ISSN: 0973-7510
E-ISSN: 2581-690X
The present research work was focused on the antibacterial activity of medicinal plants (Aegle marmelos, Citrus aurantifolia, Piper sarmentosum, Sesbania grandiflora, Carthamus tinctorius, Piper longum, Morus alba, Green tea and Oolong tea). Extracts were examined using water, methanol and ethanol as solvents and tested against six human pathogens (Staphylococcus aureus DMST4212, Bacillus cereus DMST5040, Staphylococcus epidermidis DMST518, Escherichia coli ATCC25922, Methicillin-resistant Staphylococcus aureus (MRSA) DMST20625 and Pseudomonas aeruginosa DMST4739) using the agar well diffusion method. The five day methanol extracts of green tea showed significant activity against MRSA and S. aureus of around 28.3 mm. The five day methanol extracts of A. marmelos exhibited the highest antibacterial activity against S. epidermidis (29.7 mm) and lowest in E. coli (no inhibition zone). The drop plate technique found that three day ethanol and three day methanol extracts of P. longum; water, three day and five day methanol and three day and five day ethanol extracts of green tea and oolong tea; three day and five day methanol and three day and five day ethanol extracts of C. aurantifolia; and three day ethanol extract of S. grandiflora had no growth for all six human pathogens. The results demonstrated that this plant has strong antibacterial potential against all tested bacteria.
Herb; Medicinal plant; Antibacterial; Extract.
Worldwide, significant economic value can be derived from medicinal plants. One of the important sources of control products for bacteria and fungi are natural plant products, and around 30% of the drugs used today come from natural products. Over time, plants as food or as products, such as extracts and powders, have had varying degrees of success when being used to treat and stop diseases. There are many benefits to medicines that comes from plants, such as being cheaper, non-toxic, no side effects and easily available.1 Due to the level of antibiotic resistance that has been identified in medicinally important bacteria, it is very important to provide a constant stream of new and effective agents.2
Higher plants contain antimicrobial substances that can be a source of initial ideas for novel drug compounds that can improve human health. For thousands of years humans have used nature as a source of medicinal agents and a significant amount of drugs used currently have come from natural sources, which were initially used in traditional medicine. At present, about 80% of the world’s population still relies on traditional medicine as their main source of health care. The World Health Organization has indicated that medicinal plants could be the best source from which to obtain a range of drugs. This means that these plants should be studied to ensure that their properties, safety and efficacy are well known.3
Fernandez et al.4 reported that antimicrobial effects against MRSA were found in an extract of P. sarmentosum. While an ethanol extract of green tea showed antimicrobial properties against five pathogens (Streptococcus mutans, S. sobrinus, Listeria monocytogenes, Shigella flexneri and Salmonella enterica).5 In addition, a further five pathogens (S. epidermidis, Micrococcus luteus, Brevibacterium linens, Ps. fluorescens and B. subtilis) were also affected by another green tea extract.6
There is an increasing range of bacteria, parasites, viruses and fungi that have resistance to commonly used treatments, which means that their treatment could be affected. Therefore, it is important to develop a range of compounds that can be turned into new medicines with antimicrobial properties.7 This study will determine the antimicrobial properties of a range of medicinal plants that are available in Thailand (Aegle marmelos, Citrus aurantifolia, Piper sarmentosum, Sesbania grandiflora, Carthamus tinctorius, Piper longum, Morus alba, green tea and oolong tea).
Plant materials and extraction
There were nine plants in this study with different parts sampled from different plants: the leaves of P. sarmentosum, M. alba, green tea and oolong tea; the fruits of A. marmelos, C. aurantifolia and P. longum; and the flowers of S. grandiflora and C. tinctorius. The purchases of the plants were made in May 2016 from markets in the Northeast of Thailand. The reference plant specimens were preserved at the Museum of Reference Plants at Mahasarakham University. Each sample was dried, powdered and 200g was added to 1L of methanol and ethanol for three days and five days, respectively, and then boiled for 10 min at 100°C, after which it was filtered with Whatman No. 1 filter paper. Then the crude extract was obtained via concentration at 60°C under reduced pressure in a rotary evaporator and kept at -20°C until needed.8
Antibacterial activity assays
The following strains were obtained from the Public Health Ministry, Bangkok and used to determine the antibacterial activity: S. aureus DMST4212, B. cereus DMST5040, S. epidermidis DMST518, E. coli ATCC25922, Methicillin-resistant S. aureus (MRSA) DMST20625 and Ps. aeruginosa DMST4739. After being cultured on nutrient agar, bacterial suspensions were made in 0.85% NaCl and set so their turbidities were the same as McFarland standard No. 0.5 (approximately 1.5 ×108 CFU/ml of bacteria), after which they were swabbed with sterile cotton wool onto Mueller Hinton agar. Agar wells were made with a No. 4 cork borer, and these were filled with 100 µl of the crude extract (negative control – 10% DMSO, water and positive control – gentamycin). Experiments were performed in triplicate.9,10,11
The macrodilution method was used to identify the minimal inhibitory concentration (MIC). The following Mueller Hinton broth: crude extract ratios were made 1:2, 1:4, 1:8, 1:16, 1:32, 1:64, 1:128, 1:256, 1:512, 1:1024, and 1:2048. From these, 0.9ml was taken and added to 0.9 ml of the bacterial suspension (same turbidity as McFarland standard No. 0.5 and diluted 1:2000 in broth). After 24h of incubation, the MIC was determined. The clear solutions from the MIC were then spread on nutrient agar plates to determine the minimal bactericidal concentration (MBC). The MBC was the dilution that after incubation for 24h did not result in any growth.10
Drop plate technique
The Mueller Hinton agar (liquid ller Hinton agar culture agar 25ml) was sterilized by autoclaving at a temperature of 45oC, mixed with 2.5ml medicinal plant extract concentrations of 500 µg/ml, then pour the culture media into a petri plate. Then, 10 µl of the bacterial suspension (same turbidity as McFarland standard No. 0.5, approximately 1.5×108 CFU/ml of bacteria) was dropped onto the designated quadrant of the petri plate. After the drops on the agar had dried, the petri plates were inverted and incubated at 37oC for 18h. Experiments were performed in triplicate.12.13
Statistical analysis
The significance of differences was determined by SPSS version 14.0 by analysis of variance (nonparametric test) with Krustal-wallis H and differences with P values of < 0.05 was considered to be statistically significant.
Agar well diffusion method, minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The results obtained in the present study revealed that the nine tested medicinal plant extracts possessed potential antibacterial activity against S. aureus DMST4212, B. cereus DMST5040, S. epidermidis DMST518, E. coli ATCC25922, MRSA DMST20625 and Ps. aeruginosa DMST4739 (Table 1). When tested by the agar well diffusion method, the five day methanol extracts of green tea exhibit the highest antibacterial activity against S. aureus of around 28.3 mm and the three day methanol extracts of green tea exhibited the secondary antibacterial activity against S. aureus of around 27.7 mm, but the five and three day methanol extracts of green tea did not show statistically significant differences and exhibited the highest antibacterial activity against S. aureus (Table 1). The five day methanol extracts of A. marmelos exhibit the highest antibacterial activity against S. epidermidis (29.7 mm) and lowest against E. coli (no inhibition zone) (Table 1).
Table (1):
Antibacterial activity of some medicinal plant extracts (concentrations 500 µg/ml) against bacterial species tested by agar well diffusion method..
| Microorga-nisms | Extracts | Inhibition zone diameter (mm) on Petri plates of medicinal plant | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P. longum | A. mar-
melos |
M. alba | Green tea | Oolong tea | C. tinctorius | C. auran tifolia | P. sarmen tosum | S. gran diflora | ||
| S. aureus | Water | – | – | – | 21.67±1.53c | 18.3±0.58de | – | – | – | 19.7±1.15cd |
| 3 day ethanol | – | 16.3±0.58ef | – | 22.7±2.31bc | 23.7±0.58bc | – | 16.7±1.95ef | 17.3±1.15ef | 16±1f | |
| 5 day ethanol | – | 16±1f | – | 24.3±1.15b | 18.3±0.58de | 13.3±0.58g | 17±1.73ef | 13±1g | 15±1fg | |
| 3 day methanol | – | 14.7±0.58fg | – | 27.7±0.58a | 25.7±1.15ab | 13.3±0.58 g | 19±1de | 16.7±0.58ef | 15±1fg | |
| 5 day methanol | 14.33±0.58g | 23±1bc | – | 28.3±0.58a | 26±1a | 14.7±0.58fg | 19±1de | 16±1f | 14±1g | |
| Positive control | 19.7±2.57cd | 17.9±1.85de | 20.3±1cd | 22.5±5.41bc | 20.5±0.40cd | 19.9±1.25cd | 20.8±0.81cd | 21.8±1.08bc | 19.7±1.82cd | |
| S. epidermidis | Water | – | – | – | 22.7±1.5bcd | 18±1de | – | – | – | – |
| 3 day ethanol | – | 20.7±0.6bcde | – | 27±1ab | 23±2bcd | – | 20±1bcde | 17.7±0.6de | 17.7±2.3de | |
| 5 day ethanol | – | 18.7±0.6cde | – | 27.7±1.2ab | 18.7±1.5cde | 14.3±0.6e | 22±2bcde | 13±1ef | 16.3±1.2de | |
| 3 day methanol | – | 18.7±1.2cde | – | 26.3±0.6ab | 25.3±0.6abc | 11.7±1.2f | 23.7±0.6bc | 19.3±1.2cde | 17±1de | |
| 5 day methanol | – | 29.7±1.2a | – | 26.7±1.5ab | 25.3±1.5abc | 14.3±1.2e | 23.7±1.2bc | 19.7±0.6cde | 16.7±0.6de | |
| Positive control | 21.6±2.6bcde | 21.4±3.4bcde | 22.3±1.7bcd | 22±2bcde | 23.3±1.8bc | 22.5±0.7bcd | 24.2±1.9bc | 23.3±3.5bc | 23.1±1.8bc | |
| MRSA | Water | – | – | – | 25.7±0.6a | 22±1bc | – | – | – | – |
| 3 day ethanol | – | – | – | 24.3±1.5b | 22.7±0.6b | – | 19±1de | 18±2e | 16.3±0.6f | |
| 5 day ethanol | – | – | – | 24.7±1.5b | 18.3±0.6e | 15±1f | 21.7±1.5c | 15±2f | 15.3±0.6f | |
| 3 day methanol | – | 16±1f | – | 26.7±0.6a | 25±1b | 11.7±0.6f | 20.3±0.6de | 21±1.73cd | – | |
| 5 day methanol | 15.3±0.6f | 27±1a | – | 28.3±0.6a | 27±1a | 13.7±0.6f | 20.3±0.6de | 19.7±2.3de | – | |
| Positive control | – | – | – | – | – | – | – | – | – | |
| B. cereus | Water | – | – | – | 21.7±1.5bcde | 20.7±0.6bcde | – | 14.3±1.2ef | – | – |
| 3 day ethanol | 17.3±0.6def | 16±1ef | 14.3±1.5ef | 22.3±1.5abcde | 24.3±1.2abcd | 13±1f | 17.7±0.6de | 19.3±1.2bcde | 17.3±0.6def | |
| 5 day ethanol | 17.7±0.6de | 16.7±0.6ef | 13.3±0.6f | 25.3±0.6ab | 20.3±1.5bcde | 17.7±0.6de | 19.7±0.6bcde | 17.3±0.6def | 17.7±0.6de | |
| 3 day methanol | 14.3±0.6ef | 16.7±0.6ef | – | 24.3±0.6abcd | 23.7±0.6abcd | – | 21±1bcde | 18.7±0.6cde | 17.7±0.6de | |
| 5 day methanol | 19±1cde | 19.7±0.6bcde | – | 22.7±0.6abcde | 25.3±0.6ab | – | 20.3±0.6bcde | 18.3±1.5cde | 17.7±0.6de | |
| Positive control | 20.7±0.7bcde | 20.1±1.6bcde | 20.3±0.7bcde | 19.7±0.9bcde | 19.9±1.3bcde | 19.7±1.8bcde | 20.2±0.7bcde | 19.6±2.5bcde | 19.8±0.2bcde | |
| E. coli | Water | – | – | – | – | – | – | – | – | – |
| 3 day ethanol | – | – | – | 12.7±0.6e | – | – | – | 14.3±0.6c | – | |
| 5 day ethanol | – | – | – | – | – | 13.7±1.2d | – | – | – | |
| 3 day methanol | – | – | – | – | – | – | 14.7±0.6c | 12.7±0.6e | – | |
| 5 day methanol | – | – | – | – | – | – | 15.7±0.6b | 13±1.5e | – | |
| Positive control | 18.9±1.2a | 19±0.9a | 17.8±0.9a | 18.2±0.2a | 19.5±2.4a | 18±0.9a | 18.6±0.8a | 16.9±1.1a | 18.2±1.6a | |
| Ps. aeruginosa | Water | – | – | – | – | – | – | – | – | – |
| 3 day ethanol | – | – | – | – | – | – | 15.7±1.2ab | 13.3±0.6e | 14.7±0.6bcd | |
| 5 day ethanol | – | – | – | – | – | 12.7±0.6e | 15.3±1.5ab | – | 14.7±0.6bcd | |
| 3 day methanol | 13.7±0.6de | – | – | – | 11.7±0.6e | – | 18.3±0.6a | 15±2bc | – | |
| 5 day methanol | 17.3±0.6a | 15.3±1.2ab | – | – | 10±1f | – | 18.3±0.6a | 12.3±0.6e | – | |
| Positive control | 14.9±0.8bc | 13.6±1.5e | 12.9±2.8e | 16.2±1.3ab | 13.8±1de | 16.9±1.7ab | 15.8±1.5ab | 15.1±0.7bc | 14.4±1.2bcde | |
The five day methanol extracts of green tea exhibited the highest antibacterial activity against MRSA of around 28.3 mm, the secondary, the five day methanol extracts of oolong tea, three day methanol extracts of green tea and water extract of green tea exhibited antibacterial activity against S. aureus of around 27.0, 26.7 and 25.7 mm respectively, but the five and three day methanol extracts, water extract of green tea and five day methanol extracts of oolong tea showed no statistically significant differences and exhibited the highest antibacterial activity against MRSA. The five day ethanol extracts of green tea and five day methanol extracts of oolong tea showed the significantly highest antibacterial activity against B. cereus of around 25.3 mm (Table 1). The three day and five day methanol extracts of C. aurantifolia exhibited the highest antibacterial activity against Ps. aeruginosa (18.3mm) and 14.7-15.7 mm with similar zones of inhibition as observed in E. coli. The five day green tea ethanol and five day oolong tea methanol extracts showed similar zones of inhibition against all the tested bacteria except B. cereus, which showed the highest activity (25.3 mm) (Fig. 1). The medicinal plant water extracts (A. marmelos, P. sarmentosum, C. tinctorius, P. longum and M. alba) did not have inhibition zones.
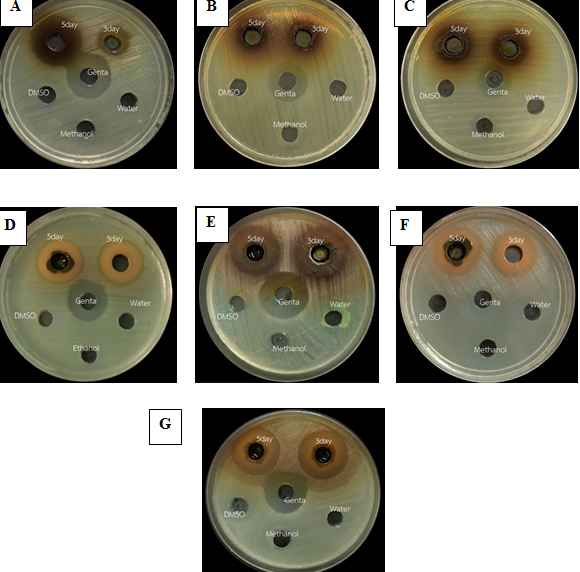
Fig. 1. Antibacterial activity of medicinal plant extracts with (A) methanol extract of A. marmelos against S. epidermidis, (B) methanol extract of C. aurantifolia against E. coli, (C) methanol extract of C. aurantifolia against Ps. aeruginosa, (D) methanol extract of green tea against B. cereus, (E) methanol extract of green tea against S. aureus, (F) methanol extract of green tea against MRSA and (G) methanol extract of oolong tea against B. cereus.
Table 2 shows the MIC and MBC of nine medicinal plant extracts against the six tested bacteria. The MIC of the green tea five day methanol extract was 1:64 (1.56 µg/ml) and the MBC was 1:2 (50 µg/ml), and it did not inhibit S. epidermidis. The oolong tea three day methanol extract MIC was 1:32 (3.125 µg/ml) and MBC was 1:2 (50 µg/ml), and it did not inhibit S. aureus.
Table (2):
MIC (µg/ml) and MBC performance of different medicinal plants extracts against pathogenic organisms.
| Extracts | Bacteria | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sa | Se | MRSA | Bc | Ec | Ps | ||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| A. marmelos | Water | – | – | – | – | – | – | – | – | – | – | – | – |
| 3 day Ethanol | 12.5 | >50 | 25 | >50 | – | – | 12.5 | >50 | – | – | – | – | |
| 5 day Ethanol | 12.5 | >50 | 25 | >50 | – | – | 12.5 | >50 | – | – | – | – | |
| 3 day Methanol | 12.5 | >50 | 25 | >50 | 25 | >50 | 12.5 | >50 | – | – | – | – | |
| 5day Methanol | 6.25 | >50 | 25 | >50 | 12.5 | >50 | 6.25 | >50 | – | – | 25 | >50 | |
| C. aurantifolia | Water | – | – | – | – | – | – | – | – | – | – | – | – |
| 3 day Ethanol | 25 | >50 | 12.5 | >50 | 25 | >50 | 25 | >50 | – | – | 25 | >50 | |
| 5 day Ethanol | 12.5 | >50 | 12.5 | >50 | 12.5 | >50 | 12.5 | >50 | – | – | 25 | >50 | |
| 3 day Methanol | 12.5 | >50 | 25 | >50 | 12.5 | >50 | 12.5 | >50 | 25 | >50 | 25 | >50 | |
| 5 day Methanol | 6.25 | >50 | 12.5 | >50 | 6.25 | >50 | 6.25 | >50 | 25 | >50 | 12.5 | >50 | |
| C. tinctorius | Water | – | – | – | – | – | – | – | – | – | – | – | – |
| 3 day Ethanol | – | – | – | – | – | – | 25 | >50 | – | – | – | – | |
| 5 day Ethanol | 12.5 | >50 | 12.5 | >50 | 25 | >50 | 12.5 | >50 | 25 | >50 | 25 | >50 | |
| 3 day Methanol | 12.5 | >50 | 12.5 | >50 | 12.5 | >50 | – | – | – | – | – | – | |
| 5 day Methanol | 6.25 | >50 | 6.25 | >50 | 12.5 | >50 | – | – | – | – | – | – | |
| M. alba | Water | – | – | – | – | – | – | – | – | – | – | – | – |
| 3 day Ethanol | – | – | – | – | – | – | 25 | >50 | – | – | – | – | |
| 5 day Ethanol | – | – | – | – | – | – | 25 | >50 | – | – | – | – | |
| 3 day Methanol | – | – | – | – | – | – | – | – | – | – | – | – | |
| 5 day Methanol | – | – | – | – | – | – | – | – | – | – | – | – | |
| P. longum | Water | – | – | – | – | – | – | – | – | – | – | – | – |
| 3 day Ethanol | – | – | – | – | – | – | 25 | >50 | – | – | – | – | |
| 5 day Ethanol | – | – | – | – | – | – | 12.5 | >50 | – | – | – | – | |
| 3 day Methanol | – | – | – | – | – | – | 25 | >50 | – | – | 12.5 | >50 | |
| 5 day Methanol | 25 | >50 | – | – | 25 | >50 | 25 | >50 | – | – | 12.5 | >50 | |
| P. sarmentosum | Water | – | – | – | – | – | – | – | – | – | – | – | – |
| 3 day Ethanol | 12.5 | >50 | 25 | >50 | 25 | >50 | 25 | >50 | 25 | >50 | 25 | >50 | |
| 5 day Ethanol | 12.5 | >50 | 12.5 | >50 | 25 | >50 | 25 | >50 | – | – | – | – | |
| 3 day Methanol | 12.5 | >50 | 25 | >50 | 25 | >50 | 25 | >50 | 12.5 | >50 | 25 | >50 | |
| 5 day Methanol | 6.25 | >50 | 12.5 | >50 | 12.5 | >50 | 25 | >50 | 25 | >50 | 25 | >50 | |
| S. grandiflora | Water | 6.25 | >50 | – | – | – | – | – | – | – | – | – | – |
| 3 day Ethanol | 6.25 | >50 | 25 | >50 | 12.5 | >50 | 12.5 | >50 | – | – | 25 | >50 | |
| 5 day Ethanol | 6.25 | >50 | 25 | >50 | 12.5 | >50 | 6.25 | >50 | – | – | 25 | >50 | |
| 3 day Methanol | 6.25 | >50 | 12.5 | >50 | – | – | 12.5 | >50 | – | – | – | – | |
| 5 day Methanol | 6.25 | >50 | 25 | >50 | – | – | 12.5 | >50 | – | – | – | – | |
| green tea | Water | 1.56 | >50 | 1.56 | >50 | 1.56 | >50 | 6.25 | >50 | – | – | – | – |
| 3 day Ethanol | 12.5 | >50 | 12.5 | >50 | 6.25 | >50 | 6.25 | >50 | 12.5 | >50 | – | – | |
| 5 day Ethanol | 6.25 | >50 | 6.25 | >50 | 12.5 | >50 | 12.5 | >50 | – | – | – | – | |
| 3 day Methanol | 3.125 | >50 | 1.56 | >50 | 3.125 | >50 | 3.125 | >50 | – | – | – | – | |
| 5 day Methanol | 3.125 | >50 | 1.56 | 25 | 3.125 | >50 | 3.125 | >50 | – | – | – | – | |
| oolong tea | Water | 1.56 | >50 | 3.125 | >50 | 0.78 | >50 | 12.5 | >50 | – | – | – | – |
| 3 day Ethanol | 12.5 | >50 | 12.5 | >50 | 12.5 | >50 | 12.5 | >50 | – | – | – | – | |
| 5 day Ethanol | 6.25 | >50 | 6.25 | >50 | 6.25 | >50 | 6.25 | >50 | – | – | – | – | |
| 3 day Methanol | 3.125 | 25 | 1.56 | >50 | 3.125 | >50 | 6.25 | >50 | – | – | 12.5 | >50 | |
| 5 day Methanol | 3.125 | >50 | 0.78 | >50 | 3.125 | >50 | 6.25 | >50 | – | – | 12.5 | >50 | |
– =not tested
The oolong tea water extract and five day methanol extract showed the least MIC values of 0.78 µg/ml against MRSA and S. epidermidis, respectively. The MICs of the water extract of oolong tea and green tea extracts were 1.56 µg/ml against S. aureus; three and five day green tea methanol extracts were 3.125 µg/ml against B. cereus; the P. longum three and five day and the C. aurantifolia five day methanol extracts were 12.5 µg/ml against Ps. aeruginosa; and the green tea three day ethanol extract and P. sarmentosum three day methanol extract were 12.5 µg/ml against E. coli (Table 2).
Drop plate technique
The results obtained in the present study relieved that the nine tested medicinal plant extracts possessed potential antibacterial activity against S. aureus DMST4212, B. cereus DMST5040, S. epidermidis DMST518, E. coli ATCC25922, MRSA DMST20625 and Ps. aeruginosa DMST4739 (Table3). When tested by the drop plate technique, the three day ethanol extract and three day methanol extract of P. longum; water extract, three day and five day ethanol extracts and three day and five day methanol extracts of green tea and oolong tea; three day and five day ethanol extracts and three day and five day methanol extracts of C. aurantifolia; and three day ethanol extract of S. grandiflora did not affect the growth of the bacteria (Table 3 and Fig. 2). The three day and five day ethanol extracts of A. marmelos and M. alba did not inhibit the growth of the six bacteria species (Table 3 and Fig. 2). The five day ethanol extract of P. longum and five day methanol extract of P. sarmentosum can inhibit the growth of gram positive bacteria, but did not inhibit the growth of gram negative bacteria (Table 3). The water extracts of P. longum, A. marmelos, C. tinctorius, P. sarmentosum and M. alba as well as three day and five day methanol extracts of M. alba did not result in any inhibition zones in any of the bacteria (Table 3).
Table (3):
Antibacterial activity of some medicinal plant extracts against bacterial species tested by drop plate technique (10 µL approximately 1.5×108 CFU/ml of bacteria)..
| Medicinal plant | Extracts | Bacteria | |||||
|---|---|---|---|---|---|---|---|
| Sa | Se | MRSA | Bc | Ec | Ps | ||
| A. marmelos | Water | n
+ + 0 + |
n
+ + 0 + |
n
+ + + + |
n
+ + 0 0 |
n
+ + + + |
n
+ + + + |
| 3 day Ethanol | |||||||
| 5 day Ethanol | |||||||
| 3 day Methanol | |||||||
| 5 day Methanol | |||||||
| C. aurantifolia | Water | +
0 0 0 0 |
+
0 0 0 0 |
+
0 0 0 0 |
0
0 0 0 0 |
+
0 0 0 0 |
+
0 0 0 0 |
| 3 day Ethanol | |||||||
| 5 day Ethanol | |||||||
| 3 day Methanol | |||||||
| 5 day Methanol | |||||||
| C. tinctorius | Water | n
+ + 0 + |
n
+ + + 0 |
n
+ + + + |
n
0 0 + 0 |
n
+ + + + |
n
+ + 0 + |
| 3 day Ethanol | |||||||
| 5 day Ethanol | |||||||
| 3 day Methanol | |||||||
| 5 day Methanol | |||||||
| M. alba | Water | n
+ + n n |
n
+ + n n |
n
+ + n n |
n
+ + n n |
n
+ + n n |
n
+ + n n |
| 3 day Ethanol | |||||||
| 5 day Ethanol | |||||||
| 3 day Methanol | |||||||
| 5 day Methanol | |||||||
| P. longum | Water | n
0 0 0 + |
n
0 0 0 + |
n
0 0 0 + |
n
0 0 0 0 |
n
0 + 0 + |
n
0 + 0 + |
| 3 day Ethanol | |||||||
| 5 day Ethanol | |||||||
| 3 day Methanol | |||||||
| 5 day Methanol | |||||||
| P. sarmentosum | Water | n
+ + 0 0 |
n
+ + 0 0 |
n
+ + 0 0 |
n
0 0 0 + |
n
+ + + 0 |
n
+ + + 0 |
| 3 day Ethanol | |||||||
| 5 day Ethanol | |||||||
| 3 day Methanol | |||||||
| 5 day Methanol | |||||||
| S. grandiflora | Water | +
0 0 0 + |
0
0 0 0 + |
+
0 + + + |
0
0 0 0 0 |
+
0 + + + |
0
0 + 0 + |
| 3 day Ethanol | |||||||
| 5 day Ethanol | |||||||
| 3 day Methanol | |||||||
| 5 day Methanol | |||||||
| green tea | Water | 0
0 0 0 0 |
0
0 0 0 0 |
0
0 0 0 0 |
0
0 0 0 0 |
0
0 0 0 0 |
0
0 0 0 0 |
| 3 day Ethanol | |||||||
| 5 day Ethanol | |||||||
| 3 day Methanol | |||||||
| 5 day Methanol | |||||||
| oolong tea | Water | 0
0 0 0 0 |
0
0 0 0 0 |
0
0 0 0 0 |
0
0 0 0 0 |
0
0 0 0 0 |
0
0 0 0 0 |
| 3 day Ethanol | |||||||
| 5 day Ethanol | |||||||
| 3 day Methanol | |||||||
| 5 day Methanol | |||||||
n = not tested because medicinal plant extract had no inhibition zone for bacteria; + = growth of bacteria; and 0 = no growth of bacteria
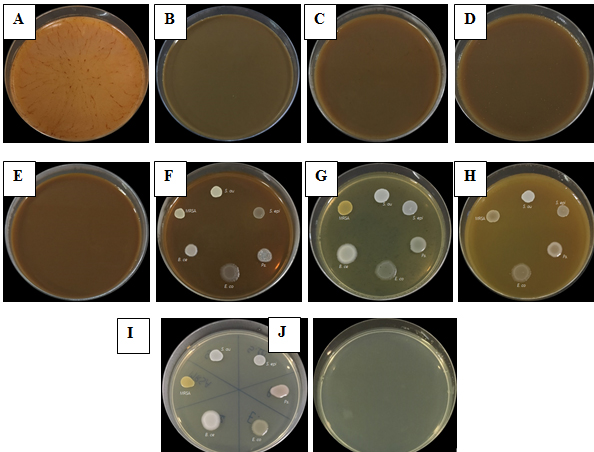
Fig. 2. Antibacterial activity of medicinal plant extracts by drop plate technique with three day ethanol extracts of P. longum (A), green tea (B), oolong tea (C), C. aurantifolia (D), S. grandiflora (E) and M. alba (G) and five day ethanol extracts of A. marmelos (F) and C. tinctorius (H) as well as negative control (I) and positive control (J).
In this study, there were nine plants with different parts sampled from different plants: the leaves of P. sarmentosum, M. alba, green tea and oolong tea; the fruits of A. marmelos, C. aurantifolia and P. longum: and the flowers of S. grandiflora and C. tinctorius. The medicinal plant extracts have the ability to inhibit the growth of microorganisms. In this study the medicinal plant extracts have the ability to inhibit the growth of gram positive bacteria and gram negative bacteria. The efficiency varies depending on the type of medicinal plant and species of bacteria. The medicinal plant extracts can inhibit the gram positive bacteria better than the gram negative bacteria. This is consistent with Parekh et al.14 where the plant extracts were more active against gram positive bacteria than against gram negative bacteria. This is because the gram positive bacterial cell wall consists of the main substance, peptidoglycan. While the gram negative bacterial cell wall structure is more complex, mainly composed of 80% outer membrane and 20% peptidoglycan.
The present study relieved that the nine tested medicinal plant extracts possessed potential antibacterial activity against S. aureus DMST4212, B. cereus DMST5040, S. epidermidis DMST518, E. coli ATCC25922, MRSA DMST20625 and Ps. aeruginosa DMST4739. When tested by the agar well diffusion method, the five day methanol extracts of green tea showed significant activity against MRSA and S. aureus of around 28.3 mm (Table1) and MIC values of 3.12 and 25 µg/ml, respectively, and BMC values of more than 50 µg/ml (Table2). The five day methanol extracts of A. marmelos exhibited the highest antibacterial activity against S. epidermidis (29.7 mm) with MIC values of 25 µg/ml and BMC values of more than 50 µg/ml and lowest in E. coli (no inhibition zone). This contrasts with the research of Prasannabalaji et al.15 where the methanol extract (0.4 g/ml) of A. marmelos showed significant inhibitory activity of 22.5 mm and MIC value of 0.156 mg/ml against E. coli.
In this study, the three day and five day methanol extracts of C. aurantifolia exhibited the highest antibacterial activity against Ps. aeruginosa (18.3mm) with MIC values of MIC 25 and 12.5 µg/ml, respectively, MBC values of more than 50 µg/ml and 14.7-15.7 mm zones of inhibition similar to those observed in E. coli. The study demonstrates that the three day and five day methanol extracts of C. aurantifolia exhibited antibacterial activity on S. aureus, S. epidermidis, MRSA, B. cereus, Ps. aeruginosa and E. coli. This is consistent with the research of Pathan et al.16 whose study demonstrates that the hydroalcoholic extract of the C. aurantifolia leaf exhibited antibacterial activity on Klebsiella pneumonia, Pseudomonas sp. and S. aureus. This suggests good support to use this plant as a medicinal plant and as a base for the development of new drugs, such as phytomedicine. Preliminary phytochemical screening of the leaf extract of C. aurantifolia revealed the presence of carbohydrates, alkaloids, flavonoids, steroids and tannins. This is well known since tannins and saponins are important plant metabolites, which are mainly responsible for antimicrobial activity.16,17
In this study, the five day green tea ethanol and five day oolong tea methanol extracts showed almost similar zones of inhibition against all the tested bacteria, except B. cereus, which showed the highest activity (25.3 mm) (Figure 1), MIC values of 12.5 and 6.25 µg/ml, respectively, and MBC values of more than 50 µg/ml (Table2). This is consistent with Sharma et al.6 who stated Camellia sinensis (tea) is known for its therapeutic properties (anti-inflammatory, anti-microbial, anti-tumour, anti-oxidative and anti-ageing). Although the antimicrobial properties of green tea have been studied, its role against bacterial strains related to skin infections and mechanism of action is not well understood. We focused on exploring the anti-microbial activity and the basic mechanism of the aqueous green tea leaf extract on the selected bacterial strains. S. epidermidis, Micrococcus luteus, Brevibacterium linens, Ps. fluorescens and B. subtilis were found to be sensitive to green tea extract via the disc diffusion assay (zone of inhibition > 7 mm). The MIC was determined via a nitro blue tetrazolium (NBT) assay (0.156–0.313 mg/ml). Moreover, the aqueous extract was found to not be toxic to the Vero cell-line up to a concentration of 500 µg/ml. The study of Sasakia et al.18 found that the activity originated from a monomeric polyphenolrich fraction, and it was stronger than that of pure polyphenols. Moreover, some combinations of monomeric polyphenols showed the highest level of antibacterial activity. These results suggest that the antibacterial activity of the oolong tea extract is caused by a synergistic effect of the monomeric polyphenols, which can easily bind to proteins.
In this study, the three day methanol extract of P. sarmentosum showed activity against MRSA, S. epidermidis, S. aueus, B. cereus, Ps. aeruginosa and E. coli for antimicrobial compounds. P. sarmentosum inhibited the growth of S. epidermidis, S. aueus, B. cereus, Ps. aeruginosa and E. coli with inhibition zones of 21.0, 19.3, 18.7, 18.7, 15.0 and 12.7 mm, respectively. This is consistent with Fernandez et al.4 where the crude methanolic extract of P. sarmentosum showed activity against MRSA, E. coli, Vibrio cholera and S. pneumonia. Plant based products have been effectively proven for their utilization as sources of antimicrobial compounds. P. sarmentosum inhibited the growth of MRSA with an inhibition zone of 10.0 mm.
Drop plate technique
This study relieved that the nine tested medicinal plant extracts possessed potential antibacterial activity against S. aureus DMST4212, B. cereus DMST5040, S. epidermidis DMST518, E. coli ATCC25922, MRSA DMST20625 and Ps. aeruginosa DMST4739 (Table 3). When tested by the drop plate technique, the three day ethanol extract and three day methanol extract of P. longum; water extract, three day and five day ethanol extracts and three day and five day methanol extracts of green tea and oolong tea; three day and five day ethanol extracts and three day and five day methanol extracts of C. aurantifolia; and three day ethanol extract of S. grandiflora inhibited the growth of the bacteria (Table 3 and Fig. 2). This is consistent with Kumar et al.19 whose study showed the green tea extract had antimicrobial activity by the drop plate technique and found that the green tea extract can inhibit the growth of Staphylococcus spp., Streptococci spp., Pseudomonas spp., Proteus spp., Bacillus spp. and E. coli.
In this study, the three day and five day ethanol extracts of A. marmelos and M. alba did not inhibit the growth of six species of bacteria (Table 3 and Fig. 2). The five day ethanol extract of P. longum and five day methanol extract of P. sarmentosum can inhibit the growth of gram positive bacteria, but did not inhibit the growth of gram negative bacteria (Table 3). The water extract of P. longum, A. marmelos, C. tinctorius, P. sarmentosum and M. alba as well as three day and five day methanol extracts of M. alba did not produce inhibition zones in the bacteria (Table 3).
Plants are important sources of potentially useful structures for the development of new chemotherapeutic agents. The first step towards this goal is the antibacterial activity assay. Many reports are available on antibacterial properties. Some of these observations have helped in identifying the active principle responsible for such activities and in the development of drugs for therapeutic use in human beings. The results of the present investigation clearly indicates that the antibacterial and antifungal activity varies with the species of plant and plant material used. Thus, the study ascertains the value of plants used in ayurveda, which could be of considerable interest to the development of new drugs.20
This work has shown the antibacterial properties of nine medicinal plant extracts that exhibit the highest antibacterial activity against S. aureus, S. epidermidis, MRSA, B. cereus, E. coli and Ps. aeruginosa. This work has shown that it would be possible to develop antimicrobial properties, because the use of antibiotics is often toxic with many side effects and are expensive. This work has shown that medicinal plant extracts can be added to the culture medium as antibacterial inhibitors for unwanted bacteria, so the preferred bacteria can be grown on the culture medium. This can also be used as the basis for future use.
ACKNOWLEDGMENTS
This research was financially supported by Mahasarakham University grant year 2017.
- Patel, S.H., Suthar, J.V., Patel, R.K., Zankharia, U.S., Jani, V.R., Gajjar, K.N. Antimicrobial activity investigation of Aegle marmelos, Couroupita guianesis, Manilkara hexandra, Cow Urine and Dung. Research Journal of Pharmaceutical, Biological and Chemical Sciences, 2015; 6(4): 1014-1022.
- Palombo, E.A., Semple, S.J. Antibacterial activity of traditional Australian medicinal plants. Journal of Ethnopharmacology, 2001; 77: 151–157.
- Abraham, J., Thomas, T.D. Antibacterial activity of medicinal plant Cyclea peltata (Lam) Hooks & Thoms. Journal of Asian Pacific Journal of Tropical Disease, 2012; S280-S284.
- Fernandez, L., Daruliza, K., Sudhakaran, S., Jegathambigai, R. Antimicrobial activity of the crude extract of Piper sarmentosum against methicilin-resistant Staphylococcus aureus (MRSA), Escherichia coli, Vibrio cholera and Streptococcus pneumonia. Journal of European Review for Medical and Pharmacological Sciences, 2012; 16(Suppl 3): 105-111.
- Jungmin, O., Heonjoo, J., Ah-Reum, C., Kim, S.J., Jaejoon, H. Antioxidant and antimicrobial activities of various leafy herbal teas. Journal of Food Control, 2013; 31: 403-409.
- Sharma, A., Gupta, S., Sarethy, I.P., Dang, S., Gabrani, R. Green tea extract: Possible mechanism and antibacterial activity on skin pathogens. Journal of Food Chemistry, 2012; 135: 672-675.
- Farjana, A., Zerin, N., Kabir, S. Antimicrobial activity of medicinal plant leaf extracts against pathogenic bacteria. Journal of Asian Pacific Journal of Tropical Disease, 2014; 4(2): S920-S923.
- Djeussi, D.E., Noumedem, J.A.K., Seukep, J.A., Fankam, A.G., Voukeng, I.K., Tankeo, S.B., Nkuete, A.H.L., Kuete, V. Antibacterial activities of selected edible plants extracts against multidrug-resistant Gram-negative bacteria. Journal of BMC Complementary and Alternative Medicine, 2013; 13:164.
- Rahman, S.F.S.A., Sijam, K., Omar, D. Identification and Antibacterial Activity of Phenolic Compounds in Crude Extracts of Piper sarmentosum (Kadok). Journal of pure and applied microbiology, 2014; 8(2): 483-490.
- Siriwattanametanon, W., Kanchanarach, W., Thiwthong, R., Dodgson, J.L.A. Culture Filtrates from Laboratory Grown Phellinus Mushrooms for Use as Antibacterial Agents. Journal of Chiang Mai Journal Science, 2014; 41(1): 243-247.
- Balouiri, M., Sadiki, M., Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis, 2016; 6: 71-79.
- Herigstad, B., Hamilton, M., Heersink, J. How to optimize the drop plate method for enumerating bacteria. Journal of Microbiological Methods, 2001; 44: 121–129.
- Naghili, H., Tajik, H., Mardani, K., Rouhani, S.M.R., Ehsani, A., Zare, P. Validation of drop plate technique for bacterial enumeration by parametric and nonparametric tests. Journal of veterinary research forum, 2013; 4(3): 179-183.
- Parekh, J., Jadeja, D., Chanada, S. Efficacy of aqueous and methanol extracts of some medicinal plants for potential antimicrobial activity. Journal of Turkish Journal Biology, 2005; 29: 203-210.
- Prasannabalaji, N., Muralitharan, G., Sivanandan, R.N., Kumaran, S., Pugazhvendan, S.R. Antibacterial activities of some Indian traditional plant extracts. Journal of Asian Pacific Journal of Tropical Disease, 2012; S291-S295.
- Pathan, R.K., Gali, P.R., Pathan, P., Gowtham, T., Pasupuleti, S. In vitro Antimicrobial Activity of Citrus aurantifolia and its Phytochemical Screening. Journal of Asian Pacific Journal of Tropical Disease, 2012; S328-S331.
- Tschesche, R. (ed): Advances in chemistry of Antibiotics susbstance from higher plant; pharmacognosy and phytochemistry proceeding of the 1st international congress, New York: Verlong, Berlin, Heidelbeg, 1971; pp 274-276.
- Sasakia, H., Matsumotoa, M., Tanakac, T., Maedac, M., Nakaic, M., Hamadab, Ooshimaa, T. Antibacterial Activity of Polyphenol Components in Oolong Tea Extract against Streptococcus mutans. Journal of Caries research, 2004; 38: 2-8.
- Kumar, A., Kumar, A., Thakur, P., Patil, S., Payal, C., Kumar, A., Sharma, P. Antibacterial activity of green tea (Camellia sinensis) extracts against various bacteria isolated from environmental sources. Journal of Recent research in science and technology, 2012; 4(1): 19-23.
- Mahesh, B., Satish, S. Antimicrobial Activity of Some Important Medicinal Plant Against Plant and Human Pathogens. World Journal of Agricultural Sciences, 2008; 4(S): 839-843.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


