phlei. Zentralblatt für Mikrobiologie. 1983; 138(4): 317-325.
- Davami F, Eghbalpour F, Nematollahi L, Barkhordari F, Mahboudi F. Effects of Peptone Supplementation in Different Culture Media on Growth, Metabolic Pathway and Productivity of CHO DG44 Cells; a New Insight into Amino Acid Profiles. Iran Biomed J, 2015; 19(4): 194 –205.
- Chattopadhyay D, Sinha BK, Vaid LK. Antibacterial activity of Syzygium species. Fitoterapia. 1998;69:365-367.
- Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14(5):323-328.
Crossref - Gulluce M, Aslan A, Sokmen M, et al. Screening the antioxidant and antimicrobial properties of the lichens Parmelia saxatilis, Platismatia glauca, Ramalina pollinaria, Ramalina polymorpha and Umbilicaria nylanderiana. Phytomedicine. 2006;13(7):515-521.
Crossref - Zhang Z, Gao W, Yan Y, Huang L. Study on the relationship between chemical compositions and antioxidant activity of Ziziphus jujuba mill. by chemometric approach. Int J Food Prop. 2015;18(2):277-289.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.