ISSN: 0973-7510
E-ISSN: 2581-690X
The outbreak of Monkeypox was declared a public health emergency of worldwide concern by WHO following the (COVID-19) pandemic. The number of reported cases of both suspicion and confirmation has increased in recent years, from over 19,000 between 2000 and 2019 to over 15,600 between 2021 and 2022, and day by day the cases of monkeypox have been reported in 12 member states to three WHO regions. As of May 21, 2022, 92 confirmed cases of monkeypox and 28 suspected cases had been reported to the WHO from more than 12 countries. By the 21st of June 2022, a total of 2677 confirmed cases had been recorded from the UK and other European and non-European countries. According to an extensive literature survey, the total number of registered cases of MPXV was 59,147 between January 2022 to September 14th, 2022, demonstrating that MPXV can spread significantly amongst people and may as a result pose a serious threat to public health with international repercussions. In clade II MPXV virus is currently occurring outside of Africa the WHO reported 25,047 confirmed cases as of August 2nd, 2022. Here, we review current better understanding, and studies on monkeypox, including its history, current scenario, epidemiology, causative agent, symptoms, diagnosis, treatment, limitations, and the new face of monkeypox, its unusual outbreak attributed to the transformation of transmission and also discussed case studies is discussed in this article.
Current Scenario, WHO, Epidemiology, Transmission, Outbreak, New Face
The outbreak of monkeypox was declared a public health emergency of worldwide concern by the World Health Organization following the pandemic (PHEIC).1 According to the World Health Organization (WHO), PHEIC is a rare event that could pose a public health danger to other countries and require a concerted international response.2 Cases of monkeypox have been reported in non-endemic countries as recently as the beginning of May 2022, while the disease has also been found in several endemic nations. The virus responsible for this illness is the extremely rare infectious monkeypox virus (MPXV), which can infect both humans and non-human primates. It often mimics the symptoms of the flu, including a high temperature, swollen lymph nodes, and skin rashes. Blister clusters packed with fluid spread rapidly across the entire body, including the face, and then burst and crust over in a matter of days. As of September 14th, 2022, 103 member states from six regions had registered a total of 59,147 confirmed MPXV cases and 22 fatalities since January 2022. The World Health Organization (WHO) has declared MPXV to be a worldwide health emergency (WH0 2022).3
What is Monkeypox?
The World Health Organization (WHO) declared the monkeypox10 epidemic a public health emergency of worldwide concern following the (COVID-19) pandemic (PHEIC).4 According to the World Health Organization (WHO), PHEIC is an extremely rare event that could require a concerted international response due to its potential for worldwide transmission and public health danger to other states. Beginning in May 2022, cases of monkeypox were reported in nations where it was previously unknown,5 and the disease was also found in other previously unaffected nations. The disease is brought about by the extremely rare infectious monkeypox virus (MPXV), which can infect both humans and other primates. This illness sometimes mimics the flu,6 complete with fever, enlarged lymph nodes, and skin rashes. The face is usually not immune to the rapid appearance of fluid-filled blister clusters that,7 after a few days, burst and crust over. Since January 2022, 103 member states from six regions have documented a total of 59,147 confirmed MPXV cases, including 22 fatalities, as of September 14th, 2022.8 The World Health Organization has declared the pandemic spread of MPXV a public health emergency of international concern.9
Origin and History
The MPX virus was first identified in 1958 at the State Serum Institute of Copenhagen, and it was found in monkeys.10, 11 The first case of monkeypox in humans was reported in 1970 in the Democratic Republic of the Congo in a 9-month-old boy who had been admitted to the Basankusu hospital for probable smallpox. The virus has two distinct lineages, with the western African strains (SL-V70, COP-58, and WRAIR-61) often causing a less severe illness than the central African strains (1-5% vs. 10% case fatality).12 In addition to the previously reported cases of MPXV in Liberia, Sierra Leone, and Nigeria, a total of six human cases were discovered, including one sick adult and five healthy children. In another instance, a 4-year-old girl from the Ihie- Imuduru community in Abia State, South-East Nigeria, developed generalized vesiculopustular skin lesions along with a feverish illness on April 4, 1971. Nine days later, her 24-year-old mother also developed a fever and skin rash. Laboratory testing revealed that the child was the first known case of monkeypox in Nigeria and that her mother’s illness was the first ever suspected instance of monkeypox transmission between people. Both the mother and the infant lacked smallpox vaccinations, when a third case was found in the Omifounfoun community in the Oyo state of southwestern Nigeria. The patient, an unvaccinated 35-year-old traditional herbalist, became unwell while visiting Omifounfoun. Later, after employing viral isolation to identify monkeypox, he went back to his native Benin Republic. Like other members of his household, he occasionally consumes bush meat, but the infection’s source is unknown13.
Current monkeypox
Reports of many outbreaks of human monkeypox, primarily in the DRC and Nigeria, have been widely disseminated in the region of Equatorial Africa. According to a systematic review of the literature, the number of reported cases of both suspicion and confirmation has increased in recent years, from over 19,000 between 2000 and 2019 to over 15,600 between 2021 and 2022. The initial incidence of monkeypox in the current outbreak was linked to travel to Nigeria, the UK Health Security Agency reported on May 7. Two further patients were discovered in the UK on May 14, both of whom had a common residence but had no known connection to the May 7 case or recent connections to Africa. Cases of monkeypox have been reported in a continuing fashion by 12 member states to three WHO regions. As of May 21, 2022, 92 confirmed cases of monkeypox and 28 suspected cases had been reported to the World Health Organization from the United Kingdom, the United States, Canada, France, Germany, Belgium, Spain, Portugal, Italy, Sweden, and Australia. [30] By the 21st of June 2022, a total of 2677 confirmed cases had been recorded from the UK and other European and non-European countries. Prairie dogs contracted the disease from Ghanaian rats, which spread the disease throughout the country. People who don’t travel internationally and males who have sex with other guys are the key populations where this global pandemic can be spread from one person to another.14-20 The continued human-to-human transmission of monkeypox among non-travelers has been caused for concern, especially among males who have extensive intercourse with other males. One case of syphilis coinfection in the Czech Republic has been linked to MSM who are HIV positive. As of September 21st, 2022, the current outbreak had confirmed 64,290 cases of monkeypox in 106 countries. These include 579 confirmed cases of monkeypox in seven countries with an endemic population.21-24
Epidemiology of Monkeypox Virus
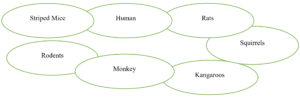
In Germany on 22 June 2022, medical labs confirmed the cases of Monkeypox Virus which were reported to the local public health and electronically submitted also in the state health department to the Robert Koch Institute according to Germany. The cases were between 20 to 67 years of age in men the median age was 38. Of 38 cases that were hospitalized 455 cases, other cases were reported that they had a mode of transmission and symptoms like monkeypox transmission by sexual or other intimate contact with the men.33 Which were notified the 14 of the 16 German federal states with the highest incidence in Berlin. The first case was determined in 1958 in an outbreak of the vesicular disease in captive “monkeys”. The largest carriers for monkeypox were not monkeys but they were Rodents and squirrels also kangaroos.25,26
Rodents were the most of largest group of Mammals with a range of 1500 species. It was detected in different species of animals, squirrels, rats, and striped mice and monkey. Monkeys look like mammals so, monkeys were considered the host of that virus. But at that time this virus transmission carrier from animals to human beings is still unclear.27-29
Animals were used to illustrate aerosol transmission, which was known as a nosocomial outbreak in the Central African Republic. The monkey virus was regarded as a contagious illness or virus that can be transmitted from one person to another through contact with sores, bleeding, mouth droplets, respiratory droplets, and body fluid. In August 1970, in the equatorial town of Bukenda, Zaire, the first case of monkeypox sickness was discovered in a 9-year-old child, who had the same vesicular skin disease as smallpox. Humans were quarantined during this time for a minimum of 5 days and a maximum of 21 days. Considering that 60 years ago, it was not much more attention-grabbing and contagious. The number of human cases of monkeypox has recently increased.30,31 The number of human monkeypox cases has steadily increased over the last 20 years, and in the first 45 years following the discovery of the monkeypox virus, there has been an accumulation of instances. The disease has been endemic throughout Central and West Africa since its discovery. The virus was introduced to humans by wild animals. Following the smallpox vaccine in the 1980s, the population’s immunity was low as a result of the rise in cases in Central and West Africa.33,34 The number of cases has increased in non-endemic regions like Singapore, Singapore, Israel, and the UK. These non-endemic regions had a history of importing rodents or people when they traveled from non-endemic regions to endemic regions.35
In May 2022, most of the cases were from the Non-endemic areas, and no cases were detected from the endemic areas also they were not connected to any epidemiological relation of monkeypox. WHO declared that various countries mainly reported men having sex with men.36 Recent research includes epidemiology, causes of the virus, and the transmission mode of a virus. The first case of monkey Virus in Taiwan was a man who was studied in Germany and developed symptoms on June 20 after 4 days he arrived in Taiwan. Symptoms were fever, Sore throat, muscle pain, lymph node swelling, and Rashes on the skin. On 24 June he was positive for Monkeypox. It was confirmed as a monkeypox case.
Causative Agents
Monkeypox is caused by animals to humans and humans to humans by the intimate contact of respiratory droplets, bleeding, lesions, and body fluid anyone can be infected by this virus aside from age, sex, race, and gender or sexual orientation, as mentioned in Figure 1.
Signs and Symptoms
The monkeypox isolation period is 7-14 days and up to 21 days. Symptoms are similar to smallpox but milder.37 It starts from the fever which is the initial stage of fever, rashes start within 1-4 days and upto facial rashes then spread all over the body. Monkeypox virus has some signs and symptoms that are:
- Fever
- Rashes
- Swelling in lymph nodes (Lumphadenopathy),
- Desquamation
- Oropharyngeal
- Ulcer in ocular mucosa because of rashes,
- Dehydration
- Malnutrition
- Cough
- Headache
- Lethargy
Secondary symptoms of bacterial infections like:
- Skin lesion
- Exfoliation of large skin areas required surgical grafting.
- Pneumonia,
- Sepsis
- Encephalitis
On the basis of Age factor some of the sign and symptoms are mentioned in Table 1.
Table (1):
According to Age Signs and Symptoms37.
| Age | Sign and Symptoms |
|---|---|
| In Children 1-16 years old | Fever |
| Cough | |
| Headache | |
| Rashes on skin | |
| Itching | |
| Sore Throat | |
| Burning sensation in the body. | |
| adults 18-30-year-old | Fever |
| Rashes on genital areas | |
| Burning sensation in eyes when contact with light | |
| Muscles pain | |
| Sore throat | |
| Exhaustion | |
| Stress, Anxiety is mostly in females. | |
| Sexual incompatibility. |
Diagnosis
Monkeypox virus first confirmed the positive test of PCR by the cotton swab or scabs of skin mucosa. Serum and CSF are either confirmatory by some diagnostic techniques such as38-39:
- Nucleic acid amplification testing
- Antibody detection
- Electron Microscopy
- Virus Isolation
Nucleic acid testing: It is the primary method to detect the monkeypox virus which detects the unique sequence of monkepox viral DNA. According to the WHO the monkeypox virus test is unavailable then if the polymerase chain reaction result for orthopoxvirus in non endemic virus countries.
⇓
Antibody detection: In this method plasma and serum is not used alone. The acute and convalescent sera used for the detection of antibody that is Immunoglobulin M and Immunoglobulin G used for Monkeypox Virus. That will show anti genic cross linking activity between the monkeypox and orthopoxvirus
⇓
Virus Isolation: It is the standard method of isolation of monkeypox virus for the detection of monkeypox. The virus is carried out P2 level of biosafety laboratories for isolation.
⇓
Flow chart of Diagnosis Techniques
Transmission of Monkeypox Virus
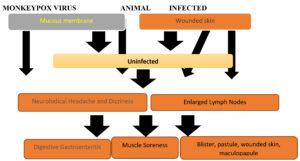
Monkeypox disease is a zoonotic disease that is spread by humans to humans and animals to humans and the disease increasing day by day efficiently. Respiratory droplets in close proximity to lesions and crust materials can spread the disease. This has infection in the skin, eye, nose, throat, and other sensory.40 It is spread by the face to face contact and in a pregnant woman to the fetus through its placenta or by the infected parents from their contact with skin after childbirth. This disease is infected by contact with infected animals that are bitten or scratched skin and also from eating infected animal meat that is not properly cooked41.
Recent research observed that it is also spread from human to human through sexual contact, especially men-to-men sexual contact. In this stage, firstly you have got fever after those rashes on the skin occur the virus enters the human body which firstly damages your skin and expansion of lymph and blood by the leukocytes. The virus will be replicated and multiplied to the lymph node as demonstrated in Figure 2. It will reach the epithelial tissues and cells through systemic circulation. Viral replication and invasion of the epithelial cell cause the superficial inflammation of the dermis layer, leading to vasodilation, hyperemia, and inflammation of cell infiltration, which result in rashes on the cutaneous layer. 42
There was a time when the virus was actively replicating its DNA and growing in population. The virus will be recognized by the host’s immune system, which will then launch an innate and adaptive immunological response. Initiate innate immune response and activate inflammation signaling pathways; strongly secrete pro-inflammatory cytokines, chemokines, and complement protein (in the complement system); these are the functions of pattern recognition receptors (PRRs) and sense pathogen-associated molecular pattern (SPAMP). When the secretion of the cytokine and chemokine’s attracted more immune cells it shows the symptoms like rashes and skin lesions on the infected site.43 Assortment Immune-modulator which works in various ways avoids host antiviral response which activates the PPRs. Monkeypox inhibits the T- cells receptor preventing the inflammatory cytokines in humans. Antibodies start to circulate, leading to clinical recovery and viral eradication.
Treatment of Monkeypox Virus Prevention
- Firstly, it may be prevented by making a distance from the infectious person who is infected by the Monkeypox virus.
- Men-to-men sexual contact should be cautious or avoided for the time being if someone has an infection.
- Should isolate the person for 1 week and not have contact with another healthy person.
- Wearing a mask for prevention of the outer environment.
- Properly use disinfectants for sanitization.
- Don’t share personal accessories and items with a healthy person.
- Isolate the infected person for a complete quarantine.
- Health specialists suggest to the caretakers of that person who is infected by the Monkeypox contact precautions by the eye.
- The person should be aware of the sexual contact with other persons and multiple sexual partners who were infected in the past 2 weeks.
- Laboratories workers should also take precautions because they are testing the patients who are infected by the MPXV.
- ACAM2000 vaccine should take for the treatment of MPXV.
- In Immunosuppression, Cardiac disease, and Pregnancy ACAM2000 can’t be used in these several conditions.
- Antiviral drugs also take for the treatment of MPXV.
- Must avoid Skin to skin contact and use gloves for contact with an infected person.
Table (2):
Treatment given in the Monkeypox38.
Name of drug+ Dosage |
M.O.A |
Administration |
Side effects |
Drug Interaction |
|---|---|---|---|---|
ANTIVIRAL DRUGS:
1. Cidofovir: 5mg\kg per week |
It inhibits the DNA polymerase |
I.V |
Fever, Nephrotoxicity, iritis, hypotony of the eye, neutropenia |
Nephrotoxic agents |
2. Tecovirimate:
200 mg for 2 weeks, 400 mg twice a day for 2 weeks, 600 mg twice a day for 2 weeks in adults. |
It inhibits envelop formation to the targeted action on the P37 viral protein |
I.V, Per Oral |
Nausea, Vomiting, Abdominal pain, and headache, from I.V infusion reaction, may be possible |
Midazolam decreases the Tecovirimate action.
Repaglinide cause hypoglycemia |
3. Brincidofovir:
For Pediatric 200 mg for 14 days, 400 mg twice a day for 2 weeks. In Adults, 200 mg is given once each week in 2 doses. |
It also inhibits or acts on DNA polymerase |
Per Oral |
Abdominal Pain, fever, Nausea, and Diarrhea |
Brincidofovir exposure is enhanced by lB3 and OATPlBl inhibitors, which may promote Brincidofovir-associated reactions. |
VACCINES:
1. MVA-BN Total of 2 doses (0.5 ml per dose) |
It induces the human response based on the life |
I.H |
Nausea, vomiting |
——————– |
2. ACAM 2000
2.5µl dose by the percutaneous route. |
It also induces the immune response |
TDDS |
—————– |
——————- |
BLOOD PRODUCTS:
1. Vaccinia immunoglobulin |
It is derived from the healthy adult blood product that is vaccinated to the vaccinia neutralization and it inhibits viral infection and provides passive immunity. |
I.V |
———- |
—————– |
Table 2 mentioned summary of all the drugs along with their mechanisms of action, mode of administration, side effects and drug interaction in monkeypox treatment
Limitations
The majority of instances are handled by self-limitation, with only minimal support required from management. It required isolation for severe oropharyngeal pain, and sore throat, and serves as the therapy of administration for severe disease (Altindis M et. al). Treatment of monkeypox included antiviral drugs which inhibit the DNA formation process by the step of polymerase all of these are considered investigational for the indication. Cidofovir, brincidofovir, blood products, and vaccines are used for the doses limits for the treatment of Monkeypox or Orthopoxvirus.44-46
New Face of Monkeypox Virus The 2022 MPXV Outbreak
The less virulent clade II MPXV virus is currently experiencing an unexpected outbreak outside of Africa. Independent MPXV infections with local transmissions have been documented starting in May 2022. 47-49 By August 2nd, 2022, the WHO (World Health Organization) had reported 25,047 confirmed cases outside of Africa. The median age of these was 36 years, and 99.9% of them were males. 98% of those who revealed their sexual preference were guys who had sex with men (MSM). The WHO designated the MPXV pandemic as a public health emergency of global concern in July 2022 (PHEIC).
Genomic Changes in the 2022 MPXV Genomes
Two separate genetic clades make up the MPXV virus: one originates in Central Africa or the Congo Basin and is referred to as clade I, while the other originates in West Africa and is referred to as clade II. The CB clade and the WA clade are both known as clade I. The case fatality rate for haplogroup I is 10.6 percentage points greater than the rate for haplotype II, which is 3.6 percentage points.50,51 A comparison of the genomes of the two clades reveals that the MPXV virus of the less hazardous clade II contains a deletion of 10 kilobase pairs.
There is only one MPXV lineage that is known to exist, and all of the present MPXV isolates belong to that lineage. This indicates that all of the MPXV strains originated from the same virus. During the years 2017 and 2018, viruses with very similar characteristics were identified in Nigeria, the United Kingdom, Singapore, and Israel. As a result of the 30–50% proofreading exonuclease activity of the viral DNA polymerase, orthopoxviruses have mutation rates that are orders of magnitude lower than those of RNA viruses. There is a highly conserved region in the middle of orthopoxvirus genomes that is home to genes that are necessary for virus replication. Nevertheless, hypervariability is present in the two terminal sections, and both deletions and sequence rearrangements are possible in these regions. These highly changeable regions include the vast majority of the genes that are responsible for the pathogenicity and host specificity of the organism. Viruses with a double-stranded DNA genome can adapt to environmental constraints such as switching hosts through a process called recombination, which involves the duplication and deletion of genes. However, as of right now, there is no indication from the MPXV genome sequences that the virus has greatly reduced the size of its genome. This is the case although the virus has been studied extensively.52-54
The ratio of A to T in poxvirus genomes is extraordinarily high, which is a strong indicator that mutations have not occurred at random. These mutant signals can be amplified by enzymes with the scientific name APOBEC3, which stands for apolipoprotein B mRNA-editing catalytic polypeptide-like. In infected cells, increased amounts of APOBEC3-G, -F, and -H may be observed in the cytoplasm, which is the location where the replication of the poxvirus takes place. Even though APOBEC3G does not have an immediate effect on the replication of VACV, there is a possibility that it may increase the pace of hypermutation and the likelihood of creating viral variants with a variety of characteristics. MPXV may have adapted to people based on the findings of a phylogenomic analysis of 2022 MPXV genomes. These alterations were shown to be microevolutionary.55-57 Since 2017, the 2022 MPXV has been in circulation, even though it has a mutational bias consisting of approximately 50 SNPs, which may indicate the presence of APOBEC3 activity. The number of SNPs that have been observed is greater than what should be anticipated based on the known substitution rates of orthopoxviruses that have occurred in the past. Although it is known that numerous point mutations have been found in the genome of the 2022 MPXV strain, it is not yet known how these changes affect the virus’s ability to spread from one person to another.58
New challenges of modernity of the Monkeypox virus
Experiments of a complex nature addressing organizational, legal, therapeutic, and pharmacological issues were carried out in different parts of the world. These studies, which employed more traditional approaches to studying, addressed the problem of the monkeypox virus. This study focuses on the areas of overlap that exist between patient management, forensic pharmacy, clinical pharmacy, healthcare administration, and pharmacy administration.59 Evidence-based medicine and pharmacy standards were adhered to throughout the process. The severity of the monkeypox pandemic, together with its wide geographic presence, poses major risks to the general population’s health and safety. The longer the monkeypox virus is around, the higher the danger of it spreading to new places, and the more quickly it will spread within those areas if it. Researchers have discovered that the monkeypox virus may infect a wide variety of different animal species. Animals that fall within this category include, but are not limited to, dormice, tree squirrels, rope squirrels, tree squirrels, Gambian pouched rats, and other nonhuman primates. It is necessary to conduct additional research into the history of the variola virus as well as its methods of spread.60 Your risk of getting monkeypox from someone who already has the disease increases in direct proportion to the number and length of times you come into contact with the infected individual. People who don’t spend much time together in close quarters have a lower risk of becoming infected with a contagious disease. The death rate among young people, particularly those who are under the age of 35, is higher than the death rate among older adults. Those who have immune systems that aren’t functioning properly are more likely to become unwell.61
It is generally accepted that transmission poses very little to no risk in today’s culture. The major means through which an infection is passed from person to person is through airborne droplets. The vast majority of infectious diseases are passed from person to person through the exchange of droplets. Scratching an infected region, touching an infected wound, breathing in contaminated droplets, or coming into contact with infected materials like bedding are all ways in which the monkeypox virus can be transmitted from one person to another.62
Comparisons with the COVID-19 pandemic
The stigma against specific social groups and the necessity for scaled-up (devolved) testing, financial support for those who live alone, and prompt guidance in the face of ongoing clinical and epidemiological concerns are probably already familiar challenges at this point. Even though this outbreak is widespread, it was initially discovered in the UK. The earliest instances, which had no connection to the others, were connected by happenstance by clever doctors.63 There are several causes for optimism regarding this outbreak, such as the recent training received by UK hospitals on how to manage cases. Both pre- and post-exposure protection can be achieved with the help of treatment and vaccines, both of which are already accessible and in use. The smallpox vaccine offers protection against MPX to about 85% of individuals. The concept that the virus may have been widely transmitted in people instead of animals for a period has emerged due to the virus’ rapid sequencing, and this was made feasible by the sharing of sequence data from several nations. Additionally, because some subgroups are more susceptible to illness, efforts to promote good health can be directed more specifically. 64 Finding contacts, particularly sexual partners, can be difficult in this outbreak, and it’s unclear how to identify cases that aren’t GBMSM. Due to the high degree of similarity among the appearances of acute rashes, clinical diagnosis has grown more challenging. This is notably true for herpes simplex viruses (HSV-1 and 2), varicella zoster virus (chickenpox), enterovirus (hand, foot, and mouth), secondary syphilis, lymphogranuloma venereum (LGV), and granuloma inguinale.
Neurological manifestations of MPXV
Preliminary findings also indicated a broad variety of neurological side effects, from headaches, myalgia, and fatigue to more serious issues including seizures and encephalitis. Though the signs and symptoms varied greatly, headaches, myalgia, weariness, photophobia, discomfort, encephalitis, and seizures were less frequent.64 The patient’s state of consciousness was lower, his pupils were larger, his optic disc was dilated, his corneal reflexes had vanished, and his deep tendon reflexes were weaker during the neurological examination. The right posterior parietal cortex, brainstem, and thalamus all displayed hyperintensity on magnetic resonance imaging (MRI), which is consistent with mixed cytotoxic and vasogenic brain edema. Pleocytosis was also discovered when the cerebrospinal fluid was examined (CSF).65
Atypical lymphocytes associated with monkeypox virus infection
Some of our patients (6/14) had atypical lymphocytes in their blood films, which multiplex PCR tests for in cases of active monkeypox infection (herpes simplex virus type 1/2, varicella-zoster virus, monkeypox). Hematological analysis was done on 11 of the 14 cases, all of which were Male and had a median age of 31 years and a range of 20–45 Six of the eleven cases were identified by the Sysmex XN-3100 analyzer, prompting a blood film analysis. The blood film of every sample that was flagged contained abnormal cells (the median was 14% of all lymphocytes, but the range was 7–36). There was little evidence of lymphocytosis, except for one patient, whose absolute lymphocyte count was 4.59109/l (median 2.71109/l, range 1.28- 3.12109/l). Immune activation causes mature cells to alter in form, which indicates an abnormal lymphocyte. The cytoplasm of the larger, more basophilic cells ranges from dark blue to light grey. In comparison to mature lymphocytes, the chromatin is finer and less developed.66 They can also be found in other infectious diseases that, depending on the stage of the disease, may cause an eruptive presentation, such as infectious mononucleosis (caused by the Epstein-Barr virus), viral hepatitis, toxoplasmosis, and malaria (chicken pox, herpes simplex virus, human immunodeficiency virus, syphilis…). We believe that the presence of atypical lymphocytes in a blood film may indicate a monkeypox virus infection in a patient with an eruptive disease based on recent knowledge. Atypical lymphocytes do not necessarily indicate the presence of a specific infectious disease, so serological and/or molecular tests should be conducted to confirm the diagnosis.
The main issue is that the most crucial element in halting the spread of disease in a population hasn’t yet been identified: the primary source of infection. Additionally, it is unclear how widespread local transmission is. This implies that there could be more cases or that some people could be affected yet not show any symptoms. Herd immunity wouldn’t be very strong because the infection is uncommon in the UK.67
Future perspective
Due to its propensity for rapid worldwide spread, monkeypox is increasingly becoming a hazard to the health of people all over the world. The most efficient approaches to warding against and curing this potentially fatal sickness are still in the research and development stage. According to the World Health Organization, although MPXV is often a minor virus that clears up on its own within a month, the virus offers a bigger risk to children than it does to adults because it has a fatality rate ranging from 3-10%.
There is still a dearth of knowledge regarding the accurate prevalence of MPX because of case ascertainment bias. This bias can be caused by a lack of diagnostic testing, clinical misdiagnosis, and poor surveillance. Even though transmission chains have been relatively short in previous outbreaks, the current outbreak demonstrates that a decrease in population immunity can lead to protracted epidemics with a basic reproduction rate (R0) > 1. This is the case even when the basic reproduction rate is greater than one. The transmission of infections from domesticated animals, particularly rats kept as pets, to wild species is essential to the spread of the MPX across the United Kingdom. It is recommended by the UKHSA human animal infections and risk monitoring (HARIS) group that unwell houses test these rats for contamination after keeping them outside for up to 21 days. This is to ensure that they are not infected with any pathogens.
MPX should always be on the list of illnesses doctors check for when a patient enters a genitourinary medicine clinic both during this outbreak and in the future. Multiplex PCR assays, which can be carried out swiftly in the clinic or a lab, should also include specialized primers for pox viruses. With a prompt diagnosis from the clinic, patients could be isolated, infections could be stopped, and contacts could be identified. As a preventative measure for those who are at risk, such as HIV patients receiving HIV pre-exposure prophylaxis (PreP), vulnerable healthcare workers in the industry, and even diagnostic laboratory staff who handle infectious material, people are still considering getting immunized with smallpox or even an MPX-specific vaccine. Immunization may become more prevalent as more people become aware of how harmful MPX is.
In conclusion, MPXV has the potential to enter the brain parenchyma and spread to other regions of the brain. Two potential mechanisms for this are hematogenous penetration through infected monocytes or macrophages and the olfactory epithelium. MPX sufferers may experience neurological symptoms and brain damage as a result of MPXV’s ability to enter the nervous system. Understanding how the virus causes neuroinvasion and even neurotropism can be accomplished by concentrating on the long-term effects of MPXV on the CNS by employing in vitro, in vivo, and post-mortem investigations. Finally, neurologists and other medical professionals should be aware that MPXV may result in neurological issues.
In the end, the outcomes of comparable queries posed by COVID-19 in March 2020 will determine the course of this outbreak. These include the extent of the epidemic internationally, the rate at which it is spreading, the extent (if any) of the involvement of animals, and the preventative measures that will be required.68
Case Studies69
The Hispanic MSM patient, age 26, had a history sex with 10 male partners between the year and 90 days before to his visit. The use of medicines (ecstasy and ketamine) within the previous 30 days was noted. When having sex with anonymous partners, anal and oral receptive and insertive intercourse were used; condoms were never used. Chlamydia, gonorrhea, and syphilis infections in the past. His external sexual organ had developed greater swelling from oedema, and his penis had increased in size, number of lesions. He also suffered from further bodily lesions and a two-night fever that was followed by fatigue and a loss of appetite. Chlamydia was discovered in the patient’s urine despite the fact that neither gonorrhoea not chlamydia had been detected in the patient’s throat or rectal region, RPR was non-reactive, and HSV had not been cultured (and negative for gonorrhea). He discovered that one of his sex partners is a Torontonian who was ill and had been diagnosed with monkeypox. The patient takes oral doxycycline 100 mg BID (twice daily) for 7 days to treat urogenital chlamydia. The patient was told to get in touch with the clinic a day or two later to go over the reassessment of their symptoms and the result.
Sex Partner
The partner, a White MSM 23-year-old from Toronto, stated that a new rash had arisen when he went to the doctor for a checkup. The previous medical background was same as patient. They were reunited the day before the index patient’s first symptoms. The sex partner admitted having sex with three men in the previous ninety days and ten times in the year before that. He having sex with fictional, anonymous people. He never wore a condom and only ever had receptive and insertive oral and receptive anal sex. Chlamydia and gonorrhea history from the preceding year. Presented with one nodular hard papule (0.5 cm) in the right axilla, five papular, Moderately erythematous lesions over the chest. One little papule on the skin of the left lower buttock.
The sex partner was tested for monkeypox on the right axilla, chest, and left buttock, all of which exhibited lesions. As a contact to Chlamydia, the sex partner began receiving doxycycline 100 mg twice daily for seven days. The orthopoxvirus was identified at all three sites of the patient’s lesions. Orthopoxvirus tests were negative at all three areas where the partner had lesions.
The patient orthopoxvirus tests were positive. The patient called the clinic to give an update, stating that the number and size of the lesions had grown in addition to the fact that he was unable to urinate due to pain and swelling. The clinic personnel contacted the emergency department to arrange for the patient’s evaluation. The patient received oxycodone and phenazopyridine treatment. The agony was under control, and he could void on his own. According to the patient’s report, before being released, he received painkillers and an additional week of doxycycline for “cellulitis.” Nine days following his final interaction with the now-confirmed case of monkeypox, the patient’s partner visited the clinic for JYNNEOS (vaccine) post-exposure prophylaxis. There was nothing left to re-swab for the orthopoxvirus because the partner’s lesions had mostly recovered. The spouse was given the diagnosis of folliculitis due to the careful sample collection, the negative Orthopoxvirus results, and the quick remission.
We described two clinical cases of monkeypox with a quick resolution of symptoms using the Oxycodone and phenazopyridine and an additional doxycycline. The quick rise of MPX cases in non-endemic areas raises concerns about the potential threat this zoonotic virus poses to public health worldwide. According to a systematic review of the literature, the number of reported cases of both suspicion and confirmation has increased in recent years, from over 19,000 between 2000 and 2019 to over 15,600 between 2021 and 2022. Over 59,147 new non-endemic MPX cases were recorded globally between January to14 September 2022 and WHO has declared the pandemic spread of MPXV a public health emergency of international concern. Men who have sex with men (MSM) who have recently had contact with new or numerous sexual partners have been identified as a new high-risk group in the ongoing non-endemic outbreak. This indicates that MPXV is spread via close physical touch. However, it’s crucial to note that both previous and current MPXV epidemics demonstrate that this virus may infect and spread between people of any sex, age, and orientation. The idea that the clinical effects of the current outbreak are less severe than those seen in endemic regions is supported by the absence of documented MPX mortality in non-endemic regions. A sudden outbreak of the less dangerous clade II MPXV virus is currently occurring outside of Africa. Starting in May 2022, independent MPXV infections with local transmissions have been recorded. Outside of Africa, the WHO reported 25,047 confirmed cases as of August 2nd, 2022. Monkeypox virus is diagnosed by Nucleic acid amplification testing, Antibody detection, Electron Microscopy, and Virus isolation. There are many reasons to be optimistic about this outbreak, including the recent training that UK hospitals received on how to handle infections. Treatment and vaccinations, both of which are already available and in use, can assist achieve both pre- and post-exposure protection. About 85% of people are protected against MPX by the smallpox vaccine. Multiplex PCR tests for abnormal cells in blood films of several of our patients (6/14) in cases of active monkeypox infection. 11 of the 14 cases, all of which were male and had a median age of 31 years and a range of 20–45, underwent hematological examination and Six of the eleven cases were identified by the Sysmex XN-3100 analyzer.
Although monkeypox epidemics have been documented in non-endemic countries before, the current outbreak has spread widely and its rate of spread is not backed up by any scientific evidence. Concern has been raised due to the sustained human-to-human transmission among non-travelers, especially among males who have sex with men, and there have been cases described without a history of travel to areas where the condition is endemic. There has been a steady rise in reported cases; however, the pandemic may not be as widespread as the 2009 COVID-19 outbreak. This is because the monkeypox virus spreads through direct contact with bodily fluids like coughing saliva.
ACKNOWLEDGMENTS
The authors would like to thank Management of Uttaranchal University, Dehradun, India for providing facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Dhawan M, Priyanka, Choudhary OP. Emergence of monkeypox: risk assessment and containment measures. Trav Med Infect Dis. 2022;49:102392.
Crossref - WHO. 2022, Monkeypox Outbreak: Global Trends; World Health Organization: Geneva, Switzerland. 2022. https://worldhealthorg.shinyapps.io/mpx_global/ Accessed September 14, 2022.
- WHO Monkeypox Declared a Global Health Emergency by the World Health Organization. https://news. un.org/en/story/2022/07/1123152. Accessed July 23, 2022.
- Moss B. Poxviridae: the viruses and their replication. Williams FVL, ed. Lippincott Williams and Wilkins. Published online 2007:2905-2946.
- Damon IK. Fields’ Virology. (Knipe DM, Howley PM, eds.). Lippincott Williams and Wilkins; 2007.
- Realegeno S, Puschnik AS, Kumar A, et al. Monkeypox virus host factor screen using haploid cells identifies essential role of GARP complex in extracellular virus formation. J Virol. 2017;91(11):e00011-17.
Crossref - Magnus P von, Andersen EK, Petersen KB, Birch-Andersen A. A pox-like disease in Cynomolgus monkeys. Acta Pathol Microbiol Scand. 2009;46(2):156-176.
Crossref - Guarner J, Del Rio C, Malani PN. Monkeypox in 2022- what clinicians need to know. JAMA. 2022;28(2):139- 140.
Crossref - Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22(8):1153-1162.
Crossref - Leon-Figueroa DA, Bonilla-Aldana DK, Pachar M, et al. The neverending global emergence of viral zoonoses after COVID-19? The rising concern of monkeypox in Europe, North America and beyond. Travel Med Infect Dis. 2022;49:102362.
Crossref - Haider N, Guitian J, Simons D, et al. Increased outbreaks of monkeypox highlight gaps in actual disease burden in Sub-Saharan Africa and in animal reservoirs. Int J Infect Dis Off Publ Int Soc Infect Dis. 2022;122:107-111.
Crossref - Al-Tawfiq JA, Barry M, Memish ZA. International outbreaks of Monkeypox virus infection with no established travel: A public health concern with significant knowledge gap. Travel Med Infect Dis. 2022;49:102364.
Crossref - Bizova B, Vesely D, Trojanek M, Rob F. Coinfection of syphilis and monkeypox in HIV positive man in Prague, Czech Republic. Travel Med Infect Dis. 2022;49:102368.
Crossref - Centers for Disease Control and Prevention (CDC). 2022 Monkeypox Outbreak Global Map. Availableonline: https://www. cdc. gov/poxvirus/monkeypox/ response/2022/worldmap.html. Accessed September
- Chen N, Li G, Liszewski MK, et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340(1):46-63.
Crossref - AM Likos, SA Sammons, VA Olson, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86(Pt 10):2661-2672.
Crossref - Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Lourie B, Bingham PG, Evans HH, Foster SO, Nakano JH, Hermann KL. Human infection with monkeypox virus: laboratory investigation of six cases in West Africa Bull. World Health Organ. 1972;46:633-639.
- Marennikova SS, Seluhina EM, Mal’ceva NN, Cimiskian KL, Macevic GR. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in manBull. World Health Organ. 1972;46:599-611.
- Ogoina D, MBBS, FWACP, FMCP, FACP, FIDSA. A brief history of monkeypox in Nigeria. Idsociety.org. Accessed December 21, 2022. https://www.idsociety.org/science-speaks-blog/2022/a-brief-history-of-monkeypox-in-nigeria/#/+/0/publishedDate_na_dt/desc/
- Heymann DL, Szczeniowski M, Esteves K. Re- emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54(3):693-702.
Crossref - Hutin YJF, Williams J, Malfait P, et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996-1997. Emerg Infect Dis. 201;7(3):434-438.
Crossref - Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19(8):872-879.
Crossref - Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13(10):e0007791.
Crossref - Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2):e0010141.
Crossref - World Health Organization. Regional office for Africa. Weekly Bulletin on Outbreak and other Emergencies: Met Week, 4. 2021:18-24.
- World Health Organization.Regional office for Africa. Weekly Bulletin on Outbreak and other Emergencies: Met Week, 4. 2022:17-23.
- UK Health Security Agency. Monkeypox cases confirmed in England-latest updates. 2022. https:// www.gov.uk/government/news/monkeypox-cases- confirmed-in-england-latest-updates.
- WHO. Multi-country monkeypox outbreak in non- endemic countries. 2022. https://www.who.int/ emergencies/disease-outbreak-news/item/2022- DON385.
- Update: Multistate outbreak of Monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. JAMA. 2003;290(4):454.
Crossref - Centers for Disease Control and Prevention. 2022 Monkeypox and orthopoxvirus outbreak global map. https://www.cdc.gov/poxvirus/monkeypox/ response/2022/world-map.html. Accessed 18 June 2022
- Luo Q, Han J. Preparedness for a monkeypox outbreak. Infectious Medicine. 2022;1(2):124-134.
- Orviz E, Negredo A, Ayerdi O. Is there a need to be worried about the new monkeypox virus outbreak? Abrief review on the monkeypox outbreak. Ann Med Surg. 2022;85(4).
- del Rio C, Preeti N. Malani. Update on theMonkeypox Outbreak. JAMA. 2022;328(10):921-922.
Crossref - Selb R, Werber D, Falkenhorst G, et al. A shift from travel- associated cases to autochthonous transmisiion with Berlin as epicenter of the monkeypox outbreak in Germany, May to June 2022. Euro Surveill. 2022;27(27):2200499.
- Farahat RA, Abdelaal A, Shah J, et al. Monkeypox outbreaks during COVID 19pandemic: are we looking at an independent phenomenon or an overlapping pandemic? Ann Clin Microbiol Antimicrob. 2022;21(1):26.
Crossref - Zhu M, Ji J, Shi D, et al. Unusual global outbreak of monkeypox: what should we do? Front Med. 2022;16(4):507-517.
Crossref - Pastula DM, Tyler KL. An Overview of Monkeypox Virus and Its Neuroinvasive Potential. Ann Neurol. 2022;92(4):527-531.
Crossref - Lai CC, Hsu CK, Yen MY, Lee PI, Ko WC, Hsueh PR. Monkeypox: An emerging global threat during the COVID-19 pandemic. J Microbiol Immunol Infect. 2022;55(5):787-794.
Crossref - Poland GA, Kennedy RB, Tosh PK. Prevention of monkeypox with vaccines: a rapid review. The Lancet. 2022;22(12):E358-E349.
Crossref - Idris I, Adesola RO. Current efforts and challenges facing responses toMonkeypox in United Kingdom. Biomed J. 2022;S2319-4170(22):00118-4.
Crossref - Altindis M, Puca E, Shapo L. Diagnosis of monkeypox virus – An overview. Travel Med Infect Dis. 2022;50:102459.
Crossref - Li H, Zhang H, Ding K, et al. The evolving epidemiology of monkeypox virus. Cytokine Growth Factor Rev. 2022;68:1-12.
Crossref - Huang YA, Howard-Jones AR, Durrani S, Wang Z, Williams PC. Monkeypox: A clinical update for paediatricians. J Paediatr Child Health. 2022;58(9):14401754.
- Tiwari A, Adhikari S, Kaya D, et al. Monkeypox outbreak: Wastewater and environmental surveillance perspective. Sci Total Environ. 2022;856(Pt 2):159166.
Crossref - Schnierle BS. Monkeypox Goes North: Ongoing Worldwide Monkeypox Infections in Humans. Viruses. 2022;14(9):1874.
Crossref - Rahimi FS, Afaghi S, Tarki FE, et al. The Historical Epidemiology of Human Monkeypox: A Review of Evidence from the 1970 Emergence to the 2022 Outbreak. Tohoku J Exp Med. 2022;258(4):243-255.
Crossref - Nityanand Jain, Edouard Lansiaux ,Raimonds Simanis.The new face of monkeypox virus: an emerging global emergency. New Microbe and New Infect. 2022; 47: 100989.
Crossref - Isidro J, Borges V, Pinto M, et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022;28(8):1569-1572.
Crossref - Gammon DB, Gowrishankar B, Duraffour S, Andrei, G, Upton C, Evans DH. Vaccinia Virus-Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis. PLoS Pathog. 2010;6(7):e1000984.
Crossref - Roth JR, Andersson DI. Poxvirus use a “gene accordion” to tune out host defenses. Cell. 2012;150(4):671-672.
Crossref - Alakunle EF, Okeke MI. Monkeypox virus: A neglected zoonotic pathogen spreads globally. Nat Rev Genet. 2022;20(9):507-508.
Crossref - Kremer M, Suezer Y, Martinez-Fernandez Y, Munk C, Sutter G, Schnierle BS. Vaccinia virus replication is not affected by APOBEC3 family members. Virol J. 2006;3:86.
Crossref - Jones TC, Schneider J, Muehlemann B, et al. Genetic variability, including gene du-plication and deletion, in early sequences from the 2022 European monkeypox outbreak. bioRxiv. 2022.
Crossref - Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of Monkeypox virus detected in the United States, 2021-2022. bioRxiv. 2022.
Crossref - Benvenuto D, Vita S, Pascarella S, et al. The evolution of Monkeypox virus: A genetic and structural analysis reveals mutations in proteins involved in host-pathogen interaction. bioRxiv. 2022.
Crossref - Abrahim M, Guterres A, Da Costa Neves PC, Ano Bom APD. The emergence of new lineages of the Monkeypox virus could affect the 2022 outbreak. bioRxiv. 2022.
Crossref - Desingu PA, Nagarajan K. Genomic regions insertion and deletion in Monkeypox virus causing multi- country out-break-2022. bioRxiv. 2022.
Crossref - Giorgi FM, Pozzobon D, Di Meglio A, Mercatelli D. Genomic analysis of the recent monkeypox outbreak. bioRxiv. 2022.
Crossref - Sejvar JJ, Chowdary Y, Schomogyi M, et al. Human monkeypox infection: a family cluster in the midwestern United States. J Infect Dis. 2004;190(10):1833-1840.
Crossref - Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(4):342-350.
Crossref - Anderson MG, Frenkel LD, Homann S, Gufey J. A case of severe monkeypox virus disease in an American child: emerging infections and changing professional values. Pediatr Infect Dis J. 2003;22(12):1093-1096.
Crossref - News. J Mater Eng Perform. 1997;6(2):129-140.
Crossref - Shapovalov VV (Jr.), Shapovalova VA, Shapovalov VV. Forensic and pharmaceutical research on the impact of drugs on the safety, life and health of road users within the organization of pharmaceutical business, drug technology, pharmaceutical and medical law in Ukraine. Health of Society. 2021;10(4):127-132.
Crossref - Shapovalova V, Zakharchenko I. Organizational and legal approaches to reforming of the law enforcement system of Ukraine: illegal circulation of psychoactive substances and addictive dependence. SSP Modern Law and Practice. 2021;1(1):1-22.
- Tukhar I, Shapovalova V, Shapovalov V, Shapovalov V. Pharmacological view on the problem of comorbidity in the pharmacotherapy of chronic pancreatitis. Science Review. 2017;3(38):77-83.
Crossref - Shapovalova V. An Innovative multidisciplinary study of the availability of coronavirus vaccines in the world. SSP Modern Pharmacy and Medicine. 2022;2(2):1- 17
Crossref - Login. Apmplmi.com. Accessed December 22, 2022. https://apmplmi.com/index.php/apmp/login
- CDCP. Monkeypox: Updates about Clinical Diagnosis and Treatment. 2022. https://emergency.cdc.gov/ coca/ppt/2022/062922_slides.pdf
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.