Phytic acid is an antinutritional compound that chelates P and essential divalent cations such as Fe, Ca, and Zn in cereals and major staple crops such as wheat, maize, rice, and sorghum. As a result, these cations cannot be absorbed by monogastric animals or humans: phytic acid has an inhibitory effect on nutrient uptake and its levels are negatively correlated with protein and starch digestibility. However, phytic acid can be degraded by the action of the enzyme phytase. Phytase plays important roles in the degradation of phytic acid and in increasing the nutritional quality of staple foods. Microbial phytase is a versatile enzyme that is beneficial for humans, animals, the environment, and the industry. In this review, we summarise the interaction of phytic acid with micronutrients, various approaches to enhancing the nutritional profile of staple foods by reducing the phytic acid content, and current knowledge of microbial-based phytase as a potential reducer of phytic acid.
Phytic Acid, Phytase, Antinutritional, Micronutrient
Phytic acid is the main source of bound or unavailable P in plants, mainly in seeds, grains, and vegetables.1 Phytic acid (C6H18O24P6), chemically known as myo-inositol 1,2,3,4,5,6-hexakisphosphate (PA; IP6), has a molecular weight of 660 g mol−1. The six reactive groups of P in PA chelate positive cations such as Fe, Ca, and Zn, which are important micronutrients required for proper growth and functioning in humans and animals. Because of the ability of PA to chelate essential micronutrients and store P, so that P is unavailable, it is considered an antinutritional factor.2,3 Despite PA being present in the soil, owing to its strong adsorption onto soil, it is unavailable for degradation by soil microflora or is relatively less bioavailable.4 Ruminants can use PA, but humans and other monogastric animals have a limited ability to hydrolyse phytate. As a result, PA reduces the bioavailability of P and chelates the minerals and micronutrients required for cellular metabolism and maintenance and tissue function.5 In pigs and poultry, which lack phytase, their gastrointestinal tract is unable to metabolise PA, which has two main negative impacts. Firstly, PA cannot be fully absorbed by these animals. Secondly, the release of unabsorbed inorganic P to the environment leads to problems such as the eutrophication of water bodies, production of greenhouse gases such as nitrous oxide, growth of toxin-producing microorganisms, and, ultimately, death of several aquatic species.1,6 Thus, biotechnological and microbiological methods must be applied to increase the quality and bioavailability of P in animal feed by reducing the PA content.
Majority of the world’s population, especially those in developing countries, depends on plant-based diets that include wheat, rice, maize, sorghum, and pearl millet. These are major staple crops but are also high in PA, which chelates essential micronutrients. This leads to “hidden hunger”, which is the unavailability of micronutrients from food consumption.7 To deal with this problem, fortification is often used to improve the nutritional quality of a food. Fortification is the practice of deliberately increasing the content of essential micronutrients (vitamins and minerals, including trace elements such as Fe, Ca, Zn, provitamin A, amino acids, and proteins.) in food.8
Currently, much focus is placed on biofortification for more efficient fortification. Biofortification simultaneously increases the bioavailability of micronutrients and the concentrations of promoters that stimulate absorption, while decreasing the concentrations of inhibitors that chelate essential micronutrients through the application of genetic and plant-breeding approaches.9 In addition to these approaches, microbiological and biotechnological methods can be applied to enhance the nutritional quality of staple crops after harvest or during food processing, through the use of microbial enzymes that can efficiently remove or dephytinise PA and release the chelated divalent cation. Using microbial enzymes is advantageous owing to their being lower in cost, environmentally friendly, and naturally available, and they can be manipulated to meet the increased demand for biofortification.10
Effect of PA on Nutrient Uptake and Micronutrient Digestibility
Hidden hunger is a form of undernutrition that occurs when the intake and absorption of vitamins and minerals are too low to sustain good health and development. One example is undernourishment overlapping with obesity.11 A person may not be starving owing to a lack of calories, but instead may be lacking in essential micronutrients. Hidden hunger mainly concerns deficiencies of essential food components, such as vitamin A, Zn, Fe, and I, in the diet. The most common micronutrient deficiencies across all ages are I, Fe, and Zn. Pregnant women and children under the age of 5 years are generally more affected by hidden hunger, which impairs their health throughout their life span and increases their mortality rate.
Fe, Ca, and Zn are important micronutrients required by pregnant women. However, pregnant women in developing countries such as Bangladesh are often deficient in these micronutrients because staple crops and plant-based diets are the main source of dietary energy. The foods consumed are usually high in PA, so the bioavailability of essential micronutrients is reduced by PA acting as an inhibitory or chelating agent.12
Similarly, in developing countries, the digestibility of proteins in traditional food is comparatively low due to the high concentrations of antinutritional factors, mainly PA and glucosinates, in food.13 In terms of the nutrient bioaccessibility of dehusked rice, although it has an acceptable in vitro protein level and starch digestibility, it is poor in mineral micronutrients such as Fe, Ca, and Zn owing to the high PA content in the rice cultivar.14 In genetic and plant breeding approaches, PA level is negatively correlated with the bioaccessibility of micronutrients and in vitro protein digestibility.15 In pigs and poultry, P absorption can be increased up to 30% by incorporating phytase, which reduces the PA in their diets. Phytase has similar effects on the digestibility of other phytate-bound micronutrients (Fe, Ca, and Zn), as evidenced by the results of various studies on pigs and poultry.16
Human Zn deficiency occurs worldwide, but it is more prevalent in regions where the diet is plant- or cereals-based and where less animal-based products are consumed. Although a cereal-based diet is already low in Zn, its lack of bioavailability plays a major role in Zn deficiency.17 Compared with adults, infants, and children, pregnant and lactating women require more Zn. Although Zn is known to play a crucial role in human metabolite and physiology, one-fifth of the world population do not consume enough Zn in their diet, and one-third of the Indian population are at high risk of Zn deficiency.18 PA is the principle dietary factor that limits Zn bioavailability through restricting Zn absorption in the gastrointestinal tract (GIT).19 The availability of Zn for GIT absorption depends on the combined effect of PA, proteins, and other mineral ions present in the body. Zn absorption substantially decreases with increasing doses of Fe under specific conditions.20 This inhibitory effect occurs only if a high Fe to Zn ratio is administered separate from meals, whereas protein intake in the diet enhances the Zn absorbed by the GIT. Zn uptake is mainly inhibited owing to the presence of PA when the molar ratio of phytate:Zn is 18:1. The World Health Organization reported that the optimal molar ratio of phytate to Zn is 15:1.21 However, Davidsson et al.22 demonstrated that Fe does not have any negative effect on Zn absorption. Because of its high-density negatively charged phosphate groups, PA forms insoluble salts with positively charged divalent cations. Allen et al.23 found that the presence of Ca ions worsens the bioavailability of Zn through the creation of insoluble Ca–Zn–PA complexes.20,24 Thus, to increase the bioavailability of Zn, the Zn content of the diet must be increased by reducing the content of uptake inhibitors such as PA.
Fe is an abundant element on Earth and a biologically essential component in the human body. Despite its abundance, Fe is a growth-limiting factor.25 Dietary Fe occurs in two forms: haem and nonhaem. Haem Fe, which is highly bioavailable, is mainly obtained from myoglobin and haemoglobin, whereas nonhaem Fe is obtained from cereals, legumes, fruits, and vegetables. Non-haem Fe bioavailability is strongly influenced by the presence of certain food components. The amount of nonhaem Fe in the diet is many times larger than that of haem Fe.26 Various dietary factors influence the bioavailability of Fe in the human body: ascorbate and citrate increase Fe bioavailability, whereas PA, polyphenols, and Ca are strong inhibitors of Fe absorption. PA, owing to its multiple negative charges, easily complexes with Fe, making it unavailable for absorption.27 Fe deficiency is the condition where no free or mobilizable Fe is available for absorption by the body, which can lead to the development of either the anaemic or nonanaemic form of Fe deficiency. Non-haem Fe and PA levels are negatively correlated. Notably, high Fe consumption but nonabsorption is related to the risk of colon cancer, as unabsorbed Fe in the intestine leads to GIT disease.28 In pregnant humans, Fe deficiency increases the risk of sepsis, maternal or perinatal mortality, and morbidity. In children under the age of 5 years, Fe deficiency reduces learning ability. Those especially at risk of Fe deficiency are infants, adolescents, and pregnant women.27
The bioavailability of Ca for absorption by the human body is controlled by both intrinsic and extrinsic factors. The intrinsic factors include age, sex, pregnancy, and lactation; the extrinsic factors include dietary variables such as the presence or absence of other dietary components like excess fat, protein, and sugar, which can reduce Ca ion intake. Insufficient vitamin D intake also reduces Ca ion absorption. Ca ions are present in cereals, legumes, and grains, but uptake can be inhibited by the presence of large amounts of PA. Although dairy products are the main sources of Ca in the diet, other foods also contribute to the overall Ca intake. Those following a vegan diet or in developing countries must include an adequate amount of non-dairy sources of Ca such as cereals and legumes, to fulfil Ca intake requirements. In humans, PA and Ca absorption have antagonistic effects: PA decreases Ca absorption, and PA degradation improves Ca availability, mainly owing to the formation of insoluble Ca phytate complexes. Furthermore, taking Ca with phytate-rich food is recommended, as the consumption of a diet rich in Ca but poor in whole products such as bran may cause renal stones and crystallisation inhibition.29 Dietary Ca deficiency leads to the thinning and weakening of the bones and, eventually, to osteoporosis in adults and rickets in children under the age of 5 years. About 99% of the total Ca consumed is required for bone development. Of the non-dairy sources, fortified ready-to-eat cereals can serve as a major source of Ca.30
As with divalent cations, PA can chelate proteins. Human and animal nutritionists have observed the hindered use of both amino acids and P when PA levels are high. PA, being a polyanionic molecule that can carry up to twelve dissociable protons, forms binary protein–phytate complexes. For negatively charged proteins, a cationic bridge (usually Ca) links phytate to the protein.31 Additionally, phytate can alternatively interact with protein by acting as a Hofmeister anion, also known as a kosmotrope. The Hofmeister or lyotropic series is a classification of cations and anions based on their capacity to stabilise or destabilise protein. Protein solubility can be reduced by stabilising the protein through the formation of hydrogen bonds in water.32 Alpha amino acids, basic terminal amino acids, the epsilon amino group of lysine, the imidazole group of histidine, and the guanidine group of arginine are involved in the interactions between proteins and PA, which depend on the unhindered cationic groups of protein attaching to PA.33
Various Strategies to Reduce PA Content in Food
Various strategies can be applied to enhance the micronutrient content of food, either pre- or postharvest or during food processing.
Biofortification
The preharvest strategies include crop biofortification, which can be achieved through different approaches involving agronomic practices, such as the use of plant growth-promoting microorganisms, mineral fertilisers, genetic engineering, and plant breeding. These agronomic approaches involve the combination of inorganic and organic fertilisers to improve the micronutrient content or status of the soil, which ultimately enhances plant quality.34 These approaches improve soil fertility but do not ensure the solubility or mobility, and therefore bioavailability, of various micronutrients in the edible parts of the plant.35,36 As such, genetic biofortification is the strategy applied to ensure minerals are bioavailable for human consumption. Genetic biofortification is the process though which mineral-rich cultivars are developed through genetics using breeding and transgenic approaches. In this approach, cultivars with low PA or antinutrient contents and high or improved micronutrient contents are selected for breeding. As PA levels are negatively correlated with the availability of essential micronutrients, various cereal crops such as wheat, maize, rice, and pulses have been improved through biofortification, in which the antinutrient level is reduced and the mineral profile is enhanced. Genetic biofortification has been performed under the Indian Council of Agricultural Research (ICAR) mega project entitled “On the biofortification of staple crops”. The aims of this biofortification project are to reduce all the major factors hindering mineral uptake from soil, improve the mobility of minerals in grains, reduce the content of antinutritional factors in grains, and others. Conventionally biofortified crops also contribute to enhancing human mineral deficiencies. The target crops for biofortification in India are wheat, rice, maize, sorghum, and small millet. By using biofortification strategies, various varieties of wheat, rice, and maize have been developed. The biofortified varieties are 1.5 to 3.0 times more nutritious than the traditional varieties. Rice variety CR DHAN 315 has excess Zn, wheat variety HD 3298 is enriched with protein and Fe, and DBW 303 and DDW 48 are rich in protein and Fe. The maize hybrid varieties are enriched with lysine and tryptophan. The varieties of millet (CFMV 1 and 2) are rich in Ca, Fe, and Zn, and the CCLMV1 variety of small millet is rich in Fe and Zn.37
Dietary Diversification
Although almost all plants can synthesise and accumulate micronutrients, the edible part of some crop plants contain inadequate amounts of essential micronutrients such as Fe, Zn, vitamin A, and folate. To enhance the micronutrient content of staple crops, one strategy involves dietary diversification. At the household level, this involves the consumption of food that has been prepared such as through soaking, fermentation, and germination, to hydrolyse the available micronutrients to maximise their bioavailability.38 These methods reduce or hydrolyse PA through increasing the activity of endogenous phytase in whole grain cereals and legumes.39
Commercial Food Fortification
Commercially, traces of micronutrients can be added to food or food supplements to enhance the micronutrient profile of the diet. A well-known food fortification is the addition of traces of I to salt, which has successfully increased the no. of countries from 67 in 2003 to 118 in 2020 with adequate iodine intake. Other examples of fortification includes the addition of B-group vitamins, Zn, and Fe to wheat flour, and vitamin A to cooking oil.39
Prebiotics and Probiotics
Microbial-based pro- and prebiotic approaches can also be applied to enhance the nutritional profile of staple crops and processed food. Various bacterial and fungal strains that are able to hydrolyse PA during food processing may be used to enhance the micronutrient profile. Several microbial strains are being used in the feed industry for monogastric animals to enhance the nutritional content of feed and reduce environmental pollution due to the release of P. However, no microbial strain is commercially available in India for use in the food industry, despite studies on the phytase-producing isolates of prebiotics.40 Thus, strains that can be used as a prebiotic or probiotic need to be identified and isolated, and then applied in the food industry to enhance micronutrient contents.41
Potential Sources of Phytase
Phytases are ubiquitous in nature, with sources ranging from fungi and bacteria to plants and animals. For industrial applications, many fungal and bacterial sources have been exploited. The fungal sources mainly include Aspergillus species, such as A. glaucus, A. japonicas, and A. terreus. Acremonium sp. can yield similar amounts of phytases to other widely known fungi. Endophytic fungi from agroindustrial byproducts, such as species of the genus Mucosodor sp., Peniophora lycii C1, Debaryomyces hansenii, and Kluyveromyces marxianus, can also produce enzymes.42 Phytases have been reported in various bacteria such as Aerobacter aerogenes, Bacillus spp., Bifidobacterium spp., Citrobacter braakii, Enterobacter spp., E. coli, Klebsiella spp., Lactobacillus spp., Megasphaera elsdenii, Prevotella sp., Mitsuokella spp., Pseudomonas spp., and S. ruminantium.43 Extracellular phytases are mainly produced by Bacillus species, such as B. subtilis.44 Microbial strains with probiotic attributes, such as the Pediococcus acidilactici BNS5B isolate, were reported to produce phytase.40 In addition to searching natural resources, various genomic and metagenomic approaches have be applied to identify potential phytase producers. The gene coding for phytase was isolated from red rice and castor been cake residues and expressed in E. coli.45 Overall, this process includes the use of genomic and metagenomic databases with the integration of bioinformatics and rDNA technology.
Another practical strategy categorised as a genomic and metagenomic approach is called genome hunting. Drafting the genome of various species and strains has contributed to identifying potential phytase producers through identifying the gene coding phytase from the putative draft genome sequences of Bacillus ginsengihumi, Pantoea sp., and the plant growth-promoting strain of Pseudomonas aeruginosa.46 Another advanced strategy is data mining, in which the NCBI and CAMERA databases are used for the homology alignment of representative genes with all classes of phytases known in nature to gain insight into the distribution of unexplored phytases in nature.47
Classification of Phytases
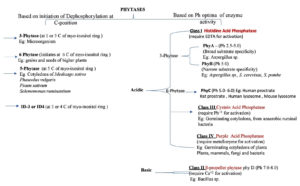
The several types of phytases that are available in nature can be broadly classified based on different properties, such as the C at which the dephosphorylation of the myo-inositol ring is initiated, the optimal pH for catalytic activity, and the catalytic mechanism of phytases (Figure).48
Phytases are categorised based on where the dephosphorylation of myo-inositol is initiated: 3-, 5-, and 6-phytases. The 3-phytases initiate dephosphorylation at either the 1- or 3-C of the myo-inositol ring and are mainly found in fungal or bacterial species. The 6-phytases initiate phosphorylation at the 6-C of the myo-inositol ring and are found in the grains and seeds of higher plants. The 5-phytases initiate phosphorylation at the 5-C of the myo-inositol ring and are found in legume cotyledons and the bacterial species Selenomonas ruminantium. Recently, another class of phytases was identified in this series, which initiates dephosphorylation at ID-3 or ID-4: protein tyrosine phosphatase (PTP)-like inositol polyphosphatases.49
On the basis of their optimal pH for catalytic activity and mechanism, phytases can be classified as acid and alkaline phytases. The acid phytases mainly include histidine acid phosphatases (HAPs), purple acid phosphatases (PAPs), PTP-like inositol polyphosphatase, and cysteine acid phosphatase (CAP).50 HAP is further divided based on whether the phytase has broad or narrow substrate specificity. PhyA is a broad-substrate HAP with an optimal pH between 2.5 and 5.0, and it has been isolated from filamentous fungi, bacteria, yeast, and plants. It has commercially been exploited from A. niger NRRL 3135 for use in the animal feed industry under the names Natuphos (BASF, Manheim, Germany) and Ronozyme (Novozymes/DSM, Copenhagen, Denmark). PhyB is a high-activity HAP with narrow substrate specificity and an optimal pH of 2.5. PhyB has been observed in A. niger, A. awamore, A. tereus, A. thermophilus, and A. fumigatus.51 Another class of acidic phytases is the PAPs, which were first isolated from germinating soybean cotyledons, and later from plants, mammals, fungi, and bacteria.49 CAP has been isolated from germinating cotyledons and anaerobic ruminal bacteria. To date, β-propeller phytases are the only phytases known to have an optimal pH in the alkaline range of 7–8 and require Ca+2 for thermostability and catalytic activity. They have mainly been reported in bacterial species, specifically, the Bacillus species B. subtilis, B. amyloliquifaciens, and B. licheniformis.52
Applications of Phytases
Food Technology
With increased awareness regarding human nutrition, more people are becoming attracted to the potential benefits of high-fibre diets focused on whole grain seeds or legumes. However, the higher fibre content in whole grain means that their PA content is also high, which is significantly negatively correlated with divalent cations, leading to either the formation of insoluble complexes or reduction in the bioavailability of these minerals.53 During the processing of whole grains into flours, some of the PA content is reduced by the action of the phytase in the cereals but not to the extent required to improve micronutrient bioavailability.54 Thus, to increase the nutritional value of cereals-based processed food products, exogenous microbial phytases or phytase-producing strains can be applied during food processing. Phytase or phytase-producing strains can be commercially used in the food processing industry to enhance the nutritional value of products. Beneficially, phytase does not affect the quality of the product; rather, it increases the protein and dialysable mineral ion contents and yield and quality of the final product.55 In the bread-making process, phytase has been added to whole rye flour, whole quinoa flour, and rye wheat sour bread.41 The use of a fungal-phytase-based processing method to produce soybean protein isolates, which are mainly used in meat products, baby food, beverages, and wheat flour products and as a gelling agent, resulted in increased percentages of dialysable Zn and Ca and a lower phytate content.56 Uses of phytase-producing Bifidobacterium strains in breadmaking from whole wheat have resulted in the reduction in phytate content and increased Fe availability.57,58 Various isolated strains with probiotic attributes show well-defined phytase activity, such as Lactobacillus, Bifidobacterium, and Enterococcus. A specific P. acidilactici BNS5B isolate strongly affects the dephosphorylation of PA, showing applicability in cereals-based processed foods, and may work as a modifier as well.40
As Food and Feed Additive
Phytase is being widely used in the feed industry and has proven its effect on weight gain and nutrient assimilation by poultry and pigs by hydrolysing phytate and releasing chelated micronutrients.59 De Souza et al.60 found that the feed intake, weight gain, and feed-to-conversion ratio of broiler chickens fed with a phytase-supplemented diet considerably improved. Supplementing the wheat–soybean–maize meal diet of egg-laying hens with phytase helps to increase egg production and maintain egg shell quality and egg content, possibly due to the mobilisation of PA, Ca, and other micronutrients by the enzyme.61-63 Ahmedi et al.64 proved that the performance and egg quality of laying hens improved when their diet was supplemented with phytase compared with those of an unsupplemented group.
Combating Environmental Pollution
Monogastric animals are unable to digest or hydrolyse the PA consumed with cereal grains, which is then excreted to the environment, causing environmental P contamination, including water bodies.65 Thus, various commercial bacterial and fungal phytases have been developed for the animal feed industry as a supplement in the form of a pelleted diet, to ensure that the P is available for uptake by monogastric animals and thus help mitigate the release of P pollution in the environment.66,67 To address the P pollution problem caused by poultry and pigs, transgenic pigs that can express phytase in their salivary glands and can completely digest PA were developed.68
Medical Applications
Phytase has been applied in anticancer treatments against various cancerous cell lines such as THP-1, Hep-G2, and MCF-7 through phytase-based platinum-coated nanodrug delivery.69 Phytases have important roles in the production of lower myo-inisitol phosphate, that is, myo-inositol trisphosphate, myo-inositol tetrakisphosphate, and inositol pentakisphosphate. These lower myo-inositol phosphates have been used to produce various beneficial health effects. They have antioxidant properties70 and are effective in treating renal stone formation,71 heart disease,72 and certain types of cancers.70,73 Furthermore, inositol triphosphates have analgesic properties.74
Miscellaneous Applications
The immobilisation of phytase over some natural support such as mineral calcium apatite (hydroxyapatite) increases the efficiency, yield, and activity of the enzyme.75 Similar results were obtained for the covalent immobilisation of phytase over multiwalled carbon nanotubes, which increased enzymatic activity by 78%.76 Phytase has been applied in the pulp and paper industry to reduce the PA in raw material and thereby create cleaner technology as the residues are not toxic.77 Phytase also positively affects bioethanol production, as the removal of PA reduces the P content in the disposed residue, and it can be used to create high-value-added ethanol and coproducts with improved efficiency and recovery.78
PA is an antinutritional factor that chelates essential mineral ions so that they are unavailable for absorption. Various strategies, such as genetic fortification, dietary diversification, and biofortification, are being used to reduce the contents of these antinutritional components in food and feed. Additionally, microbiological approaches in the form of microbial enzymes can be applied to reduce the PA content in food during processing. Various research groups are working to enhance the nutritional profile of staple-crops-based processed food, but no microbial strain is yet commercially available for human consumption. Thus, we need to explore, identify, and characterise suitable Generally Recognized As Safe (GRAS) grade microbial strains that can be used to enhance the mineral profile of food. Additionally, phytase-producing microbial strains can be applied in the industrial, environmental, and medical fields.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declares that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MP conceived and designed the article. MP and HS performed critical revision and approved the manuscript for final publication.
FUNDING
None.
DATA AVAILABILITY
Not Applicable.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Gupta RK, Gangoliya SS, Singh NK. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol. 2015;52(2):676-684.
Crossref - Urbano G, Lopez-Jurado M, Aranda P, Vidal-Valverde C, Tenorio E, Porres J. The role of phytic acid in legumes: antinutrient or beneficial function? J Physiol Biochem. 2000;56(3):283-294.
Crossref - Zhou JR, Erdman JW. Phytic acid in health and disease. Crit Rev Food Sci Nutr. 1995;35(6):495-508.
Crossref - Gerke J. Phytate (Inositol hexakisphosphate) in soil and phosphate acquisition from inositol phosphates by higher plants. A review. Plants. 2015;4(2):253-266.
Crossref - Annor GA, Tano Debrah KT, Essen A. Mineral and phytate contents of some prepared popular Ghanaian foods. Springerplus. 2016;5(1):581.
Crossref - McKie VA, McCleary BV. A Novel and Rapid Colorimetric Method for Measuring Total Phosphorus and Phytic Acid in Foods and Animal Feeds. J AOAC Int. 2016;99(3):738-743.
Crossref - Tulchinsky TH. Micronutrient Deficiency Conditions: Global Health Issues. Public Health Rev. 2010;32(1):243-255.
Crossref - Webb P. Impact Pathways from Agricultural Research to Improved Nutrition and Health: Literature Analysis and Research Priorities. Backgr Pap Prep ICN2 Second International Conference on Nutrition. 2013:28. www.fao.org/publications
- Brinch-Pedersen H, Borg S, Tauris B, Holm PB. Molecular genetic approaches to increasing mineral availability and vitamin content of cereals. J Cereal Sci. 2007;46(3):308-326.
Crossref - Singh R, Kumar M, Mittal A, Mehta PK. Microbial enzymes: industrial progress in 21st century. 3 Biotech. 2016;6(2):174.
Crossref - Polaquini L, Marcondes M, Rocha M. The Challenge of Hidden Hunger Global Hunger Index. FIEP Bull On-line. 2014;80(0):1-56. https://www.ifpri.org/sites/default/files/ghi/2014/feature_1818.html
- Al Hasan SM, Hassan M, Saha S, Islam M, Billah M, Islam S. Dietary phytate intake inhibits the bioavailability of iron and calcium in the diets of pregnant women in rural Bangladesh: A cross-sectional study. BMC Nutr. 2016;2(1):24.
Crossref - Gilani GS, Cockell KA, Sepehr E. Effects of antinutritional factors on protein digestibility and amino acid availability in foods. J AOAC Int. 2005;88(3):967-987.
Crossref - Lee HH, Loh SP, Bong CFJ, Sarbini SR, Yiu PH. Impact of phytic acid on nutrient bioaccessibility and antioxidant properties of dehusked rice. J Food Sci Technol. 2015;52(12):7806-7816.
Crossref - Chitra U, Vimala V, Singh U, Geervani P. Variability in phytic acid content and protein digestibility of grain legumes. Plant Foods Hum Nutr. 1995;47(2):163-172.
Crossref - Erdman JW, Poneros-Schneier A. Phytic acid interactions with divalent cations in foods and in the gastrointestinal tract. Adv Exp Med Biol. 1989;249:161-171.
Crossref - Roohani N, Hurrell R, Kelishadi R, Schulin R. Zinc and its importance for human health: An integrative review. J Res Med Sci. 2013;18(2):144-157. PMCID: PMC3724376
- Gupta S, Brazier AKM, Lowe NM. Low-and Middle-Income Countries Zinc deficiency in low-and middle-income countries: prevalence and approaches for mitigation. J Acad Nutr Diet. 2020;33(5):624-643
Crossref - Hambidge KM, Miller LV, Krebs NF. Physiological requirements for zinc. Int J Vitam Nutr Res. 2011;81(1):72-78.
Crossref - Lonnerdal B. Dietary factors influencing zinc absorption. J Nutr. 2000;130(5S Suppl):1378S-1383S.
Crossref - Ma G, Li Y, Jin Y, Zhai F, Kok FJ, Yang X. Phytate Intake and Molar Ratios of Phytate to Zinc, Iron and Calcium in the Diets of People in China. Eur J Clin Nutr. 2007;61(3):368–374.
Crossref - Davidson L, Almgren A, Sandstrom B, Hurrell RF. Zinc absorption in adult humans: the effect of iron fortification. Br J Nutr. 1995;74(3):417-425.
Crossref - Allen L, De Benoist B, Dary O, Hurrell R. Guidelines on food fortification with micronutrients. 2006:3-20. https://cesni-biblioteca.org/wp-content/uploads/2020/04/guide_food_fortification_micronutrients_optimize-2.pdf
- Hunt JR, Beiseigel JM. Dietary calcium does not exacerbate phytate inhibition of zinc absorption by women from conventional diets. Am J Clin Nutr. 2009;89(3):839-843.
Crossref - Quintero-Gutierrez AG, Gonzalez-Rosendo G, Sanchez-Munoz J, Polo-Pozo J, Rodriguez-Jerez JJ. Bioavailability of Heme Iron in Biscuit Filling Using Piglets as an Animal Model for Humans. Int J Biol Sci. 2008;4(1):58-62.
Crossref - McDowell LR. Minerals in Animal and Human Nutrition: Second Edition. Miner Anim Hum Nutr Second Ed. 2003:1-644.
Crossref - Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19(2):164-174. PMCID: PMC3999603. Accessed December 15, 2021.
- Lopez HW, Leenhardt F, Coudray C, Remesy C. Minerals and phytic acid interactions: Is it a real problem for human nutrition? Int J Food Sci Technol. 2002;37(7):727-739.
Crossref - Grases F, Prieto RM, Simonet BM, March JG. Phytate prevents tissue calcifications in female rats. Bio Factors. 2000;11(3):171-177.
Crossref - Pravina P, Sayaji D, Avinash M. Calcium and its Role in Human Body. Int J Res Pharm Biomed Sci. 2013;4(2):659-668. www.ijrpbsonline.com. Accessed December 15, 2021.
- Selle PH, Cowieson AJ, Cowieson NP, Ravindran V. Protein-phytate interactions in pig and poultry nutrition: A reappraisal. Nutr Res Rev. 2012;25(1):1-17.
Crossref - Baldwin RL. How Hofmeister ion interactions affect protein stability. Biophys J. 1996;71(4):2056-2063.
Crossref - Wang R, Guo S. Phytic acid and its interactions: Contributions to protein functionality, food processing, and safety. Compr Rev Food Sci Food Saf. 2021;20(2):2081-2105.
Crossref - de Valenca AW, Bake A, Brouwer ID, Giller KE. Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Glob Food Sec. 2017;12:8-14.
Crossref - Broadley MR, White PJ, Bryson RJ, et al. Biofortification of UK food crops with selenium. Proc Nutr Soc. 2006;65(2):169-181.
Crossref - Cakmak I, Kutman UB. Agronomic biofortification of cereals with zinc: a review. Eur J Soil Sci. 2018;69(1):172-180.
Crossref - Mishra JP, Arunachalam A, Kimothi SP. in Prime Minister of India. Significant acheivements 2019-2020. ICAR-Directorate of Knowledge Management in Agriculture, New Delhi. https://krishi.icar.gov.in/jspui/bitstream/123456789/38237/2/ICAR-Achievements-2019-20_English-min.pdf
- Hurrell RF. Fortification: Overcoming technical and practical barriers. J Nutr. 2002;132(4 SUPPL.):806S-812S.
Crossref - Jha AB, Warkentin TD. Biofortification of Pulse Crops: Status and Future Perspectives. Plants (Basel, Switzerland). 2020;9(1):73.
Crossref - Sharma B, Shukla G. Isolation, identification, and characterization of phytase producing probiotic lactic acid bacteria from neonatal fecal samples having dephytinization activity. Dephytinization Activity. Food Biotechnol. 2020;34(2):151-171.
Crossref - Iglesias-Puig E, Monedero V, Haros M. Bread with whole quinoa flour and bifidobacterial phytases increases dietary mineral intake and bioavailability. LWT – Food Sci Technol. 2015;60(1):71-77.
Crossref - Pires EBE, de Freitas AJ, Souza FFe, et al. Production of Fungal Phytases from Agroindustrial Byproducts for Pig Diets. Sci Rep. 2019 91. 2019;9(1):9256.
Crossref - Jain J, Sapna, Singh B. Characteristics and biotechnological applications of bacterial phytases. Process Biochem. 2016;51(2):159-169.
Crossref - Powar VK, Jagannathan V. Purification and properties of phytate-specific phosphatase from Bacillus subtilis. J Bacteriol. 1982;151(3):1102-1108.
Crossref - Farias N, Almeida I, Meneses C. New Bacterial Phytase through Metagenomic Prospection. Molecules. 2018;23(2):448.
Crossref - Suleimanova AD, Toymentseva AA, Boulygina EA, et al. High-quality draft genome sequence of a new phytase-producing microorganism Pantoea sp. 3.5.1. Stand Genomic Sci. 2015;10:95.
Crossref - Lim BL, Yeung P, Cheng C, Hill JE. Distribution and diversity of phytate-mineralizing bacteria. ISME J. 2007;1(4):321-330.
Crossref - Bhavsar K, Khire JM. Current research and future perspectives of phytase bioprocessing. RSC advances. 2014: 4(51);26677-26691.
Crossref - Puhl AA, Greiner R, Selinger LB. Stereospecificity of myo-inositol hexakisphosphate hydrolysis by a protein tyrosine phosphatase-like inositol polyphosphatase from Megasphaera elsdenii. Appl Microbiol Biotechnol. 2009;82(1):95-103.
Crossref - Yao MZ, Zhang YH, Lu WL, Hu MQ, Wang W, Liang AH. Phytases: crystal structures, protein engineering and potential biotechnological applications. J Appl Microbiol. 2012;112(1):1-14.
Crossref - Mullaney E, Ullah AHJ. The term phytase comprises several different classes of enzymes. Biochem Biophys Res Commun. 2003;312(1):179-184.
Crossref - Kerovuo J, Lauraeus M, Nurminen P, Kalkkinen N, Apajalahti J. Isolation, characterization, molecular gene cloning, and sequencing of a novel phytase from Bacillus subtilis. Appl Environ Microbiol. 1998;64(6):2079-2085.
Crossref - Sanz-Penella JM, Laparra JM, Sanz Y, Haros M. Bread Supplemented with Amaranth (Amaranthus cruentus): Effect of Phytates on In Vitro Iron Absorption. Plant Foods Hum Nutr. 2012;67(1):50-56.
Crossref - Haros M, Rosell CM, Benedito C. Use of Fungal Phytase to Improve Breadmaking Performance of Whole Wheat Bread. J Agric Food Chem. 2001;49(11):5450-5454.
Crossref - Yaver E, Bilgicli N. Effects of different dephytinisation methods on chemical properties of commercial and traditional breads prepared from composite flour. Food Chem. 2019;276:77-83.
Crossref - Wang H, Chen Y, Hua Y, Kong X, Zhang C. Effects of phytase-assisted processing method on physicochemical and functional properties of soy protein isolate. J Agric Food Chem. 2014;62(45):10989-10997.
Crossref - Garcia-Mantrana I, Monedero V, Haros M. Myo-inositol hexakisphosphate degradation by Bifidobacterium pseudocatenulatum ATCC 27919 improves mineral availability of high fibre rye-wheat sour bread. Food Chem. 2015;178:267-275.
Crossref - Sanz-Penella JM, Laparra JM, Sanz Y, Haros M. Assessment of Iron Bioavailability in Whole Wheat Bread by Addition of Phytase-Producing Bifidobacteria. J Agric Food Chem. 2012;60(12):3190-3195.
Crossref - Sun Z, Yue Z, Yang X, et al. Efficient phytase secretion and phytate degradation by recombinant bifidobacterium longum JCM 1217. Front Microbiol. 2019;10:796.
Crossref - de Sousa JPL, Albino LFT, Vaz RGMV, et al. The effect of dietary phytase on broiler performance and digestive, bone, and blood biochemistry characteristics. Brazilian J Poult Sci. 2015;17(1):69-76.
Crossref - Bubancova I, Englmaierova M, Skrivan M, et al. Effects of a low-phosphorus diet and exogenous phytase on performance, egg quality, and bacterial colonisation and digestibility of minerals in the digestive tract of laying hens. Artic Czech J Anim Sci. 2015;60(12):542-549.
Crossref - Humer E, Schwarz C, Schedle K. Phytate in pig and poultry nutrition. J Anim Physiol Anim Nutr (Berl). 2015;99(4):605-625.
Crossref - Kim JH, Pitargue FM, Jung H, Han GP, Choi HS, Kil DY. Effect of superdosing phytase on productive performance and egg quality in laying hens. Asian-Australas J Anim Sci. 2017;30(7):994-998.
Crossref - Ahmadi A, Tabatabaei MM, Aliarabi H, Saki AA, Hosseini Siyar SA. Performance and egg quality of laying hens affected by different sources of phytase. Pak J Biol Sci. 2008;11(18):2286-2288.
Crossref - Mullaney EJ, Daly CB, Ullah AHJ. Advances in phytase research. Adv Appl Microbiol. 2000;47:157-199.
Crossref - Kumar A, Chanderman A, Makolomakwa M, Perumal K, Singh S. Microbial production of phytases for combating environmental phosphate pollution and other diverse applications. Crit Rev Environ Sci Technol. 2016;46(6):556-591.
Crossref - Singh B, Satyanarayana T. Microbial phytases in phosphorus acquisition and plant growth promotion. Physiol Mol Biol Plants. 2011;17(2):93-103.
Crossref - Golovan SP, Meidinger RG, Ajakaiye A, et al. Pigs expressing salivary phytase produce low-phosphorus manure. Nat Biotechnol. 2001;19(8):741-745.
Crossref - Soni SK, Sarkar S, Selvakannan PR, Sarkar D, Bhargava SK. Intrinsic therapeutic and biocatalytic roles of ionic liquid mediated self-assembled platinum-phytase nanospheres. RSC Adv. 2015;5(77):62871-62881.
Crossref - Hawkins PT, Poyner DR, Jackson TR, Letcher AJ, Lander DA, Irvine RF. Inhibition of iron-catalysed hydroxyl radical formation by inositol polyphosphates: a possible physiological function for myo-inositol hexakisphosphate. Biochem J. 1993;294(3):929-934.
Crossref - Modlin M. Urinary phosphorylated inositols and renal stone. Lancet (London, England). 1980;2(8204):1113-1114.
Crossref - Ullah A, Shamsuddin AM. Dose-dependent inhibition of large intestinal cancer by inositol hexaphosphate in F344 rats. Carcinogenesis. 1990;11(12):2219-2222.
Crossref - Irshad M, Asgher M, Bhatti KH, Zafar M, Anwar Z. Anticancer and nutraceutical potentialities of phytase/phytate. Int J Pharmacol. 2017;13(7):808-817.
Crossref - Siren M. Inositol triphosphates. US Patent: 1987; 4,851,560.
- Coutinho TC, Tardioli PW, Farinas CS. Hydroxyapatite nanoparticles modified with metal ions for xylanase immobilization. Int J Biol Macromol. 2020;150:344-353.
Crossref - Naghshbandi MP, Moghimi H, Latif B. Covalent immobilization of phytase on the multi-walled carbon nanotubes via diimide-activated amidation: structural and stability study. Artif Cells Nanomed Biotechnol. 2018;46(sup1):763-772.
Crossref - BL L, CH J, YM T. Effect of immobilization on pH and thermal stability of Aspergillus ficuum phytase. Enzyme Microb Technol. 1999;25(6):517-521.
Crossref - Vasudevan UM, Jaiswal AK, Krishna S, Pandey A. Thermostable phytase in feed and fuel industries. Bioresour Technol. 2019;278:400-407.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.