ISSN: 0973-7510
E-ISSN: 2581-690X
Pneumocystis jirovecii is an opportunistic fungus, which causes Pneumocystis pneumonia (PJP) in immunocompromised, COPD and TB positive patients with a high rate of colonization, morbidity and mortality. Dihydropteroate synthase (DHPS) gene mutations are well-reported in PJP. Although sulfa prophylaxis generally is associated with DHPS mutant infection, Multiple molecular techniques applied for detect sulfa resistance single-base mutation. Conformation sensitive gel electrophoresis (CSGE) is a rapid screening method for detection of DNA sequence variation, specifically single-base changes or small insertions and deletions. The current study is investigate on the DHPS sequence single-base dislocation among strains isolated from Iranian TB positive co-infected with PJP in association to increased levels of serum Lactate Dehydrogenase. Through high serum lactate dehydrogenase (LDH) levels have been associated with established Pneumocystis pneumonia. We investigated the DHPS mismatches in five P. jirovecii isolated of TB infected patients. For genetic identification of Pneumocystis isolates and detection of intraspecific variation, we developed a method for heteroduplex analysis. Our utilizing fragments was the DHPS gene regions, amplified by PCR method with specific primers. Serum LDH indicator was analysed for lung acute damages. In our results, at least 4 suspected isolates show more slowly migrating bands containing single heteroduplexes, reveal single-base mislocation in studied sequences. LDH level Peak was higher (p<0.05) in patients with PCP (445± 155 U/L) in comparison patients with chronic TB (310±50 U/L). CSGE is a simple manual method, based on heteroduplex analysis, and compares well in terms of sensitivity with other screening technologies. Manual CSGE remains a low-cost, accessible, and effective approach for mutation screening, which can be carried out with minimal specialist equipment.
Pneumocystis jirovecii, Dihydropteroate synthase (DHPS), Gene mutations, CSGE, Heteroduplex, Lactate dehydrogenase (LDH).
Pneumocystis jirovecii pneumonia (PJP) formerly known as Pneumocystis carinii pneumonia (PCP) is a life-threatening opportunistic fungal infection of the lungs with a signiûcant morbidity and mortality among immunocompromised patients1. Pneumocystis often cause of pneumonia in immunocompromised hosts such as hematologic malignancies, organ transplants, immunosuppressive drugs users, pulmonary tuberculosis (TB), COPD and patients infected with human immunodeficiency virus (HIV)2. Tuberculosis makes a special immune background with a low supplementary oxygen pressure due to Pneumocystis expedited colonization3. The emergence of drug resistance is predicted because of the extreme usage of TMP-SMX for prophylaxis against PJP and wide-spectrum antibiotics in respiratory disorders4. In Pneumocystis sulfa drug resistance gene, single base mismatches produce treatment challenges in mutant strains. Finding a proper method to detection of complex gene’s single-base mutations remains a technical challenge, and there has been a continuing search for rapid and efficient methods for developed investigations. Although there is several molecular techniques for diversity and mismatch detection, but few was utilize in Pneumocystosis infection. Conformational sensitive gel electrophoresis (CSGE) is an operational method develop for scanning PCR products for the presence of single-base and larger base transpositions in DNA. The assay was based on the assumption that mildly denaturing solvents in an appropriate buffer can accentuate the conformational changes produced by single-base mismatches in double-stranded DNA and thereby increase the differential migration in electrophoretic gels of hetero duplexes and homoduplexes5. Here the sensitivity of assays by CSGE was improved by limiting the maximal size of the PCR products to 450 bp and making several changes in the conditions for PAGE. With the improved conditions, CSGE detect identified single-base changes in a large series of PCR products that contain multiple exons with highly repetitive and GC-rich sequences and provide a rapid detection of single-base differences in double-stranded PCR products and DNA fragments6. This analytical method has been used in medical genetics and to clarify the evolution of organisms including viruses, bacteria and human genetic disorders7. Furthermore we have noted a disconcerting tendency for physicians to rely on Usefulness of lactate dehydrogenase and it’s Iso enzymes as serum indicators of chronic lung damage or inflammation. Lactate dehydrogenase (LDH) is a cytoplasmatic enzyme present in essentially all major organ systems. Increased serum/BAL lactate dehydrogenase (LDH) activity is suggesting induced disturbances of cellular integrity that support the presumptive diagnosis of pathological conditions of Pneumocystis pneumonia (PJP) and other inflammations8-9. It may also be increased in other variety of extra pulmonary and granulomatous fungal infections10. We aimed to determine the frequency of infection; the salient clinical, laboratory; and the probable single-base mutations of isolated Pneumocystis in patients who have this pulmonary co-infection, then find a significant relationship between co-infection morbidity and LDH activity. The general attempt of the present study was to apply hetero duplex analysis for not only routine identification of Pneumocystis jirovecii mutant and drug resistant isolate, but also to modify treatment line and sensitive detection of genetic diversity within species.
Samples and patients
We study on 5 isolated strain of opportunistic fungi Pneumocystis jirovecii DNA sequences obtain from Tuberculosis co-infected patients in a former retrospective investigation.
Identification of P. jirovecii and Mycobacterium tuberculosis
Direct microscopic examination of bronchoalveolar lavage (BAL) revealed AFB and culture of the same yielded Mycobacterium tuberculosis. Also, acid fast bacillus was shown by Zeil-Neelsen staining technique and Bronchoalveolar lavage specimens were prepared using cytocentrifugation. Duplicate smears of respiratory specimens submitted for P. jirovecii examination and examined for the presence of P. jirovecii cysts with the Giemsa staining technique.
DNA extraction and PCR amplification of Pneumocystis jirovecii dihydropteroate synthase gene
PCR products were synthesized by amplification of DHPS gene according to the recent parallel research11-12. Genomic DNA from P. jirovecii was extracted using kit manufactured by Qiagen (QIAamp DNA MiniKit; Qiagen, Germany) according to the manufacturer’s instructions. DHPS loci of the P. jirovecii genome were amplified by HUM: 5′-GCGCCTACACATA TTATGGCCATTTTAAATC-3′ and DHPS-4: 5′-GGAACTTTCAACTTGG CAACCAC-3′ for first PCR and a second specific primer pairs: Cprim 5’CCCCCACTTATACA-3’and Dprim: 5′-GGGGGTGTTCATTCA-3′ for an internal amplification 269 bp fragment.
Scanning of the PCR products by CSGE technique
In order to hetero duplex analysis by CSGE, all PCR products(269 bp fragments) were electrophoresed in a 1-mm thick gel with 37-well comb (FMC) prepared with 10 or 15% polyacrylamide, 99:1 ratio of acrylamide (Intermountain Scientific, Kaysville, UT) to 1,4-bis (acryloyl) piperazine (Fluka), 10% ethylene glycol (Sigma), 15% formamide (GIBCO), 0.1% ammonium persulfate (U.S. Biochemicals), and 0.07% N,N,N9,N9-tetramethylethylenediamine (Sigma) in 0.53 TTE buffer. It was important not to autoclave the TTE buffer to obtain optimal separation of heteroduplexes and homoduplexes. Before electrophoresis, EDTA was added to each PCR product in a final concentration of 10 mM. Twenty microliters of each sample was heated to 98°C for 5 min followed by incubation at 65°C for 1 hour to generate hetero duplexes. The optimal polymerization time was about 1 hour. Four microliters of PCR products containing heteroduplexes were mixed with 3 ml of stock loading buffer (stock solution of 30% glycerol, 0.25% bromphenol blue, 0.25% xylene cyanol). Samples were separated by electrophoresis on a standard DNA sequencing gel apparatus with 37.5 × 45cm glass plates using 0.53 TTE as the electrode buffer.
Typically, a comb for 37 lanes was used, and up to five PCR products of different sizes were mixed and loaded in each lane. The gel was pre-electrophoresed for 15 minutes, and the samples were separated at room temperature using power as a limiting factor during the run with 40 W and 6 hours for 10% gels, or 40 W and 8.5 hours for 15% gels. After electrophoresis, the gel was stained on the glass plate in 1 mg/ml of ethidium bromide for 10 min followed by destaining in water5. The relevant section of the gel was cut, transferred to a piece of blotting paper, and then released from the paper onto the surface of a transilluminator by wetting with water. The gel was photographed with either a Polaroid camera or high-quality charge-coupled-device camera for gel documentation (Fotodyne, New Berlin, WI).
Lactate dehydrogenase enzyme assay
In addition, another diagnostic factor was measured as Serum/BAL LDH levels at the international unit scale (IU) with use of LDH diagnostic kit (PISHTAZTEB), colorimetric assay (BT 3000 system) . In this method lactate is used as a substrate and NAD as coenzyme. so is the “optimised standard method” according to the recommendations of the German Society for Clinical Chemistry (DGKC). For measurement procedure collected 200 BAL using standard sampling tubes heparinised 125 ¼l and adding 1000 ¼l of R1 reagent ( tris buffer pH 7.5 50 mmol/l , pyruvate 0.6 mmol/l , preservative), mix, incubate for 1 minute at 37°C temperature, then add: 250 ¼l of R2 reagent (NADH 0.18 mmol/l , preservative) mix, incubate for 1 minute and read initial absorbance start stopwatch simultaneously, test reaction show in below:
Pyruvate + NADH + H+ -LDH– Lactate + NAD+
Lactate dehydrogenase catalysis the conversion of pyruvate to lactate; NADH is oxidized to NAD in the process. The rate of decrease in NADH is directly proportional to the LDH activity and is determined photometrically by UV-assay, wavelength: 340nm, temperature: +37°C, Cuvette: 1cm, light path and Multi calibrator XL ref: PT-cal, according to a standardized method. Standard reference values for both men and women were 120-240 lU/L 9-10.
Identification of heteroduplex bands of dihydropteroate synthase gene by CSGE
To detect possible single-base mismatches in nucleoside site 165 and 171, the conformational electrophoresis analysis was performed on five PCR products that contained of previously identified as a P. jirovecii amplified DHPS gene (Fig.1). Our studied fragments (269bp) were less than 400bp so have optimum condition for correct migration. The homoduplexes in all negative control lanes and positive control sequence migrated, but at least 4 suspected isolates show more slowly migrating bands containing single heteroduplexes. Our findings propound the genetical mismatch and diversity in dihydropteroate sequence mutative sites in clinical isolate 1,2,4,5 and confirm diversity in RFLP results of former study in the event that submitted double mutation in codon 55 Thr/57 Pro.
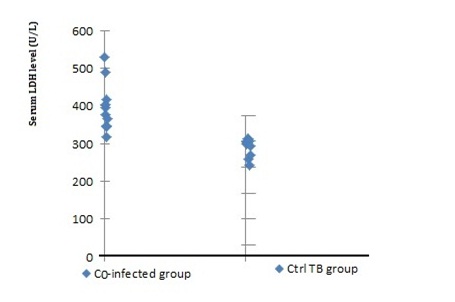 Fig. 1: PCR products of Pneumocystis jirovecii DHPS gene (269bp fragments), used for mutation detection by CSGE technique
Fig. 1: PCR products of Pneumocystis jirovecii DHPS gene (269bp fragments), used for mutation detection by CSGE techniqueDespite the negative report for the third isolation, no heteroduplex production and single-base Displacement were seen on PAGE even after recheck and doubling the incubation time in the EDTA buffer (Fig. 2).
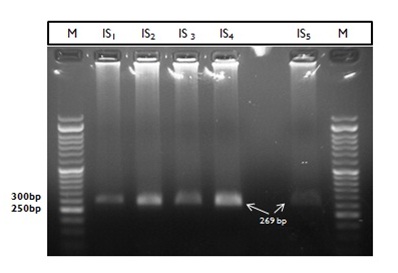
Fig. 2: Differential migration of DNA heteroduplexes and homoduplexes. Samples were PCR products (DHPS gene) of five Pneumocystis jirovecii strains obtained from TB positive patients. Templates contain different base in a single site (Nt 165, 171). Ctrl1: wild type Pneumocystis strain1, Ctrl2: wild type Pneumocystis strain2, Positive Ctrl3: submitted sulfa antibiotics resistance strain, *: Double test for Isolate 3 (after 2h incubation in 65°C in EDTA buffer)
Lactate dehydrogenase enzyme assay
The mean total LDH levels in co-infected patients (PCP in TB positive patients) was higher (445± 155 U/L) than enzyme level in patients with chronic TB (310±50 U/L). The difference was statistically significant using ANOVA analysis method (p<0.05). The ranges of LDH levels in the two groups are shown in table 1, in the control patients without PCP and TB LDH ranges was 77-240 U/L P < 0.05 (Fig. 3). The highest range of enzyme (601 IU/L) was reported in the serum and BAL samples of patient with second Pneumocystis clinical isolate.
Table (1):
Lactate dehydrogenase enzyme alteration between TB positive, negative and co-infected groups.
| Patient code | PJP | AFB* | LDH(IU/L) | |
|---|---|---|---|---|
| Co-Infected Group | 36B | + | + | 443 |
| 76B | + | + | 590 | |
| 30B | + | + | 601 | |
| 84A | + | + | 476 | |
| 126A | + | + | 418 | |
| TB positive Group | 10A | – | + | 295 |
| 17A | – | + | 299 | |
| 18B | – | + | 304 | |
| 53A | – | + | 296 | |
| 95A | – | + | 265 | |
| Negative control | 31A | – | – | 238 |
| 22B | – | – | 182 | |
| 15A | – | – | 215 | |
| 152A | – | – | 135 | |
| 88B | – | – | 159 |
AFB* Test; Acid Fast Bacillus Test followed by Ziehl-Neelsenstaining technique
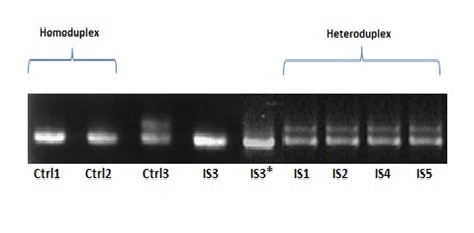 Fig. 3: Serum lactate dehydrogenase (LDH) in patients with Tuberculosis &Pneumocystis pneumonia co-infected cases in comparison to TB positive control group, LDH levels are shown for ten examples. There is obvious difference in enzyme value between two studied group that support aggravation of para clinical significance in Pneumocystis mix infection
Fig. 3: Serum lactate dehydrogenase (LDH) in patients with Tuberculosis &Pneumocystis pneumonia co-infected cases in comparison to TB positive control group, LDH levels are shown for ten examples. There is obvious difference in enzyme value between two studied group that support aggravation of para clinical significance in Pneumocystis mix infectionConformation sensitive gel electrophoresis (CSGE) is a rapid screening method for the detection of DNA sequence variation, specifically single-base changes or small insertions and deletions. It has been widely used for mutation screening in genetic disorders and for the detection of single nucleotide polymorphisms (SNPs)13. Detection of mutations in double-stranded DNA by gel electrophoresis is based on the supposition that a single-base mismatch can make conformational changes such as a bend in the double helix that causes differential migration of heteroduplexes and homoduplexes structures5. On electrophoresis in a non denaturing gel, heteroduplexes have retarded mobility compared to homoduplexes. The technique was first described for insertion/deletion mutations, but can also be applied to single-base replacement 14. It was developed on the basis of the further assumption that mildly denaturing solvents in an appropriate buffer can accentuate the conformational changes produced by single base mismatches and thereby increase the differential migration of heteroduplexes and homoduplexes15. Under the initially described our conditions, 4 single-base mismatches were detected by CSGE in a series of PCR products in size 269bp and one sequence variations (No. 3) that were not detected by CSGE was found. According to previous studies, CSGE direct use in diversity identification of fungi is rare and the technique commonly has been used to detect mutations in human hereditary diseases16-17. In a similar study about fungal diversity a modified PAGE system (HPA) be used that enables accurate identification of the species of Aspergillus section Flavi and subdivision based on highly sensitive discrimination of sequence variability7. They applied nucleotide sequence alignments, by using commercial panels to resulting heteroduplexes and found hydrolink mutation detection enhancement (MDE) gel (FMC Bio Products) in according the manufacturer’s instructions. while our resulting system was customary and manual. Conformation-sensitive gel electrophoresis (CSGE) is a variant of the HA method, employing mildly denaturing gel conditions. It is ideal for fragments differential in size range of 200–800bp, sensitivity of 88% has been detected recent developments in CSGE include the application of fluorescent labeling and detection and capillary electrophoresis14. In addition to the sensitivity, the advantage of CSGE over other molecular used techniques for scanning PCR products is no need to special equipment or preparation of PCR samples. Eventually The procedure is simple, requires little standardization, does not use radioactivity and the standard polyacrylamide gel electrophoresis is used in a solvent buffer system.
Also we consider a hypothesis, significance of serum dehydrogenase indicator and mix morbidity of M. tuberculosis and Pneumocystis pneumonia. Total LDH level of >300 U/L should not be considered diagnostic; it should instead suggest that a diagnosis other than PJP or bacterial pneumonia be considered, even the patients with PJP seldom had LDH levels of >1,000 U/L 10. In addition, we did not assess the source of the excess LDH in our patients because we did not measure LDH isoenzyme levels. Therefore, we propose to evaluate LDH isoenzymes pattern (special LDH3) for next researches. The relevance of serial LDH measurements for the management of Pneumocystis clonization or for assessing the risk of relapse is an interesting question and deserves further studies 18. As other investigations have confirmed this feature manifestations, elevated serum/BAL lactate dehydrogenase levels except low specificity have been noted in patients with PJP as high diagnostic value in novel clinical perspectives of Pneumocystosis.
ACKNOWLEDGMENTS
This study was financially supported by Research Deputy of Tarbiat Modares University. The authors wish to thank Firouz Nouroozi for helpful technical assistance.
- Kovacs JA, Masur H. Evolving health effects of Pneumocystis: one hundred years of progress in diagnosis and treatment. JAMA. 2009; 301(24): 2578-2585.
- DP KI, Valachis A, Velegraki M, Antoniou M, Christofaki M, Vrentzos GE, et al. Predisposing factors, clinical characteristics and outcome of Pneumonocystis jirovecii pneumonia in HIV-negative patients. J Infect Chemother. 2014; 20(7): 412-416.
- To KK, Hung IF, Xu T, Poon RW, Ip WC, Li PT, Li CP, Lau SK, Yam WC, Chan KH, Yuen KY. Clinical significance of Pneumocystis jirovecii in patients with active tuberculosis. Diag Microbiol Infect. Dis. 2013; 75(3):260–265.
- Sheikholeslami MF, Sadraei J, Farnia P. Dihydropteroate synthase gene mutation rates in Pneumocystis jirovecii strains obtained from Iranian HIV-positive and non-HIV-positive patients. Med Mycol., 2015; 53(4): 361–368.
- Korkko J, Annunen S, Philahamaa T. Conformation sensitive gel electrophoresis for simple and accurate detection of mutations: Comparison with denaturing gradient gel electrophoresis and nucleotide sequencing. Proc. Natl. Acad. Sci. USA .1998; 95(4): 1681–1685.
- Ganguly, A., Rock, M. J. Prockop, D. J. Conformation sensitive gel electrophoresis for rapid detection of single base differences in double stranded PCR products DNA fragments Proc. Natl.Acad. Sci. USA 1993; 90(21): 10325–10329.
- Kumeda Y, Asao T. Heteroduplex Panel Analysis, a Novel Method for Genetic Identification of Aspergillus Section Flavi Strains. Appl Environ Microbiol., 2001; 67(9): 4084–4090.
- Quist J, Hill AR, Serum Lactate Dehydrogenase (LDH) in Pneumocystis carinii Pneumonia, Tuberculosis, and Bacterial Pneumonia. Chest., 1995; 108(2):415-418.
- Butt A, Michaels S, Kissinger P. The association of serum lactate dehydrogenase level with selected opportunistic infections & HIV progression. Int J Infect Dis. 2002; 6(3):178-181.
- Corcoran GR, Abdely HA, Flanders CD. Markedly Elevated serum lactate dehydrogenase levels are a clue to the diagnosis of disseminated histoplasmosis in patients with AIDS. Clin Infec Dis. 1997; 24(5):942-944.
- Montes-Cano MA, de la Horra C, Mart1´n-Juan J. Pneumocystis jirovecii Genotypes in the Spanish Population. Clin Infect Dis. 2004; 39(1):123–128
- Tyagi K A, Mirdha R B, Luthra K. Dihydropteroate synthase (DHPS) gene mutation study in HIV-Infected Indian patients with Pneumocystis jirovecii pneumonia J Infect Dev Ctries. 2010; 4(11):761-766.
- Hill M. Conformation sensitive gel electrophoresis. Medical Biomethods Handbook, vol 3. Humana Press Inc., Totowa, NJ, 2005, P: 147-153.
- Elles R. Methods in Molecular Medicine, vol. 92: Molecular Diagnosis of Genetic Diseases, Second Edition. Edited by: R. Elles and R. Mountford. Humana Press Inc., Totowa, NJ. 2004, P: 9-44.
- Ganguly A. Prockop, D. J. Detection of mismatched bases in double stranded DNA by gel electrophoresis Electrophoresis. 1995: 16(10): 1830–1835.
- Jayandharan G, Shaji RV, Baidya S, Nair SC, Chandy M, Srivastava A. Identification of factor VIII gene mutations in 101 patients with haemophilia A: mutation analysis by inversion screening and multiplex PCR and CSGE and molecular modelling of 10 novel missense substitutions. Haemophilia, 2005; 11(5): 481-491.
- Balogh K, Patócs A, Majnik J, Rácz K, Hunyady L. Genetic screening methods for the detection of mutations responsible for multiple endocrine neoplasia type 1. Mol Genet Metab.2004; 83(1-2): 74-81.
- Matsumura Y, Shindo Y, Iinuma Y, Yamamoto M. Clinical characteristics of Pneumocystis pneumonia in non-HIV patients and prognostic factors including microbiological genotypes. BMC Infect Dis., 2011; 11:76.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


