ISSN: 0973-7510
E-ISSN: 2581-690X
Green synthesis nanoparticles were considered as an alternative effective resource instead of chemically engineered metal oxide nanoparticles. Using leaf extracts for green synthesis, essential for the reduction and oxidation process of the metals. Phyllanthus niruri (L.) and Aristolochia indica (L.) leaf extracts were used to synthesize yellowish brown coloured silver (Ag) and white coloured zinc oxide (ZnO) nanoparticles. Synthesized green nanoparticles characterized by different spectroscopic analysis (XRD, XPS, FTIR, PL) and TEM. Characterization results confirmed the particles morphology, size, structure and also their optical and photonic properties. Three different concentrations of Ag and ZnO NPs were analysed against three (gram positive) and five (gram negative) bacteria. Increased levels of green synthesized Ag and ZnO NPs showed increased zone of inhibition than amoxicillin (positive control). Our study proved that the green synthesized Ag and ZnO NPs showed similar unique physical and chemical properties with metal oxide nanoparticles but less toxic while their discharge into the ecosystem.
Green synthesis, silver, zinc, spectroscopy, TEM, antibacterial studies
Nanotechnology is a newly emerging branch of science with efficient techniques which gained lots of researcher attention as well as environmentalist due to their immense role in various fields1. In worldwide, the utilization of nanoparticles in various sectors has increased from 225,060 to 584,984t with 21.1% annual growth rate2. Based on their shape and structure, nanoparticles exhibited unique properties. Engineered chemical nanoparticles (NPs) were extensively utilized in cosmetics, medicine, food and biological sciences3 resulted in high incidence of discharge into the ecosystem either accidently or intentionally4. Deposition of engineered chemical NPs in the environment cause serious health ailments to the biota including humans5,6.
Silver (Ag) and zinc (Zn) nanoparticles belongs to the metal oxide nanoparticles category7. Ag nanoparticles considered as noble metal nanoparticles due to their good compatibility and target oriented interactions and generally used as cell compound delivery vehicle. Ag NPs also acts as an efficient antibacterial agent5. Rubber production, LCD and solar cells manufacturing and LCDs, as a whitener in pigment productions, electronics, chemical fibers and textiles8,9, antifouling paints10 whereas bulk ZnO were increasingly altered by ZnO NPs due to its potent antibacterial activities11.
Nowadays, green synthesis of nanoparticles has gained researchers attention due to their non toxic or less toxic properties which has the similar unique properties. Plant extract-mediated nanomaterials synthesis is one of the most stable and suitable alternatives than compared to physical, chemical and microbial method productions. Leaf extracts were used as an alternative source for raw materials by using less energy and toxic chemicals. Green synthesis provided a hygienic environment with more stable compounds and lesser waste products12.
The plant Phyllanthus niruri (L.) belongs to the family Phyllanthaceae, commonly seen in tropical countries and widely used in Ayurveda treatments to cure various chronic ailments in liver, kidney, spleen and stomach13. The plant Aristolochia indica (L.) belongs to the family Aristolochiaceae, their roots were used to prepare anti-venom drugs, enzyme inhibitors14.
Fourier Transform Infrared (FTIR) spectroscopy and X ray diffraction (XRD) characterization techniques were performed for the nanoparticles identification, size structure, composition elucidation through spectrum and elemental mapping15-17. Transmission Electron Microscopy (TEM) revealed nanoparticles morphology18.
Present work is framed to produce Ag and Zn nanoparticles through green method using Phyllanthus niruri and Aristolochia indica leaf extracts. The synthesized nanocomposites were characterized by XRD, TEM, X-ray photoelectron spectroscopic (XPS) studies and Photoluminescence spectroscopy. Different concentrations of green synthesized nanoparticles antibacterial activities were studied on various gram positive and gram negative bacteria.
Green synthesis of Ag and Zn nanoparticles
The plants Phyllanthus niruri (L.) and Aristolochia indica (L.) were collected from Jamal Mohamed College (Tiruchirappalli, Tamil Nadu, India). Plant leaves were cleaned with tap water and then with distilled water. Finely chopped leaves (10gm) mixed with 100ml deionized water and boiled (10mins) at 80°C and 50-60°C for P. niruri and A. indica leaves respectively. By using Whatman (No. 1) filter paper, extraction was filtered and the filtrate was collected, kept at room temperature.
10ml of Phyllanthus niruri leaf extracts mixed with 90mL of AgNO3 (0.01M) at room temperature. Ag NPs reduction was clearly observed within 10mins. Simultaneously, 100ml of Aristolochia indica leaf extracts mixed with Zn(NO3)2.6H2O (0.1M) salt and green reducing agents19 which forms white coloured homogenous solution and kept in stirrer for 4-6hrs at 80˚C and the precipitate was calcined at 800˚C for 5hrs20.
Nanoparticles characterization: FTIR, XRD, XPS, TEM & Photoluminescence studies
Green synthesized silver and zinc oxide nanoparticles characterized by X’PERT PRO PANalytical model – X-ray diffractometer and in the ranges of 30°-80° and 20°-80° for Ag and ZnO NPs samples with monochromatic wavelength of 1.54Å, the diffraction patterns were recorded21. The Carl Zeiss XPS instrument with ultra high vacuum and 250W Al Kα excitation used for XPS measurements. Nanoparticles morphology was analyzed by TEM (Carl Zeiss Ultra 55 FESEM)22. Nanoparticles FTIR spectra were recorded in the range of 400-4000cm-1 by using Perkin-Elmer spectrometer23. Photoluminescence spectra of the green synthesized nanoparticles were taken using spectrometer Perkin Elmer-LS 14 within the range between 200-1100nm.
Antibacterial assay
By well diffusion method24, the antibacterial activities of Ag and ZnO NPs were investigated. The following gram positive (Staphylococcus aureus, Streptococcus pneumoniae and Bacillus subtilis) and gram negative bacteria (Klebsiella pneumonia, Eischerichia coli, Shigella dysenteriae, Proteus vulgaris and Psuedomonas aeruginosa) were cultured on Mueller Hinton agar (MHA). The plates were inoculated with 0.1ml of log phase of respective bacterial culture. After inoculation, disc loaded with 40, 50 and 60μg/mL of Ag and ZnO NPs respectively placed on the bacterial inoculated plates, amoxicillin (10μg/mL) used as the positive control. After 24 hrs (37˚C) incubation, inhibition zone around the disc in plates were measured.
Various researchers reported the interactions of leaf phytocompounds with metallic salts or ions during ZnO NPs green synthesis25-28. During synthesis, plant antioxidants chelated the Zn ions which forms metal complex by thermoreduction process metal complex converted into ZnO NPs29. Rafique et al.19 reported green synthesis of ZnO NPs by using Syzygium cumini extracts. During biosynthesis, plant extracts act as stabilizing and capping agent and finally zinc acetate reduced into ZnO NPs.
X-ray diffraction patterns
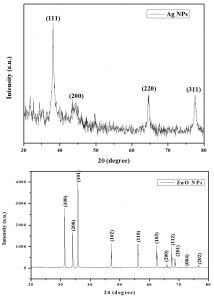
XRD patterns for green synthesized Ag and ZnO NPs were shown in Fig. 1. The standard diffraction peaks showed the center faced cubic phase of silver NPs (confirmed by JCPDS data card no: 89-3722) and hexagonal wurtzite structure for zinc NPs (JCPDS data card no. 36-1451). By using Scherer formula, the average Ag and ZnO NPs size were calculated as 35.36nm and 45nm respectively.
Fig. 1. XRD patterns of green synthesized Ag and ZnO NPs using Phyllanthus niruri and Aristolochia indica leaf extracts
Vidya et al.30 reported the leaf extracts of Calotropis gigantea used green synthesised ZnO NPs. XRD patterns showed spherical nano crystallites with average size as 30-35nm. Thema et al.31 reported the ZnO NPs green synthesis along with Agathosma betulina plant extracts XRD analysis showed quasi-spherical nanoparticles with average size 15.8nm. Selim et al.32 reported green synthesized ZnO NPs and confirmed the average size as 15.22nm by using TEM, FTIR, XRD and UV spectroscopy.
X ray photoelectron spectroscopy (XPS) analysis
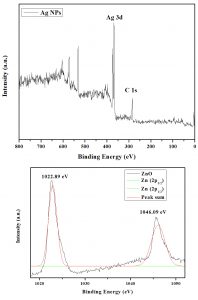
The XPS wide spectrum of Phyllanthus niruri leaf extracts mediated Ag NPs showed oxidation state of C1s and Ag 3d state. In Ag NPs, traces of leaf extract responsible for the C (1s) signals. In Gaussian fitting, XPS spectra of ZnO showed asymmetric O (1s) signals contains three symmetrical signals namely O1s1, O1s2 and O1s3. The Aristolochia indica leaf extracts synthesized ZnO NPs results showed three signals O1s1 at 531.49eV, O1s2 at 533.24eV and O1s3 at 534.65eV due to the O2– ion in Zn2+ ion (Fig. 2)
These results were evidenced by De la Rosa et al.33, Remashan et al.34 and Armelao et al. 35 reported adsorbed H2O and lattice oxygen on the ZnO surface, Zn-O-Zn surface and Zn-OH surface respectively.
FTIR spectroscopic studies
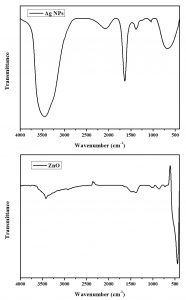
Functional groups in Ag and ZnO NPs were conferment by FTIR analysis15. FTIR spectra for Phyllanthus niruri leaf extracts synthesized Ag NPs (Fig. 3) showed the presence of phytochemical components like steroids phenols, glycosides, tannins, triterpenoids, flavonoids, saponines and glycerides. The Ag metal peak observed at 424cm-1 for silver NPs by Phyllanthus niruri leaf extracts and the C-H bending vibration observed at 839cm-1 for silver nanoparticles. FTIR spectra of green synthesized ZnO NPs by Aristolochia indica showed the O-H stretching band at 3428cm-1, asymmetric C-H stretching at 2904cm-1, C=O symmetric stretching at 1458cm-1, O-H asymmetric stretching at 1022cm-1, Zn-O stretching 440cm-1 for ZnO NPs (Fig. 3).
The medicinal plant extract are act as dual role like reducing and capping agent36. FTIR spectra of Anigozanthos manglesii green synthesized Ag NPs with broad absorption band at 3448cm-1 and hydroxyl (-OH) group present in the bio-macromolecules on leaf extract37. FTIR spectra of biosynthesis Ag NPs vibration frequency observed at 1381cm-1 and corresponding to –NO3 stretching whereas the peak at 692cm-1responsible for the reduction and capping during the synthesis of Ag NPs36,38. Zandi et al.39, Munoz-Hernández et al.40 and Xiong et al.41 reported results evidenced our ZnO NPs FTIR results.
Photoluminescence (PL) spectroscopic studies
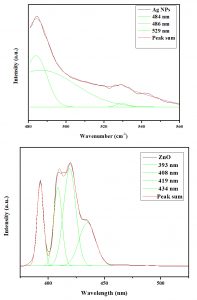
By using photoluminescence spectroscopy, synthesized nanoparticles optical and photonic properties were studied. Ag NPs excitation wavelength observed at 42nm. Three peaks observed as blue, blue-green and green emission spectrum at 484, 486 and 529nm respectively. Luminescence emission peaks observed at 440 and 600nm due to presence of antioxidant compounds in Phyllanthus niruri leaf extracts while the green emission peak observed at 529 nm due to organic matrix bound to silver nanoparticles.
Whereas photoluminescence spectra of Aristolochia indica leaf extracts green synthesized ZnO NPs excitation wavelength observed at 350nm. The emission spectra showed four peaks at 398, 408, 419 and 434nm. A peak observed at 398nm due to the radiative recombination of collision process during ZnO NPs green synthesis (Fig. 4). Fan et al.42 reported two violet emission bands at 408 and 419nm due to the electron transition between the Zn at interstitial with the high incidence ones. Mishra Sheo et al.43 also reported blue emissions peaks at 434nm, where strongly ascribed due to singly ionized Zn vacancies.
TEM analysis
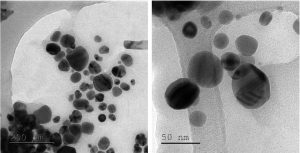
TEM images showed morphology of green synthesized Ag NPs by Phyllanthus niruri extract as spherical shape and their average size was 30nm (Fig. 5). TEM images of green synthesized ZnO NPs by Aristolochia indica extract showed spherical shaped particles with average size of 30-60nm (Fig. 6). Suresh et al.44 reported Phyllanthus niruri mediated AgNO2 NPs where the average particle size found as 30-60nm.
Antibacterial activities
Three different concentrations of green synthesized Ag and ZnO nanoparticles antibacterial activities were tested against three gram positive and five gram negative bacteria such as S. aureus, S. pneumoniae, B. subtilis and K. pneumoniae, E. coli, P. aeruginosa, P. vulgaris, S. dysenteriae respectively. Table 1 showed zone of inhibition results of green synthesized Ag and ZnO NPs with control amoxicillin in culture plates. Ag NPs showed high antibacterial activities to the all the bacterial types than compared to amoxicillin whereas increased concentrations of Ag NPs showed increased zone of inhibition. The inhibition of bacterial cells was due to the disruption of bacterial lipopolysaccharide layers with the green synthesized Ag and ZnO NPs. This interaction developed ROS in the bacterial cytoplasm leads to the disintegration of membrane potential and reduced cell growth. These results were evidenced by similar reports observed from Jung et al.45, Santhoshkumar et al.46 and Tong et al.47 Albizia procera leaf extract mediated Ag NPs showed high antibacterial activities against gram positive and gram negative bacteria48. Khalid et al.49 reported the significant antibacterial activity of Cu NPs against gram positive and gram negative bacteria.
Table (1):
Zone of inhibition (in mm) observed in various bacteria treated with green synthesized Ag and ZnO NPs.
| No. | Tested microbes | Amoxicillin* 10μl (mm) |
Green Synthesised Ag NPs |
Green Synthesised ZnO NPs |
||||
|---|---|---|---|---|---|---|---|---|
| 40μl (mm) | 50μl (mm) | 60μl (mm) | 40μl (mm) | 50μl (mm) | 60μl (mm) | |||
| 1. | S. aureus | 15 | 11.5 | 12.8 | 13.2 | 11.1 | 12.5 | 12.9 |
| 2. | S. pneumoniae | 16 | 10.3 | 11.5 | 12.7 | 9.9 | 11.1 | 12.4 |
| 3. | B. subtilis | 15 | 12.6 | 13.1 | 13.9 | 12.1 | 12.8 | 13.2 |
| 4. | K. pneumoniae | 16 | 13.9 | 14.3 | 14.9 | 13.7 | 14.0 | 14.4 |
| 5. | E. coli | 18 | 10.1 | 11.9 | 13.5 | 9.8 | 11.6 | 13.4 |
| 6. | P. aeruginosa | 16 | 10.5 | 11.8 | 13.1 | 10.2 | 11.4 | 12.8 |
| 7. | P. vulgaris | 16 | 12.6 | 13.8 | 14.6 | 12.2 | 13.3 | 14.2 |
| 8. | S. dysenteriae | 15 | 11.0 | 12.1 | 12.8 | 10.7 | 11.8 | 12.4 |
1-3: Gram positive bacteria; 4-8: Gram negative bacteria; *Positive control
Phyllanthus niruri (L.) and Aristolochia indica (L.) leaf extracts were used to synthesize Ag and ZnO nanoparticles. Green synthesized nanoparticles morphology, structure, size, optical and photonic properties were studied by various characterization analysis. Three different concentrations of Ag and ZnO NPs were tested against various gram positive and negative bacteria. Increased concentrations of green synthesized Ag and ZnO NPs showed increased zone of inhibition. Our study proved that the green synthesized Ag and ZnO NPs showed similar unique physical and chemical properties with metal oxide nanoparticles. Plant leaf extracts mediated Ag and ZnO NPs were non toxic to the non targeted species and became a non threat during their discharge into the aquatic ecosystem.
ACKNOWLEDGMENTS
The authors express our heartfelt thanks to Dr. AK Khaja Nazeemudeen Sahib, Secretary and Correspondent, Dr. S Ismail Mohideen, Principal, Jamal Mohamed College (Autonomous) and Dr. M. Ghouse Basha, Head, P.G. & Research Department of Botany, Jamal Mohamed College (Autonomous) for providing all the necessary facilities and encouragement. Authors are thankful to DST-FIST (Govt. of India) for providing funds for upgrading modern equipments which was utilized in this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
HSJ – Work designed, supervised and approved the work, TTK – Complied data and literature, MMC – worked and obtained data for Ag NPs, RA – worked and obtained data for Zn NPs.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Ju-Nam Y, Lead JR. Manufactured nanoparticles: an overview of their chemistry, interactions and potential environmental implications. Sci Total Environ. 2008;400(1-3):396-414.
Crossref - BCC Research, Global Markets for Nanocomposites, Nanoparticles, Nanoclays and Nanotubes. 2015. http://www.bccresearch.com/market-research/nanotechnology/nanocomposites-market-nan021f.html?vsmaid=203/.
- Nowack B, Bucheli TD. Occurrence, behavior and effects of nanoparticles in the environment. Environ Poll. 2007;150(1):5-22.
Crossref - Stanley JK, Coleman J, Charles GA, Weiss J, Steevens JA. Sediment toxicity and bioaccumulation of nano and micron-sized aluminum oxide. Environ ToxicolChem. 2010;29(2):422-429.
Crossref - Klaine SJ, Koelmans AA, Horne N, et al. Paradigms to assess the environmental impact of manufactured nanomaterials. Environ ToxicolChem. 2012;31(1):3-14.
Crossref - Muralisankar T, SaravanaBhavan P, Radhakrishnan S, Seenivasan C, Srinivasan V. The effect of copper nanoparticles supplementation on freshwater prawn Macrobrachiumrosenbergii post larvae. J Trace Elem Med Biol. 2016;34:39-49.
Crossref - Glenn JB, White SA, Klaine SJ. Interactions of gold nanoparticles with freshwater aquatic macrophytes are size and species dependent. Environ Toxicol Chem. 2012;31(1):194-201.
Crossref - Dastjerdi R, Montazer M. A review on the application of inorganic nano-structured materials in the modification of textiles: focus on anti-microbial properties. Colloid Surf B. 2010;79(1):5-18.
Crossref - Song W, Zhang J, Guo J, et al. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol Lett. 2010;199(3):389-397.
Crossref - IPPIC (International Paint and Printing Ink Council). Regulatory Status of Zinc Oxide in Antifouling Products. Final draft. 2012. http://www.ippic.org/site/assets/docs/Public%20AFWG/IPPIC%20%20Zinc%20oxide%20-%20regulatory%20status%20-final%20Draft%20March%201%202012.pdf.
- Padmavathy N, Vijayaraghavan R. Enhanced bioactivity of ZnO nanoparticles-an antimicrobial study. Sci Technol Adv Mater. 2008;9(3):35-40.
Crossref - Rafique M, Sadaf I, Rafique MS, Tahir MB. A review on green synthesis of silver nanoparticles and their applications. Artificial Cells, Nanomed Biotech. 2017;45(7):1272-1291.
Crossref - Nidia DP, Giovanni SM, Eduardo M, Sabrina TR, Miguel S, Denise E, William CN. Effect of Phyllanthus niruri on metabolic parameters of patients with kidney stone: a perspective for disease prevention. Int Braz J Urol. 2018;44(4):758-764.
Crossref - Payel B, Debasis B. Characterization of the aqueous extract of the root of Aristolochia indica: Evaluation of its traditional use as an antidote for snake bites. J Ethnopharma. 2013;145(1):220-226.
Crossref - Gopalakrishnan VK, Starlin T, Arul Raj C, Ragavendhran P. Phytochemical screening, functional groups and elemental analysis of Tylophora Pauciflora. Int Res J Pharm. 2012;3:6-10.
- Kenji O. UV-Vis spectroscopy for characterization of Metal nanoparticles formed from reduction of metal ions during ultrasonic irradiation. In Challa SSRK (Ed) UV-VIS and photoluminescence spectroscopy for nanomaterials characterization. Chapter IV. 2013: 151-177.
Crossref - Zheng J, Nagashima K, Parmiter D, de la Cruz J, Patri AK. SEM X-ray microanalysis of nanoparticles present in tissue or cultured cell thin sections. Methods Mol Boil. 2011; 697: 93-99.
Crossref - Williams DB, Carter CB. Transmission Electron Microscopy: A Textbook for Materials Science, Diffraction II. Springer Science & Business Media. Springer-Verlag US. 1996.
- Rafique M, Tahir R, Gillani SSA, et al. Plant-mediated green synthesis of zinc oxide nanoparticles from Syzygium cumini for seed germination and wastewater purification. Int J Environ Anal Chem. 2020:1-16.
Crossref - Mallik PK, Swain PK, Patnaik SC. Characterisation of Suspension Precipitated Nanocrystalline Hydroxyapatite Powders. IOP Conf. Series: Mat Sci Eng. 2016;115:12-25.
Crossref - Dorset DL. X-ray Diffraction: A Practical Approach. Micro Microanal. 1998;4(5):513-515.
Crossref - Molpeceres J, Aberturas MR, Guzman M. Biodegradable nanoparticles as a delivery system for cyclosporine: preparation and characterization. J Microencapsul. 2000;17(5):599-614.
Crossref - Anjali AA, Prachi PK, Manmohan K, Megha BM. Synthesis of CTAB-IPA reduced copper nanoparticles. Mat Chem Phy. 2005;91(2-3):507-512.
Crossref - Valgas C, De Souza SM, Smania EFA. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol. 2007;38(2):369-380.
Crossref - Singh AK, Pal P, Vinay G, Yadav TP, Vishu G, Singh SP. Green synthesis, characterization and antimicrobial activity of zinc oxide quantum dots using Eclipta alba. Mater ChemPhys. 2018;203:40-48.
Crossref - Nava OJ, Soto-Robles CA, Gomez-Gutierrez CM, et al. Fruit peel extract mediated green synthesis of zinc oxide nanoparticles. J MolStruct. 2017;1147:1-6.
Crossref - Matinise N, Fuku XG, Kaviyarasu K, Mayedwa N, Maaza M. ZnO nanoparticles via Moringa oleifera green synthesis: physical properties & mechanism of formation. Appl Surf Sci. 2017;406:339-347.
Crossref - Fazlzadeh M, Khosravi R, Zarei A. Green synthesis of zinc oxide nanoparticles using Peganum harmala seed extract, and loaded on Peganum harmala seed powdered activated carbon as new adsorbent for removal of Cr(VI) from aqueous solution. Ecol Eng. 2017;103(Part A):180-190.
Crossref - Bandeira M, Giovanela M, Roesch-Ely M, Devine DM, Crespo JS. Green synthesis of zinc oxide nanoparticles: A review of the synthesis methodology and mechanism of formation. Sust Chem Pharm. 2020;15: 100223.
Crossref - Hiremath S, Chandraprabha M, Antonyraj M, Venu GopalI, Jain A, Bansal K. Green synthesis of ZnO nanoparticles by Calotropisgigantea. Int J Curr Eng Technol. 2013;1:118-120.
- Thema FT, Manikandan E, Dhlamini MS, Maaza M. Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mat Lett. 2015;161:124-127.
Crossref - Selim YS, Azb MA, Ragab I, Abd El-Azim MHM. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extract of Deverratortuosa and their Cytotoxic Activities. Scientific reports. 2020;10(1):3443-3451.
Crossref - De la Rosa ES, Sepulveda-Guzman B, Reeja-Jayan A, Torres PS, Elizondo N, Jose Yacaman M. Controlling the growth and luminescence properties of well-faceted ZnOnanorods. J Physical Chem C. 2007;111(24):8489-8495.
Crossref - Remashan K, Dae-Kue H, Seong-Ju P, Jae-Hyung J. Effect of rapid thermal annealing on the electrical characteristics of ZnO thin-film transistors. Japanese J ApplPhy. 2008;47(4S):2848.
- Armelao L, Gregorio B, Laura B, et al. Proteins conjugation with ZnO sol-gel nanopowders. J sol-gel Sci Tech. 2011;60(3):352-358.
Crossref - Coates J. Encyclopedia of analytical chemistry. In: Meyers RA, (ed.), John Wiley & Sons Ltd, Chichester. 2000;10815-10837.
- Monaliben S, Gerrard EJP, Derek F. Biogenic synthesis of silver nanoparticles via indigenous Anigozanthosmanglesii (red and green kangaroo paw) leaf extract and its potential antibacterial activity. Int J Res Med Sci. 2016;4:3427-3432.
Crossref - Thomas Nesakumar J, Immanuel E, Yong Rok L, MathurGopalakrishnan S. Green synthesis of silver nanoparticles using Terminaliacuneata and its catalytic action in reduction of direct yellow-12 dye. Spectrochimica Acta A: Mol Biomol Spectro. 2016;161:122-129.
Crossref - Zandi S, Kameli P, Salamati H, Ahmadvand H, Hakimi M. Microstructure and optical properties of ZnO nanoparticles prepared by a simple method. Physica B: Cond Matt. 2011;406(17):3215-3218.
Crossref - Munoz-Hernandez G, Escobedo-Morales A, Pal U. Thermolytic growth of ZnO nanocrystals: morphology control and optical properties. Crystal Grow Des. 2008;9(1):297-300.
Crossref - Xiong G, Pal U, Serrano JG, Ucer KB, Williams RT. Photoluminesence and FTIR study of ZnO nanoparticles: the impurity and defect perspective. Physica status solid C. 2006;3(10):3577-3581.
Crossref - Fan XM, Lian JS, Zhao L, Liu YH. Single violet luminescence emitted from ZnO films obtained by oxidation of Zn film on quartz glass. Appl Sur Sci. 2005;252(2):420-424.
Crossref - Mishra SK, Rajneesh KS, Prakash SG, Raghvendra SY, Panday AC. Photoluminescence and photoconductive characteristics of hydrothermally synthesized ZnO nanoparticles. Opto-Elect Rev. 2010;18(4):467-473.
Crossref - Suresh U, Murugan K, Benelli G, Nicoletti M, Barnard DR, Panneerselvam C. Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedesaegypti (Diptera: Culicidae). Parasitol Res. 2015;114:1551-1562.
Crossref - Jung J, Gopinath K, Jongchul S. Development of functional antimicrobial papers using chitosan/starch-silver nanoparticles. Int J biologmacromol. 2018;112:530-536.
Crossref - Santhoshkumar A, Kavitha HP, Suresh R. Hydrothermal Synthesis, Characterization and Antibacterial Activity of NiO Nanoparticles. J Adv Chem Sci. 2016;24:230-232.
- Tong GX, Du FF, Liang Y, et al. Polymorphous ZnO complex architectures: selective synthesis, mechanism, surface area and Zn-polar plane-codetermining antibacterial activity. J Mat Chem B. 2013;1(4):454-463.
Crossref - Rafique M, Sadaf I, Bilal Tahir M, et al. Novel and facile synthesis of silver nanoparticles using Albizia procera leaf extract for dye degradation and antibacterial applications. Mat Sci Eng C. 2019;99:1313-1324.
Crossref - Khalid H, Shamaila S, Zafar N, et al. Antibacterial Behavior of Laser-Ablated Copper Nanoparticles. Acta Metallurgica Sinica(English Letters). 2016;29(8):748-754.
Crossref
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.