ISSN: 0973-7510
E-ISSN: 2581-690X
A natural sap from mature coconut palm known as coconut neera is enriched with essential minerals and vitamins. Rapid microbial fermentation affects neera processing industries because it spoils the physicochemical properties. There are various methods in preservation that extend the shelf life of coconut neera. The addition of nisin is one of the methods which protect neera against fermentation. Therefore, the study is focused to identify the effective combination of nisin (50 ppm) with preservatives like sodium benzoate (500, and 1000 ppm), and calcium carbonate (2500, and 3000 ppm) at two different combinations in neera. At the end of 21 d, 3000 ppm calcium carbonate with 50 ppm nisin in N4 treatment had an effective reduction of 120 × 105 CFU/ml and 143 × 102 CFU/ml for total bacteria and total yeast count. The reduced microbial survival resulted in the pH of 10.45 ± 0.05, total soluble solids of 15.43 ± 0.12 °Brix, and total acidity of 1.11 ± 0.04 mg/ L, at this combination. The treatment of nisin with 3000 ppm calcium carbonate demonstrated the high red fluorescence bacterial cells than the treatment of nisin with 1000 ppm sodium benzoate. Additionally, the microorganisms in N4 treatment precipitated 65.34% Ca2+ from 79.96% in XRF intensity analysis. The synergistic effect of nisin and calcium carbonate explored their antimicrobial activity against the heterogeneous microbial population in coconut neera. The concentration of 3000 ppm calcium carbonate and nisin 50 ppm preserves the physicochemical and sensory qualities, up to 21 d at 4°C, and offer hope for the industrial-scale implementation.
Coconut neera, Nisin, Calcium carbonate, Microorganisms, Physicochemical, Shelf life
The inflorescence sap of coconut neera is the abundant source of sucrose (16.19 g/ 100 mL), minerals such as K-68.4 mg/ 100 mL, Na-90.6 mg/ 100 mL, P-3.9 mg/ 100 mL, vitamins and proven low glycemic index drink (GI-35) with added medicinal benefits of nephroprotective, and hepatoprotective activity.1 In addition, the natural sap is the source of indigenous microorganisms such as Bacillus, Lactobacilli, Micrococci, Enterobacter, Leuconostoc, Saccharomyces, Candida, and Pichia.2 These microbes ferment the neera in a rapid way, and affected the palatability by producing the astringency, unpleasant volatile, cloudy appearance which gives economic loss to the neera entrepreneurs.3,4 However, the natural sap preserved by the existing technology through the combination of preservatives (citric acid and sodium metabisulphite), processing methods such as centrifugation, filtration and pasteurization.5 Another patented technology preserved the neera by acidification, preservatives such as nisin and pasteurization.6 Though the developed technologies bestow towards effective shelf life enhancement of neera, they might deplete the quality attributes due to thermal pasteurization.7 To overcome these disadvantages, the current study is designed to minimize the processing methods by adding antimicrobial agents for retaining the physicochemical and sensory properties.
The bio-preservative nisin (E234) from Lactococcus lactis classified as “Generally Recognized as Safe” for the food industry by World Health Organization has antimicrobial peptides that are widely used against gram-positive organisms and spores.8 Previous study reported that the application of nisin at a maximum concentration of 100 µg/ mL in wine effectively inhibited lactic acid fermentation.9 However, the microbial inactivation mechanism of ion-permeable pores in the cytoplasm is reported mainly for gram-positive bacteria than gram-negative bacteria.10 Hence, their combination with chemical preservatives can affect the heterogeneous microbial (gram-positive, gram-negative bacteria, yeast) growth in neera. Calcium carbonate (E170) is the widely used alkaline additive with a varied role in industries; nutritional supplements, curing agent, bulking agent, and modifiers. Traditionally, calcium carbonate is coated on the neera collection vessel to extend the storage period.11 As per the Food Safety and Standard Regulations 6.1.19 (FSSR, 2010), the maximum ppm of 5000 allowed for calcium carbonate in food preservation. Earlier studies reported that nanoparticles of calcium carbonate showed antimicrobial activity for gram-positive and gram-negative bacteria. The minimum inhibitory concentration (MIC) of 125 µg/ mL and 62.5 µg/ mL were reported against the gram-positive and gram-negative bacteria.12 Likewise, Sodium benzoate (E211) is another preservative used in fresh juice, margarine, sweet products against the fungi. The study in black olive fermentation reported that 1000 ppm of sodium benzoate reduced the yeast count upon storage.13 Hence, the current study is aimed to control heterogeneous microbial communities of neera by nisin in combination with preservatives (sodium benzoate, and calcium carbonate), and assess the quality changes during storage.
Addition of preservatives to the coconut sap
The collected fresh coconut neera was screened for pH and total soluble solids (≥ 6 and 15 °Brix), and then the sample was taken for further treatment. Based on the preliminary study, four different combinations (N1, N2, N3, and N4) were selected for the study. N1 and N2 contain sodium benzoate (Ganesh Benzoplast, India) of 500 ppm and 1000 ppm, respectively, while calcium carbonate (Local market, Thanjavur) of 2500 ppm and 3000 ppm were ascertained for N3 and N4 along with 50 ppm of nisin (Bimal Pharma Pvt. Ltd, India). Fresh coconut neera without preservatives was taken as control (C). The control and treated samples were stored at 4°C and analyzed for physicochemical, microbial, and sensory changes during 0, 3, 6, 9, 12, 15, 18, and 21 d of storage.
Microbiological analysis
The total viable bacteria and yeast in the control (C) and treated (N1, N2, N3, N4) samples were analyzed using a serial dilution method. For the enumeration of bacteria and yeast, plate count agar and oxytetra glucose yeast agar base (Hi-Media, Mumbai, India) were used. After spreading the diluted sample, plates were incubated at 30 ± 2°C for 24 and 72 h, respectively.14 The observed colonies were expressed in the colony-forming unit (CFU), and more than 300 CFU/ml in storage days were denoted as too numerous to count.15
Physicochemical analysis
Aseptically drawn samples were analyzed for pH through the ELICO pH meter (L1 120 – model). The percentage of CO2 was measured using PBI Dansensor. Hunter color parameters (Lightness, redness, and yellowness) of the neera samples were measured using a colorimeter (Color Flex EZ – 45/0LAV). The total soluble solids of the neera sample were measured by a hand-held refractometer (RHB – 55ATC), and results were expressed in °Brix. The total acidity of neera was analyzed against 0.1 N NaOH with the addition of a phenolphthalein indicator.
Microscopic analysis
The treatment effect on the microbial population of neera was evaluated by the live/dead cells staining using confocal laser scanning microscopy (model LSM 710 – Carl Zeiss Microscopy GmbH, Germany). For differentiating the viable and non-viable cells, the fluorescent dyes mixture of acridine orange and propidium iodide were added (1 µg in 1 µL) into the cells. Before the addition, the treated coconut neera sample was centrifuged at 10,000 × g for 5 m, and the collected pellet was washed with phosphate buffer solution.16 The stained cells were excited and detected using Argon laser (488-nm laser excitation) with a long pass from 500 to 640 nm. The image analysis was performed using Zen 2009 image software.
XRF analysis
The freeze-dried treated whole sample and sediment mineralogy was analyzed using an X-ray fluorescence spectrometer (XGT-5200, Horiba, Japan) equipped with an X-ray tube of 50 kV and a peltier cooled silicon drift detector.17
The Sensory evaluation
A trained panelist of eight judges from the Indian Institute of Food Processing Technology has scored the values using the nine point scale. The coded samples were presented in white cups, and their intensity of appearance, color, flavor, taste, after taste, consistency, and overall acceptability were scored on the respective storage days.
Statistical analysis
The experiments were carried out in triplicates. The values were evaluated statistically from 0 to 21 d by Student’s t-test (SPSS.23). The p-value < 0.05 denotes the significant difference between the storage days.
Addition of preservatives against the microbial growth in neera
The microbial population analysis in plate count revealed that the reduced total bacteria, and yeast count in N4 treatment (nisin 50 ppm with calcium carbonate 3000 ppm) than the N3 (nisin 50 ppm with calcium carbonate 2500 ppm) treatment (Table 1). Whereas, other treatments such as N1, N2, and control sample (C) had more than 300 CFU/ml for the bacteria and yeast population upon storage days. The enhanced antimicrobial activity of nisin with calcium carbonate significantly reduced microbial growth in coconut neera than the nisin with sodium benzoate treatment.18 The added concentration of 3000 ppm calcium carbonate in N4 treatment suppressed the microbial growth in highest count such as 120 × 105 CFU/ml for total bacteria and 143 × 102 CFU/ml for yeast at the end of the storage period (21 d). Whereas, the lowest ppm of 2500 calcium carbonate in N3 treatment reduced the growth as 160 × 105 and 260 × 102 CFU/ml for total bacteria and yeast.
Table (1):
Total bacterial and yeast count (CFU/ml) of fresh and treated coconut neera during the storage days at 4°C.
| Storage days |
Total Bacteria (105) | Total yeast (102) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | N1 | N2 | N3 | N4 | C | N1 | N2 | N3 | N4 | |
| 0 | 120 | 130 | 120 | 3 | 1 | 160 | 67 | 63 | 7 | 10 |
| 3 | * | 270 | 230 | 10 | 3 | * | 247 | 203 | 70 | 10 |
| 6 | * | * | * | 30 | 8 | * | * | * | 130 | 30 |
| 9 | * | * | * | 60 | 17 | * | * | * | 220 | 47 |
| 12 | * | * | * | 100 | 37 | * | * | * | 233 | 67 |
| 15 | * | * | * | 110 | 73 | * | * | * | 243 | 107 |
| 18 | * | * | * | 130 | 100 | * | * | * | 250 | 123 |
| 21 | * | * | * | 160 | 120 | * | * | * | 260 | 143 |
* Represent TNTC (Too Numerous To Count) colonies on storage days.
Abbreviation mentioned in the table throughout denotes: C – Control neera without treatment, N1 – Neera treated with 50 ppm nisin and 500 ppm of sodium benzoate, N2 – Neera treated with 50 ppm nisin and 1000 ppm of sodium benzoate, N3 – Neera treated with 50 ppm nisin and 2500 ppm of calcium carbonate, N4 – Neera treated with 50 ppm nisin and 3000 ppm of calcium carbonate. The values in the table represent the mean of triplicate.
Detection of viable and non-viable cells by microscopy
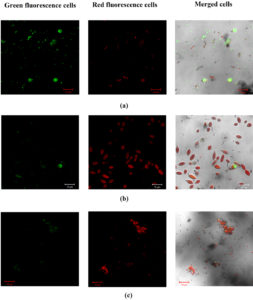
The live and dead cells between treatment demonstrated the antimicrobial activity in neera. In Fig. 1(a) the control sample (C) without preservatives exhibited large number of green fluorescence bacterial cells and it affect the coconut neera by lactic acid fermentation.19 The treatment of N2 expressed more non-viable yeast cells (red fluorescence cells) than the bacterial cells (Fig. 1b). This combination was ineffective against the bacteria because the added preservative of sodium benzoate developed acidic pH which affected the solubility of nisin , and it generally effective towards acidic foods.20 In the presence of nisin and calcium carbonate, there was an increase in the non-viable bacterial cells (red fluorescence cells) which arrest the lactic acid fermentation (Fig. 1c). The reduced bacterial viability confirms the solubility of nisin and their inhibitory activity at this combination.
Fig. 1. Detection of viable and non-viable cells in coconut neera by confocal laser scanning microscopy. Observation of cells from neera (control) without treatment (a) cells from nisin (50 ppm) and sodium benzoate (1000 ppm) added neera (b) cells from nisin (50 ppm) and calcium carbonate (3000 ppm) added neera (c). Green cells represent live microorganisms, while red cells represent dead microorganisms.
Calcium microbe precipitation by XRF analysis
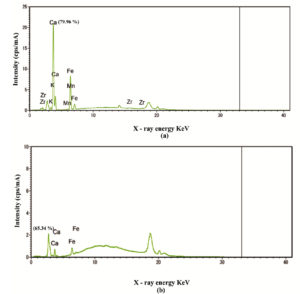
The calcium microbe precipitation in N4 (nisin 50 ppm with calcium carbonate 3000 ppm) treatment was identified through XRF intensity analysis. The intensity of the total treatment was observed with the 79.96% of calcium and sediment portion of the same treatment reveled the percentage of 65.34% (Fig. 2a, b). The resulting percentage in the sediment evident that the sugar rich medium of neera favors the mechanism of precipitation between microbes and minerals.21 The intensity of zirconium (Zr) indicated synthesis of new bio mineral from the precipitation reaction than other minerals of fresh coconut neera (K, Na, Mg, Fe, Ca, and Zn).22 These findings suggest that the precipitation of calcium with indigenous microorganisms had a better inhibition against the spontaneous fermentation.23
Fig. 2. XRF patterns of minerals from nisin (50 ppm) and calcium carbonate (3000 ppm) treated coconut neera (a) whole treated sample includes the sediment (b) sediment of treated sample. The spectra explains the various mineral determinants.
Physicochemical properties of treated neera
The combination effect of preservatives with nisin on the physicochemical qualities of coconut neera were presented in Table 2, and their significant changes between three days interval were tabulated in supporting document. The results revealed that C and N1, and N2 treatment had significantly reduced the pH. However, N4 treatment at the end of 21 d showed a higher pH of 10.45 ± 0.05 from the 0 d control. The pH reduction in sodium benzoate treatments might be due to the production of lactic acid, acetic acid, and citric acid in neera at a temperature of 4°C. Moreover, the pH changes in the C, N1, and N2 were similar to the pH of fermented neera.2 Addition of calcium carbonate in N3 and N4 increased the pH than other treatments. Along with pH, the physicochemical property of CO2 increased in storage days with the greater percentage of 55.53 ± 0.21, 32.70 ± 0.00, and 25.67 ± 0.23 in C, N1, and N2 respectively. This changes in storage indicates the conversion of sucrose by vigorous microbial action of the yeast in N1, N2, and C.24 Whereas, N3 and N4 treatments exhibited less production of CO2 due to the alkali-resistant Bacillus and yeast cells.25
Table (2):
Physicochemical changes in fresh and treated coconut neera during storage days at 4°C.
| Treatment | pH | CO2 (%) | Total acidity (mg/ L) |
Total soluble solids (°Brix) |
Color (∆E) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 d | 21 d | 0 d | 21 d | 0 d | 21 d | 0 d | 21 d | 0 d | 21 d | |
| C | 7.58±0.01 | 2.38±0.01f | ND | 55.53±0.20 h | 0.19±0.02 | 11.83±0.12 h | 19.70±1.71 | 12.17±0.06 f | * | 37.63±0.02 h |
| N1 | 7.16±0.03 | 2.53±0.03 f | ND | 32.7±0.00 h | 0.20±0.00 | 9.13±0.02 h | 17.77±0.15 | 12.27±0.06 f | 3.41±0.00 | 35.53±0.02 h |
| N2 | 7.27±0.00 | 3.48±0.14 h | ND | 25.66±0.23 h | 0.26±0.00 | 10.07±0.02 h | 18.13±0.15 | 12.37±0.06 h | 6.85±0.39 | 33.61±0.02 h |
| N3 | 9.67±0.01 | 5.62±0.01f | ND | 12.37±0.11 h | 0.14±0.01 | 1.23±0.02 h | 18.83±0.29 | 15.27±0.06 g | 7.23±0.03 | 31.37±0.01 h |
|
|
110.82±0.03 | 10.45±0.05 b | ND | 6.83±0.06 g | 0.12±0.02 | 1.11±0.04 g | 19.33±0.29 | 15.43±0.12 g | 9.41±0.29 | 28.79±0.01 h |
Note: ND means not detected. * Indicates no difference on the delta E value of fresh neera (L* 58.13 a* 0.36 and b* 9.26). Data are shown as means ± standard deviation of n = 3 independent experiments. The different alphabets in storage indicate significantly different at 0.05 level between the 0 d to 21 d. Abbreviation mentioned in the table denotes: C – Control neera without treatment, N1 – Neera treated with 50 ppm nisin and 500 ppm of sodium benzoate, N2 – Neera treated with 50 ppm nisin and 1000 ppm of sodium benzoate, N3 – Neera treated with 50 ppm nisin and 2500 ppm of calcium carbonate, N4 – Neera treated with 50 ppm nisin and 3000 ppm of calcium carbonate. The values in the table represent the mean of triplicate.
According to the fermentation phenomenon, C, N1, and N2 treatment had changes in total soluble solids and total acidity. Their quantification in storage days were similar to the trend of fermented neera than treatment of N3 and N4.26 The acidity from lactic acid fermentation was increased as 1.23 ± 0.02 and 1.11 ± 0.04 mg/ L in N3 and N4 respectively which was least acidity in storage. Addition of sodium benzoate in N1 and N2 treatments were ineffective in controlling two-stage fermentation by the bacteria and yeast.27 The better total soluble solids and acidity in N3 and N4 might be due to the precipitation reaction of microbes-calcium.28
The treatment effect on the color of the neera was depicted as color difference (∆E) from the L, a, b values (Table 2). The combination of nisin and sodium benzoate quickly increased the ∆E values between the storage period, and it causes a significant difference from the 0 d control. The addition of calcium carbonate in N3 and N4 had a high impact on whitening the neera color compared to sodium benzoate treatment.29 The undissolved and light scattering calcium carbonate particles in the treatment-induced the 0 d color of neera in N3 and N4. At the end of storage studies, the increased solubility of calcium carbonate produced the least color difference of 31.37 ± 0.01 and 28.79 ± 0.01 ∆E in N3 and N4 treatments respectively.
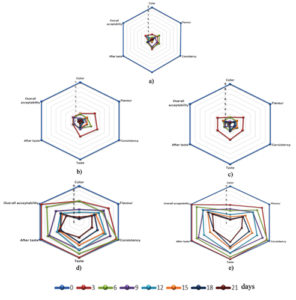
Fig. 3. Sensory properties of coconut neera during storage at 4 °C. (a) Control neera (b) Neera treated with nisin (50 ppm) and sodium benzoate (500 ppm) (c) Neera treated with nisin (50 ppm) and sodium benzoate (1000 ppm) (d) Neera treated with nisin (50 ppm) and calcium carbonate (2500 ppm) (e) Neera treated with nisin (50 ppm) and calcium carbonate (3000 ppm).
Evaluation of sensory attributes in treated neera
The treatment of N4 (Fig. 3e) exhibited better sensory properties for a longer period of 21 d as compared to other treatments. Whereas calcium carbonate concentration of 2500 ppm in N3 lost its sensorial properties on 15 d (Fig. 3d). The sample of C, N1 and N2 samples lost their sensory properties with a shorter period of 3 d (Fig. 3a, b & c). The decreased trend of sensorial properties in increasing storage days was due to the physicochemical changes. The treatment of N4 (nisin 50 ppm with 3000 ppm) with calcium carbonate had better sensory characteristics for more extended period than N3 treated sample. Finally, added 50 ppm nisin did not affect the sensorial properties in any of the neera combinations.9
The addition of sodium benzoate and nisin in neera was ineffective against the microbial growth and resulted the similar physicochemical and sensory quality of fermented neera. The enhanced antimicrobial activity of calcium carbonate with nisin against the bacteria and yeast preserved the quality of coconut neera. However, 3000 ppm of calcium carbonate with 50 ppm nisin was found to be effective to control high number of indigenous microorganisms that preserve the physicochemical, and sensory qualities.
ACKNOWLEDGMENTS
The authors wish to thank Ministry of Food Processing Industries (MOFPI), Government of India for the financial support and Bharathidasan University, Tiruchirappalli, for providing the instrumental facility of Confocal Laser Scanning Microscopy.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was supported by Ministry of Food Processing Industries (MOFPI), Government of India under the project F.No.Q-11/17/2019-R&D.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analysed during this study are included in the manuscript and the supplementary files.
- Hebbar K, Pandiselvam R, Manikantan M, Arivalagan M, Beegum S, Chowdappa P. Palm sap-Quality profiles, fermentation chemistry, and preservation methods. Sugar Tech. 2018;20(6):621-634.
Crossref - Atputharajah JD, Widanapathirana S, Samarajeewa U. Microbiology and biochemistry of natural fermentation of coconut palm sap. Food Microbiol. 1986;3(4):273-280.
Crossref - Borse BB, Rao LJM, Ramalakshmi K, Raghavan B. Chemical composition of volatiles from coconut sap (neera) and effect of processing. Food Chem. 2007;101(3):877-880.
Crossref - Aparajhitha S, Mahendran R. Effect of plasma bubbling on free radical production and its subsequent effect on the microbial and physicochemical properties of Coconut Neera. Innov Food Sci Emerg Technol. 2019;58:102230.
Crossref - Chinnamma M, Bhasker S, Hari MB, Sreekumar D, Madhav H. Coconut neera-a vital health beverage from coconut palms: harvesting, processing and quality analysis. Beverages. 2019;5(1):22.
Crossref - Ramalakshmi K, Ramesh M, Raghavan B, Prakash V. Process for the preservation of coconut sap (neera). U.S. Patent US 0191375 A1; 2004.
- Chia SL, Rosnah S, Noranizan MA, Wan Ramli WD. The effect of storage on the quality attributes of ultraviolet-irradiated and thermally pasteurised pineapple juices. Int Food Res J. 2012;19(3):1001-1010.
- Economou T, Pournis N, Ntzimani A, Savvaidis I. Nisin-EDTA treatments and modified atmosphere packaging to increase fresh chicken meat shelf-life. Food Chem. 2009;114(4):1470-1476.
Crossref - Pei J, Jiang L, Dai H, CHen P. Application of nisin-the well-known lactic acid bacteria bacteriocin-against spoilage bacteria in tangerine wine. Czech J Food Sci. 2016;34(6):488-494.
Crossref - Pokhrel PR, Toniazzo T, Boulet C, et al. Inactivation of Listeria innocua and Escherichia coli in carrot juice by combining high pressure processing, nisin, and mild thermal treatments. Innov Food sci Emerg Technol. 2019;54:93-102.
Crossref - Somawiharja Y, Purnomo H, Wonohadidjojo DM, Kartikawati M, Suniati FRT. Indigenous technology of tapping, collecting and processing of coconut (Cocos nucifera) sap and its quality in Blitar Regency, East Java, Indonesia. Food Res. 2018;2(4):398-403.
Crossref - Ataee R, Derakhshanpour J, Mehrabi Tavana A, Eydi A. Antibacterial effect of calcium carbonate nanoparticles on Agrobacterium tumefaciens. Journal of Military Medicine. 2011;13(2):65-70
- Turantas F, Goksungur Y, Dincer AH, Unluturk A, Guvenc U, Zorlu N. Effect of potassium sorbate and sodium benzoate on microbial population and fermentation of black olives. J Sci Food Agr. 1999;79(9):1197-1202.
Crossref - Hariharan B, Singaravadivel K, Alagusundaram K. Effect of Food Grade Preservatives on the Physicochemical and Microbiological Properties of Coconut Toddy during Fermentation. J Food Sci Nutr. 2014;4(5):1-5.
Crossref - Rahman T, Hasan S, Noor R. An assessment of microbiological quality of some commercially packed and fresh fruit juice available in Dhaka city: A comparative study. Stamford J Microbiol. 2011;1(1):13-18.
Crossref - Zhang T, Fang HHP. Quantification of Saccharomyces cerevisiae viability using BacLight. Biotechnol Lett. 2004;26(12):989-992.
Crossref - Lee J-A, Kim M-K, Kim H-M, et al. The fate of calcium carbonate nanoparticles administered by oral route: absorption and their interaction with biological matrices. Int J Nanomed. 2015;10(1):2273-2293.
Crossref - Datta S, Janes M, Xue QG, Losso J, La Peyre J. Control of Listeria monocytogenes and Salmonella anatum on the surface of smoked salmon coated with calcium alginate coating containing oyster lysozyme and nisin. J Food Sci. 2008;73(2):M67-M71.
Crossref - Pandiselvam R, Manikantan MR, Binu SM, et al. Reaction kinetics of physico-chemical attributes in coconut inflorescence sap during fermentation. J Food Sci Technol. 2021;58(9):3589-3597.
Crossref - Glevitzky M, Dumitrel GA, Perju D, Popa M. Studies Regarding the Use of Preservatives on Soft Drinks Stability. Chem Bull PoLI TEHNICA Univ Timisoara. 2019;54:31-36.
- Park J-M, Park S-J, Ghim S-Y. Characterization of three antifungal calcite-forming bacteria, Arthrobacter nicotianae KNUC2100, Bacillus thuringiensis KNUC2103, and Stenotrophomonas maltophilia KNUC2106, derived from the Korean Islands, Dokdo and their application on mortar. J Microbiol Biotechnol. 2013;23(9):1269-1278.
Crossref - Asghar MT, Yusof YA, Mokhtar MN, et al. Coconut (Cocos nucifera L.) sap as a potential source of sugar: Antioxidant and nutritional properties. Food sci nutr. 2020;8(4):1777-1787.
Crossref - Cacchio P, Ercole C, Cappuccio G, Lepidi A. Calcium carbonate precipitation by bacterial strains isolated from a limestone cave and from a loamy soil. Geomicrobiol J. 2003;20(2):85-98.
Crossref - Kapilan R. Determination of efficient fermentation inhibitor of the tapped inflorescence sap of Caryota urens in Sri lanka. Intl J Curr Microbiol Appl Sci. 2015;4(10):487-496.
- Combet-Blanc Y, Kalamba KK, Kergoat PY. Effect of pH on Bacillus thermoamylovorans Growth and Glucose Fermentation. Appl Environ Microbiol. 1995;61(2):656-659.
Crossref - Xia Q, Li R, Zhao S, et al. Chemical composition changes of post-harvest coconut inflorescence sap during natural fermentation. Afr J Biotechnol. 2011;10(66):14999-15005.
Crossref - Kalaiyarasi K, Sangeetha K, Rajarajan S. A comparative study on the microbial flora of the fresh sap from cut inflorescence and fermented sap (toddy) of Borrassus flabellifer Linn (Palmyrah tree) and of Cocos nucifera Linn (Coconut tree) to identify the microbial fermenters. Int J Res Pure and Appl Microbiol. 2013;3(3):43-47.
- Konopacka-Lyskawa D, Czaplicka N, Koscielska B, Lapinski M, Gebicki J. Influence of Selected Saccharides on the Precipitation of Calcium-Vaterite Mixtures by the CO2 Bubbling Method. Crystals. 2019;9(2):117.
Crossref - Hsu CK, Chiang BH. Effects of water, oil, starch, calcium carbonate and titanium dioxide on the colour and texture of threadfin and hairtail surimi gels. Int J Food Sci Technol. 2002;37(4):387-393.
Crossref
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.