ISSN: 0973-7510
E-ISSN: 2581-690X
In vitro study was conducted to explore antibacterial properties of the larval gut extracts of Rhynchophorus ferrugineus (Red Palm Weevil) Oliver. Larval gut extracts were tested against salivary bacteria causing dental carries using the agar well diffusion method. The gut extracts significantly affected the growth of both Klebsiella spp. and Streptococcus viridans. The two bacterial species revealed significant differences in their sensitivity to the extract. The extract efficacy depended upon the concentration and time of exposure. When using 100%concentration of the extract, the mean of inhibition zones for S. viridans and Klebsiella spp. at 24 h after treatment were 1.61 mm and 2.50 mm, respectively. At 48 h post-treatment, the mean of inhibition zones for S. viridans and Klebsiella spp. were 1.96 mm and 2.66 mm. After 72 hours, the means zones were 2.28 mm and 2.91 mm, respectively. Electron microscopic examinations showed morphological changes of the outer membrane of bacteria with a noticeable damage as a result of exposure to the gut extract. The results suggest potential use of these extracts against dental caries bacteria.
Rhynchophorus ferrugineus, Red Palm Weevil, Antimicrobial activity, Streptococcus viridians, Klebsiella spp.
Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) commonly known as the red palm weevil, is a destructive insect pest to the palm trees worldwide. Previous studies demonstrated that extracts from the alimentary canal of the weevil larvae have shown potency against different species of Gram-positive and Gram-negative bacteria1. Based on those results, we decided to extend the study to evaluate the extracts’ activity against bacteria present in dental carries which are considered a major reservoir for infectious agents2. Dental caries are known to harbor Streptococcus mutans and their levels in carries are indicative of the degree of contamination. This species and other streptococci species are major pathological agents of human dental caries3. It has evolved to be able to develop multiple mechanisms to colonize the teeth surfaces and has become a significant species in cariogenic biofilm4.
Dentists have prescribed antibiotics to manage and treat dental caries. However, the misuse and overuse of these drugs led to the development of bacterial resistance to antibiotics which became an increasingly challenging issue and an alarming concern around the world and has led to problems associated with using antibiotics for the treatment of endocarditis4-6. Streptococcus mutans (839 isolates) have shown particular resistance to cefuroxime, penicillin, tetracycline, and mercury in 209 human subjects7. In the light of new pathogens and diseases emerging and the increasing threats of bacteria developing resistance against antibiotics, it has become substantially very important to discover and develop new forms of antibiotics and antimicrobial drugs8.
Insects are well known for being resistant to a wide range of pathogenic microorganisms, specially bacteria. They produce certain proteins and peptides that play a role in insect immunity against pathogens either by activating the innate immune system or by directly attacking the invading pathogens9,10. These molecules are known as Antimicrobial peptides (AMPs). Fat bodies and the hemocytes of insects produce their AMPs and transport them to the hemolymph11. The fat body peptides secreted into the hemolymph is an effective and important mechanism, which occurs in the wounded or infected insects. Antimicrobial peptides are also secreted by the gut epithelial cells as well as those of the salivary and venom glands10,12. Throughout history, extracts from different insect organs and their secretions were used as alternative medicine to cure various diseases. Colon cancer13 and drug resistance14 are still being treated by using aqueous, alcoholic or Serratia peptidase- treated extracted from larvae of Chrysomya rufifacies (blow fly maggot) (Macquart) and Musca domestica (house fly) (Linnaeus).
Insect AMPs are intensively studied as promising and potential alternatives for conventional drugs to treat infectious diseases, cancer and to overcome bacterial resistance to antibiotics15-17. This in vitro study was conducted to evaluate inhibitory effects of the products extracted from the red palm weevil larvae against specific bacteria responsible for dental caries.
Saliva Samples
Saliva samples were collected from the adult patients who volunteered in the outpatient dental clinic, Faculty of Oral and Dental Medicine at Cairo University, Egypt, where they were treated for dental caries and periodontal diseases. The saliva samples were collected in the microbiology laboratory in the department of microbiology in Faculty of Medicine at Cairo University. The volunteer patients were selected according to standard procedures. The patients, free of any systemic and local diseases, were aged between 20-40 years. Saliva swabs for culturing were placed in tubes containing transport media to keep the bacterial swabs vital until their arrival to the laboratory. The swabs containing saliva were divided into two groups; the first group was plated on Mitis Salivarius Agar, blood, MacConkey and mannitol salt types of agar. Plates in both the groups were aerobically incubated for 24 hours at 37°C. The second group was inoculated on selective medium and incubated for 36 hours at 35°C for the isolation of other bacterial species18.
Isolation and Characterization of Saliva Bacteria
Saliva samples were suspended in 0.05 M phosphate buffer and vortexed. This was followed by preparing 10-fold serial dilution of the mixture to be cultured on the desired agar. Aliquots of 100µl were cultured on agar plates containing the base of Mitis Salivarius, which was added with a solution of 1% Potassium tellurite. In order to isolate Streptococcus sp., the above aliquot was further added with 0.2 units/ml of bacitracin by raising sucrose concentration to 20% (MSB).The plates were then sealed and incubated in an anaerobic container along with a gas generating kit for 24 h at 37°C. The purification of the bacterial cultures was done by continuous streaking on particular agar media, ensuing phase-contrast microscopic examination. Colonies of Streptococcus sp. appeared as dark blue, small, raised, and adherent with an irregular margin19. Colonies of morphological types of Streptococcus sp. were sub-cultured on modified Mitis Salivarius Agar base as previously described. Streptococcus sp. was identified using pure isolates grown on a plate containing Biology Universal Growth (BUG) agar + 5% sheep blood, which was kept at 37°C with 6.5% CO2. Micro-plates containing wells were then pipetted with 150 μl of bacterial suspension, which were then left for 24 hours at 30°C.
Insect Sampling and Dissection
Ismailia Governorate situated about 100 km east of Cairo was selected as the collection site for the larvae of Red Palm Weevil; large sized larvae (7-10th larval instars) were collected from infested palms. In the laboratory, the larvae were topically sterilized with 70% ethanol, left to dry, immobilized on ice, and dissected under a stereoscopic microscope in cold distilled water using sterile tools. About 20 g of gut tissues (content excluded), were collected separately and homogenized using a manual glass grinder. The supernatant, collected after centrifuging the homogenates at 10,000×g for 15 minutes at 4°C, was stored in the sterilized Eppendorf tubes at -80°C for further use20-22.
Evaluation of Antibacterial Effects
The minimum inhibitory concentration (MIC) is considered a basic measure of the activity of any antimicrobial agent and its ability to inhibit the growth of the tested pathogen23. The antimicrobial activity was evaluated by measuring clear zones of growth inhibition using the agar well diffusion method24.
TEM Examination
The effect of the gut extracts on Streptococcus viridans was observed using Transmission Electron Microscopy (TEM). Cells of S. viridans were scraped off the culture, exposed to gut extract, and incubated at 37°C for 24 hours. Cells were fixed with 5% formaldehyde cacodylate buffer and 3% glutaraldehyde. The treated and untreated cells were washed several times before fixing them in 1% (w/v) osmium tetroxide in cacodylate buffer. The cells were examined with a Zeiss transmission electron microscope (EM 910) after counterstaining them with 1% lead citrate and 0.5% uranyl acetate.
Statistical Analysis
A randomized design was applied to analyze the data. Assitat software25 was used to compare treatments by using the Least Significant Difference Test (LSD)26. The effect of gut extracts and changes occurring on the inhibition zones were analyzed by using ANOVA test. The relationship between the time of exposure and growth inhibition was also determined.
Antimicrobial Activity
The bacterial species S. viridans and Klebsiella spp. were isolated from patients’ saliva samples. The treatment with Red Palm Weevil extracts showed antimicrobial activity and affected the growth of both types of the isolated bacteria. Table 1 shows the inhibitory effects of the extracts against both bacterial species. The Multivariable analysis (ANOVA) of the data showed significant differences in the effectiveness of these extracts against the studied bacteria. The inhibition zone area for both bacterial species i.e., S. viridans and Klebsiella spp., increased proportionately with exposure time up to 72 hours.
Table (1):
Antimicrobial activity of gut extracts from larvae of the red palm weevil R. ferrugineus against S. viridans and Klebsiella spp. according to the well diffusion method.
| Days | Conc. | Inhibition Zones (mm.) | Mean | |
|---|---|---|---|---|
| Streptococcus viridians | Klebsiella spp | |||
| 1 | 10% | 1.68 | 2.58 | 2.13 |
| 25% | 1.56 | 2.40 | 1.98 | |
| 50% | 1.54 | 2.38 | 1.96 | |
| 100% | 1.65 | 2.65 | 2.15 | |
| Mean | 1.61 | 2.50 | 2.05 c | |
| 2 | 10% | 2.00 | 2.69 | 2.34 |
| 25% | 1.90 | 2.59 | 2.24 | |
| 50% | 1.83 | 2.50 | 2.16 | |
| 100% | 2.11 | 2.88 | 2.49 | |
| Mean | 1.96 | 2.66 | 2.31 b | |
| 3 | 10% | 2.09 | 2.75 | 2.42 |
| 25% | 2.00 | 2.64 | 2.32 | |
| 50% | 1.93 | 2.59 | 2.26 | |
| 100% | 2.28 | 2.91 | 2.59 | |
| Mean | 2.07 | 2.72 | 2.40 a | |
| 10% | 1.92 | 2.67 | 2.30 b | |
| 25% | 1.82 | 2.54 | 2.18 c | |
| 50% | 1.76 | 2.49 | 2.13 d | |
| 100% | 2.01 | 2.81 | 2.41 a | |
| Mean | 1.88 b | 2.63a | ||
LSD value at 0.05:
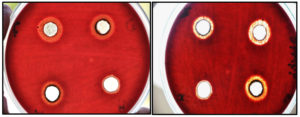
Results presented in Table 1 show that the bacterial growth inhibition caused by gut extract treatments was concentration and time dependent. At all concentrations of the tested extracts, S. viridans was significantly much less affected than Klebsiella spp. When using high concentration (100%), the mean of inhibition zones for S. viridans and Klebsiella spp. at 24 h after treatment were 1.61 mm and 2.50 mm, respectively. At 48 h post-treatment, the mean of inhibition zones for S. viridans and Klebsiella spp. were 1.96 mm and 2.66 mm. After 72 hours, the means zones were 2.28 mm and 2.91 mm, respectively. The statistical analysis showed significant differences between the effect of different concentrations of weevil gut extract on both S. viridans and Klebsiella spp. . These results are illustrated in Fig. 1.
Fig. 1. Zone of inhibition of S. viridans caused by gut extracts from red palm weevil larvae at 1 day after treatment.
There was a significant difference between the two bacterial species when treated with different concentrations of the gut extract. The highest concentration caused a significant growth inhibition zone compared with the lowest concentration. The statistical analysis showed significant differences in inhibition zones as a result of the duration of exposure.
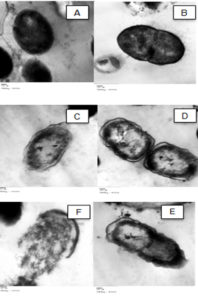
Fig. 2. TEM photo showing the effect of gut extracts from the larvae of red palm weevil on S. viridans 24 h after treatment. Untreated bacteria at (A) 168000x and (B) 41400x magnification; treated bacteria at (C) 36600x and (D) 36600x magnification.
The TEM examination showed that the untreated cells had normal cell shapes and undamaged structures (Fig. 2 A and B). However, morphological changes (Fig. 2 C and D), and malformation of the cell membrane of the bacterial cells and damages due to gut extract exposure were observed 24 h after treatment (Fig. 3). There was also separation between the cell membrane and the cytoplasm of S. viridans (Fig.s 3c-f) associated with cell hydrolyses (Fig. 3f).
A mixture of Streptococci and a number of species of the Viridans group can be found in the oral cavity, including S. mitis, S. mutans, S. milleri, S. sanguis, and S. salivarius27. S. viridans is considered an important micro-organism that can cause infections leading to dental caries and heart valve damage27. The ability of S. viridans to cause carious lesions is well documented. However, effective treatment with antibiotics is faced with the emergence of new resistant bacteria28. In the present study, the effect of the gut extract of the red palm weevil R. ferrugineus Olivier was tested against S. viridans (gram positive) and Klebsiella spp. (gram negative). The agar well diffusion method clearly demonstrated that the gut extract had antimicrobial activity in vitro and caused zone of growth inhibition for both bacterial species. The effectiveness of the extract depended on the concentrations applied and duration for which the bacteria were exposed.
The electron microscopy examination of treated S. viridans cells showed the presence of malformation of the cell membrane and leakage of the intracellular constituents due to cell hydrolysis. These results are supported by previous studies which indicated that antibiotics have antibacterial activity that caused membrane disruption for different bacterial strains29-31. The sensitivity of S. viridans to the gut extract can be attributed to the fact that Gram positive bacteria are only surrounded by a peptidoglycan cell membrane and lack the presence of the outer cell membrane which makes it vulnerable to the effect of antimicrobial peptides of the gut extract. This peptidoglycan layer is relatively porous and allows the AMP molecules to pass through and penetrate the cell1. On the other hand, gram negative bacteria have an additional membrane known as the outer membrane. This outer membrane works as a barrier that plays an important role as a protective mechanism against antimicrobial agents and antibiotic selection pressure thus promoting bacterial antibiotic resistance32. Interestingly in this study, the gram negative bacteria Klebsiella spp. were more sensitive and had significantly larger zones of inhibition compared to the gram positive bacteria S. viridans after being treated with the gut extract. This result is similar to the findings reported after treating both E. coli (gram negative bacteria) and S. aureus (gram positive bacteria) with the external secretions of the red palm weevil33. Similar results were also reported when studying the antimicrobial effects of T. molitor secretions on different microbes including gram positive and gram negative bacteria34. The effect of the gut extract on gram negative bacteria might be attributed to the presence of other factors or components in addition to the antimicrobial peptides that can interact together to enhance and strengthen their immunosuppressive efficacy. Further studies are needed to explore and isolate these components responsible for the inhibition effect.
In summary, developing countries face major challenges in developing and maintaining health care, for which they need novel, effective, and affordable procedures, treatments and medications to treat various infections. The gut extract from larvae of the red palm weevil R. ferrugineus have the potential to serve as a natural antibacterial agent to control and prevent the growth of different pathogenic micro-organisms including dental carries bacteria.
This study presents an effective treatment for dental carries bacteria using crude gut extract of R. ferrugenius. These extracts showed potential antimicrobial activity against S. viridans and Klebsiella spp., which may be further explored and evaluated as a potential alternative to conventional antibiotics.
ACKNOWLEDGMENTS
The authors acknowledge the financial support provided by King Abdulaziz University’s Deanship of Scientific Research (DSR). The authors are thankful to Prof. Steve Harakeh special infectious agents unit, King Fahd Medical research Center for manuscript revision.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
GS and HAA wrote the manuscript, collect data and statistical analysis. HMH and ZAM did the experimental work.
FUNDING
This study was supported by grants no.G:113-305-1439.from the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript
- Sewify GH, Hamada HM, Alhadrami HA. In Vitro Evaluation of Antimicrobial Activity of Alimentary Canal Extracts from the Red Palm Weevil, Rhynchophorus ferrugineus Olivier Larvae. BioMed Res Int. 2017;2017:8564601.
Crossref - Okada T, Takada K, Fujita K, et al. Differentiation of banding patterns between Streptococcus mutans and Streptococcus sobrinus isolates in rep-PCR using ERIC primer. J Oral Microbiol. 2011;3:7190.
Crossref - Loesche, W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986; 50: 353-80.
- Jubair HH. The relationship between biofilm forming and antibiotics resistance of Streptococcus mutans isolated from dental caries. Int J Curr Microbiol App Sci. 2015;4(5):568-574.
- William B, Rwenyonyi CM, Swedberg G, Kironde F. Cotrimoxazole Prophylaxis Specifically Selects for Cotrimoxazole Resistance in Streptococcus mutans and Streptococcus sobrinus with Varied Polymorphisms in the Target Genes folA and folP. Int J Microbiol. 2012;2012:916129.
Crossref - Tsuda H, Yamashita Y, Shibata Y, Nakano Y, Koga T. Genes Involved in Bacitracin Resistance in Streptococcus mutans. Antimicrob Agents Chemother. 2002;46(12):3756-3764.
Crossref - Leistevuo J, Jarvinen H, Osterblad M, Leistevuo T, Huovinen P, Tenovuo J. Resistance to Mercury and Antimicrobial Agents in Streptococcus mutans Isolates from Human Subjects in Relation to Exposure to Dental Amalgam Fillings. Antimicrob Agents Chemother. 2000;44(2):456-457.
Crossref - Manniello MD, Moretta A, Salvia R, et al. Insect antimicrobial peptides: potential weapons to counteract the antibiotic resistance. Cell Mol Life Sci. 2021.
Crossref - Wu Q, Patocka J, Kuca K. Insect Antimicrobial Peptides, a Mini Review. Toxins. 2018;10(11):461.
Crossref - Ganz T. The role of antimicrobial peptides in innate immunity. Integrative and Comparative Biology. 2003;43(2):300-304.
Crossref - Yi H-Y, Chowdhury M, Huang Y-D, Yu X-Q. Insect antimicrobial peptides and their applications. Appl Microbiol Biotechnol. 2014;98(13):5807-5822.
Crossref - Mylonakis E, Podsiadlowski L, Muhammed M, Vilcinskas A. Diversity, evolution and medical applications of insect antimicrobial peptides. Philos Trans R Soc B: Biol Sci. 2016;371(1695):20150290.
Crossref - Hou L, Shi Y, Zhai P, Le G. Antibacterial activity and in vitro antitumor activity of the extract of the larvae of the housefly (Musca domestica). J Ethnopharmacol. 2007;111(2):227-231.
Crossref - Park SO, Shin JH, Choi WK, Par BS, Ohi JS, Jung A. Anti- bacterial activity of housefly maggot extracts against MRSA (Methicillin- resistant Staphylococcus aureus) and VRE (Vancomycin- resistant enterococli). J Envron Biol. 2010;31(5):865- 871.
- Ratcliffe NA, Mello CB, Garcia ES, Butt TM, Azambuja P. Insect natural products and processes: New treatments for human disease. Insect Biochem Mol Biol. 2014;41(10):747-769.
Crossref - Roy S, Saha S, Pal P. Insect natural products as potential source for alternative medicines – A Review. World Scientific News. 2015;19;69-83.
- Brady D, Grapputo A, Romoli O, Sandrelli F. Insect cecropins, antimicrobial peptides with potential therapeutic applications. Int J Mol Sci. 2019;20(23):5862.
Crossref - Wong SH, Cullimore DR, Bruce DL. Selective medium for the isolation and enumeration of Klebsiella spp. Appl Environ Microbiol. 1985;49(4):1022-1024.
Crossref - Nikawa H, Makihira S, Fukushima H, et al. Lactobacillus reuteri in bovine milk fermented decreases the oral carriage of mutans streptococci. Int J Food Microbiol. 2004;95(2):219-223.
Crossref - Valzano M, Achille G, Burzacca F, et al. Deciphering microbiota associated to Rhynchophorus ferrugineus in Italian samples: a preliminary study. Journal of Entomological and Acarological Research. 2012;44(3):e16.
Crossref - Oren Z, Shai Y. Mode of action of linear amphipathic helical antimicrobial peptides. Biopolymers; 1998; 47:451–63.
- Cudic M, Otvos L Jr. Intracellular targets of antibacterial peptides. Curr Drug Targets. 2002;3(2):101-106.
Crossref - Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(Suppl 1):5-16.
Crossref - Turillazzi S, Perito B, Pazzagli L, Pantera B, Gorfer S, Tancred M. Antibacterial activity of larval saliva of the European paper wasp Polistes dominulus (Hymenoptera,Vespidae). Insect Soc. 2004;51:339-341.
Crossref - Silva FAS. The ASSISTAT software: statistical assistance, in Proceedings of the International Conference on Computers in Agriculture, pp. 249-298, American Society of Agriculture Engineers, 6 Cancun, Mexico, 1996.
- Snedecor GW, Cochran WG, Statistical Method. Published by The lowa State University Press, 1980.
- Y. Refoua, A Study of Streptococcus viridans in the maxillofacial region. Journal of Dentistry of Tehran Uni. of Med. Sci. 2005; 2(4):174-179.
- WHO. High levels of antibiotic resistance found worldwide, new data. Shows. http://www.who.int/mediacentre/news/releases. 2018.
- Yanping W, Bai J, Zhong K, et al. Antibacterial activity and membrane-disruptive mechanism of 3-p-trans-coumaroyl-2-hydroxyquinic Acid, a novel phenolic compound from pine needles of Cedrus deodara, against Staphylococcus aureus. Molecules. 2016;21(8):1084.
Crossref - Hartmann M, Berditsch M, Hawecker J, Ardakani MF, Gerthsen D, Ulrich AS. Damage of the bacterial cell envelope by antimicrobial peptides gramicidin S and PGLa as revealed by transmission and scanning electronmicroscopy. Antimicrob Agents Chemother. 2010;54(8):3132-3142.
Crossref - KarikalanS, Mohankumar A. Antibiogram of streptococcus mutans isolated from dental caries patients. International Journal of Medical and Health Research. 2016;2(3): 79-83, 2016.
- Blair JM, Webber MA, Baylay AJ, Ogbolu DO, Piddock LJV. Molecular mechanisms of antibiotic resistance. Nat Rev Microbiol. 2015;13(1):42-51.
Crossref - Pu YC, Xiang HJ, Liang XY, et al. External immune inhibitory efficiency of external secretions and their metabolic profiling in Red Palm Weevil, Rhynchophorus ferrugineus (Coleoptera: Curculionidae). Frontiers in Physiology. 2020;10(1624).
Crossref - Qiang CK, Yang ZF, Du YZ. Study on the antimicrobial activity of the defensive secretion of Tenebrio molitor L. Biotechnology. 2006a(16):22-24.
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.