Since the beginning of the New Year 2020, countries around the world are stumbling due to the coronavirus disease (COVID-19) pandemic. Better approaches of diagnostics and medical facilities have helped some countries recover early. Previous exposures to epidemics have imparted lessons to handle such a pandemic with a high level of preparedness. The World Health Organization (WHO) and national health authorities are taking great efforts via efficient and impactful interventions to contain the virus. Diagnostic tests such as reverse transcription-polymerase chain reaction are increasingly being used to confirm the diagnosis because testing biological samples for the presence of the virus is the definitive method to identify the disease, analyze the risk for transmission, and determine whether someone has been cured or not. It is also important to screen asymptomatic individuals to get the exact overview of the virus spread. Antibody detection plays a pivotal role in diagnosis; however, using it at the wrong time yields negative results and conveys dissenting opinion about the tests. Although the scaling up of testing has been significant, overall testing has been limited by the availability of diagnostics. Rapid diagnoses and discontinuation of transmission are keys to ending this pandemic. Diagnostics manufacturers are developing test kits and distributing them to different countries. Therefore, more than 500 commercial test kits for molecular- and immunoassays, most with Emergency Use Authorization, are now becoming available in the market. In this review, we discuss the importance of diagnostics, approaches of different countries toward the epidemic, global testing situation, and lessons to countries at the start of the epidemic for better preparedness.
COVID-19, SARS-CoV-2, Sensitivity, Specificity, RT-PCR, Diagnosis
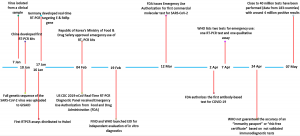
The coronavirus disease (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first notified to the Country office of the World Health Organization (WHO) in China on December 31, 2019, as pneumonia of unknown etiology. One week later, Chinese scientists identified the cause as a novel coronavirus and made its genome sequence publicly available on January 7, 20201. This sequence was distinct from those of the coronaviruses that caused severe acute respiratory syndrome (SARS) (2003–2004) and Middle East respiratory syndrome (MERS) (2012) outbreaks. COVID-19 rapidly spread worldwide, mainly due to global travel, and was in all continents except Antarctica within a month; hence, WHO declared the epidemic as a Public Health Emergency of International Concern (PHEIC) on January 30, 2020. As on May 7, 2020, the COVID-19 pandemic is in around 210 countries, territories, or areas, with more than 3.8 million cases and 265,000 deaths2. The full genomic sequence of the virus enabled the development of diagnostic tests, such as reverse transcription polymerase chain reaction (RT-PCR), by pharmaceuticals and diagnostics manufacturers in different countries. In 2003, the development of diagnostics for the SARS outbreak took about six months3. However, COVID-19 test kits were available in a matter of three days in China and six weeks in several other countries (Fig. 1), owing to technological advancements in the last 17 years4. It is well evident that these diagnostics are playing a major role in screening, monitoring, and most importantly, epidemiological surveillance5. Previous outbreaks have shown the consequences of not implementing testing protocols rapidly. Testing is mandatory not only to identify those who have contracted the virus but also to understand its spread and transmission rates. However, the availability of the diagnostics depends on their affordability by nations6. The COVID-19 case numbers reported to WHO are based on the testing approaches of individual countries. Most countries are giving importance to testing those who are critically and severely ill.
Diagnostics and their demands
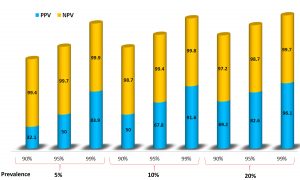
Diagnostics are the key to identify, contain, and ultimately resolve disease outbreaks. Basic diagnostic tests for any infectious disease typically fall into two categories. The first is pathogen detection. They comprise either molecular assays that detect the genetic material of the pathogen or antigen detection assays that detect the proteins present in the infectious organisms. The second category detects host biomarkers such as antibodies7. For diagnostic tests to be useful, they must be accurate and suitable for the population and setting for which they are intended. The efficiency of the diagnostic tests is reflected by their sensitivity and specificity. They are usually determined when evaluating a new test against a reference standard test (sometimes referred as a “gold standard test”) used to determine subjects, which are truly infected or non-infected. Additional characteristics of diagnostic tests that are important to consider are the positive predictive value (PPV) and negative predictive value (NPV). The PPV is the percentage of truly infected persons among those that tested positive in the test and the NPV is the percentage of truly non-infected persons among those that tested negative in the test. Both the PPV and NPV depend not only on the sensitivity and specificity of the test but also on the prevalence of infection in the population studied. False-negative test results are more likely when the prevalence of the disease is high, whereas false-positive test results are more likely when the prevalence is moderate to low8. For example, when calculating theoretically, with the disease prevalence at 5% and the sensitivity and specificity of a test at 90% level of probability the NPV and PPV will be 99.4% and 32.1%, respectively. It shows that the probability that the disease is present when the test is negative is 0.6% and the probability that the disease is absent when the test is positive is 67.9% (Fig. 2). The important determinants considered for evaluating the role of diagnostic tests are their use with the right samples, in the right clinical settings, on the right patients, and at the right time.
Fig. 2. Positive predictive values (PPV) and negative predictive values (NPV) of COVID-19 testing at different prevalence levels of the disease and diagnostic sensitivity and specificity of the test
The ability of the diagnostics is also affected by extrinsic factors, such as the time of illness, virus concentration, change in the quality of the sample from collection to transport until the processing, and specific formulation of the reagents in the test kits9. Early studies have shown that antibodies may not be detectable before 7–10 days from infection and before 3 days from the onset of symptoms10. The IgM antibodies (indicating recent infection) are detectable from approximately 7 days of infection and IgG (past infection) can be detected 14 days from infection. The combination of RNA + IgM tests or antigen + IgM tests can be used to widen the window of detection for acute infection11. The main understanding is that the negative results of RT-PCR and antibody tests need not necessarily agree with each other. Any disagreement can be traced to identify the time lag between the infection being acquired and the test being performed. In most cases, the time of infection is unknown; hence, a combination of RT-PCR and IgM/IgG testing may improve the diagnosis, because RT-PCR can detect the acute phase and antibody tests can detect the chronic phase of the infection12. Sometimes confusion is created when deciding whether to use a test for diagnosis or screening purposes. The difference between diagnostic and screening tests depends on their purpose and characteristics. For example, the target population for screening tests is of asymptomatic individuals, whereas those for diagnostic tests are either symptomatic individuals or asymptomatic individuals who tested positive in screening tests. The foremost requirement for a screening test is high sensitivity toward the potential disease and that for a diagnostic test is high specificity13. Diagnostic tests such as PCR detect the presence of the pathogen rather than the body’s immune response. They give a good indication of whether a person is infected. On the contrary, screening tests are not suitable to make the final diagnosis because this may delay identification, by which time the infection may be transmitted to a larger population. In contrast, the use of RT-PCR for screening a large population is not feasible; it would only indicate less prevalence of the virus among the population14.
Some studies have suggested the complementary role of chest computed tomography (CT) in the diagnosis of SARS-CoV-2; it is more sensitive than RT-PCR15. In chest CT, the characteristic ground-glass opacity, consolidation, reticulation/thickened interlobular septa, and nodules have been noticed bilaterally with varying degrees in each cohort. In a study from China, with RT-PCR results as a reference, chest CT showed sensitivity, specificity, and accuracy in indicating COVID-19 infection of 97% (580/601), 25% (105/413), and 68% (685/1014), respectively. The PPV and NPV were 65% (580/888) and 83% (105/126), respectively16. In comparison to RT-PCR, chest CT is more reliable, practical, easily accessible, and rapid for COVID-19 diagnosis in the current pandemic situation. It would be easy to initially screen the epidemic area using chest CT and then confirm infection with RT-PCR. However, CT lesions are not specific to COVID-19. They have been observed in other cases of viral pneumonia caused by influenza viruses and non-infectious etiologies. Although CT has a clinical benefit, it may lead to false security if the test results are negative. On the contrary, the use of CT for COVID-19 diagnosis may cause a shortage of resources to vulnerable patients who need imaging for other diseases, and create a distraction during the pandemic17.
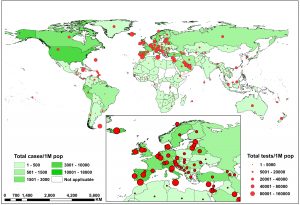
Fig. 3. Number of COVID-19 cases and tests per million population in different countries as on 6 May, 2020
Global status on COVID-19 testing
Diagnostic testing of COVID-19 is important for tracking the virus, understanding the epidemiology, informing case management, and interrupting transmission. WHO has stated that testing, isolation of the virus, and contact tracing should be the backbone of the global response to COVID-19, as social distancing and personal hygiene measures alone will not help overcome the epidemic18. Fig. 3 shows the countries, areas, and territories with COVID-19 cases, and tests per millions of population until May 7, 2020. On March 16, 2020, Dr. Tedros Adhanom, the Director-General of WHO, told countries to “test, test, test.” The ability to test and to identify asymptomatic cases is critical for our future. However, testing asymptomatic and convalescent individuals (i.e., screening) with a diagnostic test poses some inherent problems as well, such as over-diagnosis of cases, which may overburden the system working to control the disease19. Several groups all over the world have questioned the efficacy of using COVID-19 diagnostic tests for screening healthy people20. The community transmission of COVID-19 is mainly due to asymptomatic individuals or those with mild symptoms, and the extent to which testing is available; access to those who exhibit mild disease affects the number of cases and the estimated case fatality rate reported from each country, and can further drive testing policies. WHO has classified the schematic use of diagnostic testing in 4 different types of transmission scenarios, including how testing must be carried out when a shortage of reagents or testing facilities forces prioritization of certain populations or individuals for testing, as:
No cases
Countries with no cases.
Sporadic cases
Countries with one or more cases, imported or locally detected.
Clusters of cases
Countries having clusters of cases over time, location, and exposure.
Community transmission
Countries with massive outbreaks or sustained and pervading local transmission21.
Currently, confirmation of COVID-19 cases is based on the detection of a unique sequence of viral RNA using nucleic acid amplification tests (NAAT) such as rRT-PCR. Viral genes such as N, E, S, and RdRP are targeted in the sequence22. If the NAAT test is negative and there is strong epidemiological relation to COVID-19 infection, serological surveys with paired sera samples are recommended with validated tests. However, cross-reactivity of other coronaviruses may be challenging and the tests should be validated to facilitate accurate diagnosis23. WHO has announced that the molecular and serological diagnostic products developed for COVID-19 are being validated and verified for quality and safety, using the WHO Prequalification Emergency Use Listing Procedures, through collaboration with the Foundation for Innovative New Diagnostics (FIND)24. WHO has warned countries against relying on antibody tests to make policy decisions and has recommended that they should be used only for surveillance and epidemiological purposes in hotspot areas. Details of the COVID-19 closed platform diagnostic test kits authorized for use by national regulatory panels with manufacturer-based test performances are listed in Table 1. SARS-CoV-2 testing at the global level is based on either molecular assays (RT-PCR, rRT-PCR) or immunoassays (manual or lateral flow, manual ELISA, and rapid tests specific for SARS-CoV-2 antigen or antibodies). Testing policies vary in different countries based on government structures and transition through the aforementioned transmission scenarios. Countries that had previously faced the SARS and MERS outbreaks were equipped with better healthcare facilities and a greater healthcare worker force. Their preparedness for the ongoing pandemic is well reflected in their testing capacities and ability to bring down the epidemic wave at the earliest (Fig. 4). The 360Dx platform(https://www.360dx.com/coronavirus-test-tracker-launched-covid-19-tests) is continuously providing updates on COVID-19 diagnostics.
Table (1):
Manufacturers’ reported specifications and test performances of closed platforms.
| Closed platform manufacturer | Test name | Specimen type | Target | Limit of detection | Capacity | TAT | Positive percent agreement (95% CI)a | Negative percent agreement (95% CI)b | N, poscneg |
|---|---|---|---|---|---|---|---|---|---|
| Abbott Laboratories | Abbott Real-Time SARS- CoV-2 test | Nasal, nasopharyngeal and oropharyngeal swabs | RdRp and N genes | < 100 copies / ml | 24-96 specimens | 470 patient samples in 24 hours | 100%, (94.0% – 100%) | 100% (88.8% – 100%) | 61/34 |
| Abbott ID NOWTM COVID-19 test | Nasal, nasopharyngeal and throat swabs | RdRp gene | 125 copies / ml | Single test | 5-13 minutes | 100% (72.3% – 100%) | 100% (88.7%- 100%) | 30/30 | |
| Becton, Dickinson and Company (BD) | BioGX SARS- CoV-2 | Nasopharyngeal and oropharyngeal swabs | N gene | 40 copies / ml | 24 specimens | <3 hours | 100% | 100% | 30/30 |
| Cepheid Inc | Xpert® Xpress SARS- CoV-2 | Nasopharyngeal swabs, nasal aspirates, and nasal washes | NA | 250 copies / ml | Single test | 45 minutes | 100% | 100% | 30/30 |
| Hologic Inc | Panther Fusion° SARS- CoV-2 | Nasopharyngeal and oropharyngeal swabs | ORF1ab gene | 0.01 TCID50/mL | 1150 tests in 24 hours | 2.4 hours | 100% (94.7% – 100%) | 100% (96.6% – 100%) | 69 /109 |
| QIAGEN | QlAstat- Dx® Respiratory SARS- CoV-2 | Nasopharyngeal swabs | Orf1b and Rdrp genes | 0.01 -3.6 TCID50 /ml. depending on the strain | Single test | 1 hour | 100% (85.8% – 100%) | 100% (85.8% – 100%) | 30/130 |
| Roche Holding AG | cobas° SARS- CoV-2 | Nasal, nasopharyngeal and oropharyngeal swabs | ORF1ab gene | 0.009 TCID5.3/mL | 960 tests in an eight- hour shift (on the Cobas° 8800) | 3 hours | 100% (92.9% – 100%) | 100% (96.3% – 100%) | 50/100 |
NA – Not available; TAT – Turnaround time; Source: https://www.finddx.org/covid-19-2/dx-data/ and company websites
- Similar calculation as clinical sensitivity
- Same calculation as clinical specificity
- Contrived samples, unless otherwise specified
Different countries and their epidemic approaches
The following examples illustrate different approaches that countries are taking toward the COVID-19 epidemic:
China
China followed both testing and contact tracing strategies that intended to identify the affected individuals and isolate them from the rest of the population25. The outbreak happened just before their lunar New Year holidays, which is a period where there is generally high population movement. However, the government acted quickly, and its approach was reported as “draconian.” Some provinces cancelled the celebrations and followed traditional public health measures such as isolation, social distancing, quarantine, and community containment26. They screened as many people as possible and hospitalized everyone who tested positive. As an example, when a person was tested, they had to wait in the hospital to get the results. The results of the tests were made available within a day. If the test result was positive, the person had to stay in the health facility until they were free of the virus (indicated by two successive negative PCR tests). If the test results were negative, the individuals were required to self-isolate for two weeks27.
Singapore
Singapore has a better health index because of its experience with the SARS outbreak in 2003. The national center for infectious diseases was well established to handle outbreaks; health facilities such as isolation hospitals were built, more negative-pressure rooms were created, and expertise was built up via training sessions with WHO and US CDC28. National exercises such as “sparrowhawk” were conducted to increase awareness and test preparedness among health professionals. Once COVID-19 arrived in Singapore, which was the first nation affected outside China, the government tested all visitors and returning nationals with symptoms, and conducted effective contact tracing and testing, even for asymptomatic cases29. The Disease Outbreak Response System Condition (DORSCON) alert level was elevated to orange, as the disease was severe and spreading30. This comprehensive approach was used to identify all the cases and their links within a cluster, and put them into quarantine, to avoid further transmission. Effective control was maintained over the initial wave by restricting positive cases from returning to the community31. This is the main tactic that European countries, USA, and Australia did not follow because their cases were mild. The Singaporean government imposed self-quarantine on those with no symptoms, conducted contact tracing, and monitored them twice a day using a mobile phone platform. By the end of January, all the public hospitals were equipped to perform tests; they then moved to the enhanced screening of patients with respiratory illness32.
Republic of Korea
One of the countries that effectively controlled the pandemic using lessons learned from the 2015 MERS outbreak. The government conducted vigorous testing among its citizens and made testing centers easily accessible to the population, enabling effective implementation of public health measures33. From January 19 to February, the nation recorded a total of 30 cases with no deaths. However, after February 18, the 31st case was followed with a spike of 2300 cases. The 31st case was called as super spreader. Despite the initial rapidity of the COVID-19 spread, authorities framed well-established strategies and put them into place34. Testing was one of the major steps taken against the fast-spreading virus. The country has conducted more than 350,000 tests and repeated them many times in some patients before their release. The swift response of health authorities included the following:
- Organizing more than 100 testing locations, including public facilities, 81 healthcare facilities, and 5commercial laboratories, around the country, to provide testing and diagnosis services35.
- Implementation of the drive-through and walk-through screening centers, allowing rapid sample collection and referral for testing36.
- Rapid development and manufacturing of diagnostics within the country’s biotechnology industry, including fast-tracking five COVID-19 diagnostic tests through regulatory approval, which allowed rapid availability of tests37.
- Use of data (e.g., from mobile phones) to monitor COVID-19-positive individuals and close contacts, facilitating self-isolation and contact tracing.
Germany
Germany has one of the largest medical industries in the world; it benefited from following the footsteps of South Korea, and exhibited a very low death rate. Widespread testing and isolation helped to flatten the epidemic curve with less than 5% fatality rate38. As of March 20, domestic laboratories were equipped to conduct as many as 160,000 tests per week. After the announcement of human-to-human transmission of the coronavirus in mid-January by WHO, German scientists efficiently developed the first reliable test, which was later adopted by WHO itself39. It is said that German institutes are among the fastest and most efficient in the world. Bosch, a multinational company from Stuttgart, has assigned its medical engineers to develop a new diagnostic test that will be able to detect the coronavirus in 2.5 hours40. A major advantage of the country’s healthcare sector is that doctors’ practices can extend beyond hospitals. This enabled the setting up of an impressive number of test centers across the country41.
African countries
They initially experienced a slow rise in COVID-19 cases, except South Africa, but the facilities were not adequate to map the actual number of cases. The African CDC has reported that more than 1 million coronavirus tests will be carried out to mend the “huge gap” in assessing the true number of cases in the continent42. Africa is facing difficulty in the global race to obtain diagnostic kits and other much-needed health equipment such as ventilators43. Until now, South Africa, the most self-assured African nation in testing, has carried out 190,000 tests.
Nigeria
Some low- and middle-income countries in North Africa understood their constraints in funding, capacity, and infrastructure and aggressively ramped up measures to contain COVID-19 spread as early as possible44. These measures included the deployment of total lockdown and a willingness to procure rapid, easy-to-use tests, even if they were not as accurate as conventional PCR tests, which require relatively expensive laboratory equipment and supplies and greater laboratory capacity45.
Peru
Peru has only two COVID-19 testing facilities, both in the capital, Lima46. Although they are not as accurate as PCR tests, the government is seeking a million rapid diagnostic tests and plans to use them to help hospitals gauge how many healthcare workers have been infected, to be able to test and identify individuals and locate their close contacts. The Peruvian government also started constructing isolation centers in advance of any confirmed COVID-19 cases47.
Italy
Italy has the most elderly population in Europe and the second most elderly population in the world after Japan. It is a known fact that Italy has an excellent health care system; however, it faced a widespread disease occurrence48. The analysis showed that attributes such as smoking, heart disease, and high median age made the condition severe in the country despite the well-established health system49. The country developed mobile apps for voluntary contact tracing, which alerted people when they had close contacts with individuals who tested positive for the virus50. The blood tests for identifying antibodies against the virus are still being developed. Virologists have made aware that these tests will not assess the immunity but will give a snapshot of whether a person has been in contact with the virus51. Approximately a million people in Italy will be tested for natural antibodies to determine whether they have developed immunity to the virus and can return to normal life. Providing “immunity passports” will speed up the return of key workers to essential duties52. However, WHO has warned countries against using immunity passports in this situation as it is immature.
USA
USA established three different priorities for testing patients with suspected SARS-CoV-2 infection once the first case was reported from Snohomish County, Washington on January 20, 202053. Currently, the country has conducted more than 7.7 million tests, more than any other country in the world. They transitioned from having no tests or assays at the start of this decade to conducting COVID-19 diagnostics with multiple tests in a span of just a few weeks54. More labs are using indigenously developed kits. It is stated that WHO warned governments to prepare for COVID-19 in January and distributed a Germany-based PCR test for the virus. Rather than using those tests, the US CDC developed indigenous tests in February and allowed only those in the country. In late February, it was revealed that the CDC tests were in short supply and faulty. National scientists are blaming the worsening of the pandemic in the country on the deficiency of national strategies and failure to increase testing at the right time55. The COVID Tracking Project, which is a volunteer organization launched by The Atlantic, collects and issues the comprehensive testing data available for US states and territories56. The policy is to test all in a locality where any case of COVID-19 is reported, home-quarantine those that test positive (no action on contacts if they test negative), hospitalize those that are sick and need medical help, and implement selective lockdown.
India
The first case of COVID-19 was reported on January 30, 2020; the number increased to three cases on February 3, and all were students from Wuhan, China, which was the epicenter of the pandemic then. Thereafter, there were no cases in February, and the government only screened visitors from affected countries. On March 4, 22 new cases were identified and then the disease escalated57. India adopted a cluster and containment strategy, similar to that implemented during previous epidemics, to control the virus and break the chain of transmission. The National Institute of Virology (NIV), Pune headed the strategy and initially trained 15 labs across the country. As of March 17, the country had 65 labs equipped to test the coronavirus (many of them are not fully functional)58.
India became the fifth country to isolate the virus in mid-March at NIV, Pune and submitted two full SARS-CoV-2 genome sequences to the Global Initiative on Sharing All Influenza Data (GISAID)59. The Indian Council of Medical Research (ICMR) has claimed that isolation of the virus will pave the way for diagnostics, vaccine development, and rapid testing in the country60. As of May 7, India reported nearly 53,000 cases and over 1700 deaths. Despite the number of cases detected in India, it is still in an early stage of outbreak monitoring, with below 1000 tests per million populations. The Ministry of Health and Family Welfare (MoHFW) has implemented rapid scaling up of testing by:
- Increasing government-approved PCR testing laboratories from 19 to over 327 (as on May 6) and designating other labs as sample collection centers.
- Utilizing 118 approved private-sector labs (as on May 6) to test for COVID-19, and asking that they support the home-collection of samples.
- Increasing personnel shifts in select laboratories to operate at all times.
- Using decentralized testing platforms and those used for other disease programs.
- Using TrueNAT, a test based on nucleic acid amplification test (NAAT) technology for tuberculosis detection; it has been considered a screening test for COVID-19. Laboratories that have installed TrueNAT will upload their results; positive samples will be sent for confirmation with RT-PCR and negative results will be deemed as final for that episode.
- Promoting the use of cartridge base nucleic acid amplification test (CBNAAT)for tuberculosis with FDA-approved Cepheid Xpert Xpress SARS-CoV-2.
- Implementing high-throughput molecular testing platforms from other disease programs, such as IDSP and NVBDCP (e.g., Roche’s cobas® 6800 and Abbott RT-PCR), in government laboratories61.
- Using rapid antibody tests in hotspots and containment areas.
- Issuing guidance on the suitability of using pooled samples for molecular testing of COVID-19.
- Establishing multiple depots for reagent supply to laboratories.
- Prioritizing in vitro COVID-19 diagnostic test kits for expedited regulatory approval.
- Rapid screening of the community with antibody tests; positive cases are isolated and negative cases are tested again with RT-PCR to decrease the cost and time of testing62.
The Central Drugs Standard Control Organisation (CDSCO) has approved coronavirus (COVID-19) diagnostic test (RT-PCR) kits developed by Pune-based Mylab Discovery. They claim that the RT-PCR kits can screen 1000 samples in large labs and 200 in smaller labs. The test takes 2.5 hours after the initial sample preparation, and the company is pricing it around ₹1,200 (US$17), or ₹80,000 for a 100-test kit63. The Department of Science and Technology (DST) is playing a pivotal role in funding Seagull Bio-Solutions, a start-up developing an Active Virosome (AV) vaccine and immunodiagnostic kits for identifying asymptomatic individuals64.
India has used digital platforms to create awareness among the public via celebrity talks, text-messaging of vital health points to people directly on their mobile phones, and mobile apps such as Aarogya Setu for contact tracing and containing the spread.
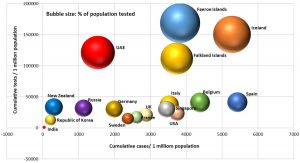
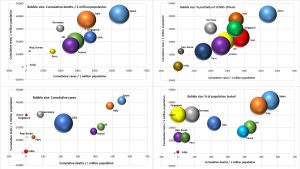
The cumulative number of cases, deaths, and tests per million populations, with the percentage positivity of the tests and percentage of the population tested in the aforementioned countries as of May 7 is depicted in Fig. 5.
Fig. 5. Cumulative cases, cumulative tests, cumulative deaths per million populations with % positivity of tests and % of population tested in different countries
How countries are augmenting COVID-19 molecular testing
Although the increase in testing has been significant, overall testing has been limited due to the unavailability of diagnostics65. Countries are trying to establish the use of in-house tests and protocols that were developed and rapidly introduced in centralized PCR labs. Commercial kits, including rapid serological tests and closed platforms, some of which can be deployed to more remote settings, are now becoming increasingly available. These commercial kits have been developed to facilitate testing outside the laboratory and they are based on virus protein detection in respiratory samples (e.g., sputum and throat swabs) or detection of human antibodies in blood or serum. However, the efficiency of these kits is still questionable, and WHO recommends their use only in research settings. Such rapid detection kits for antigens enable diagnosis in 30 minutes. However, they can detect the virus only in the replication stage. Therefore, they are recommended for identifying early and acute infections66. During an epidemic, rapid testing of antibodies will be vital in determining any immunity that develops among the population, and antigen detection test kits can be used to confirm active infection without PCR’s arduous process of laboratory testing.
Rapid confirmation of COVID-19 cases and the tracing of all contacts linked to a confirmed case, regardless of symptoms, should be a top priority for all countries, especially given the large number of asymptomatic and mild cases (81%) of COVID-1967. Countries are making rapid antibody test kits (IgM/IgG) available to conduct surveillance and get a clear picture of the distribution of the virus and developed herd immunity. However, they should be used at the right time, as many negative results in the early stage of the infection may not serve the purpose. Countries will understand the stage of the epidemic and be adequately prepared for appropriate public health measures, including accurate forecasting of demand for diagnostics, which includes swabs for collection of samples, personal protective equipment for healthcare workers, critical-care beds, and ventilators in hospitals, only through strong and well-implemented testing policies.
Owing to the shortage of test kits, countries are moving toward “group testing,” which is done by preparing “super samples” from a group of test samples. However, the use of group tests depends on the assumption that swabs of some COVID-19-positive individuals mixed with those of COVID-19-negative individuals should always result in a positive in a test of mixed swabs. The test is not useful if this is not the case. Other approaches such as CRISPR-based68, carbohydrate-based69, and lateral flow assays; automated molecular diagnostic tests; convalescent plasma therapy; use of Chest CT70; and molecular techniques such as RT-LAMP have not been validated. Studies are proposing the use of faecal samples for the detection of coronavirus with high accuracy, even in recovered patients71.
Based on the data for 178 countries and the total tests conducted (as on May 7, 2020), it is evident that only Iceland (15.04%) and Faeroe island (16.86%) have tested >15% of their population. UAE has tested 12.13% and the Falkland Islands has tested nearly 11.14% of their population. The remaining countries have tested only less than 10% of their population (Fig. 2). Although the United States has conducted approximately 7.7 million tests, they have covered only 2.33% of their population. India has conducted approximately 1,300,000 tests so far but has covered only 0.09% of the total population and needs 13.5 million more tests to reach 1% of the population, which may take more than a year at the current pace. It has been revealed that Algeria, a North African country, has conducted only 6500 tests and identified 4838 cases, a 74% positivity. Other countries are observing less than 50% of their tests return positive. Developed and severely affected countries such as France (15.5%), United Kingdom (14.09%), Spain (13.12%), United States (16.02%), Italy (9.48%), and Germany (6.5%) have questioned the efficiency of their diagnostics. India is witnessing 3.8% test positivity.
Although the expansion of testing for COVID-19 will differ based on the country and socio-economic status, acknowledging and learning from the experiences of different countries is the key to designing and implementing test expansions within a country. Since many countries are still in the early stage of infection and its monitoring, they can contain the epidemic if they apply the measures that have proven efficacious in countries that have already experienced the epidemic. Countries should have some realistic goals with respect to testing strategies, such as identifying hotspots to contain the explosion of the disease and estimating the disease prevalence in asymptomatic carriers and the general population. There exists a competing interest between public health and economics in an ongoing crisis. However, by minimizing human suffering, the economy can be boosted in the future, provided the country can sustain the burden of the disease and its diagnosis in the present. Some are arguing that herd immunity may play a major role in containing the spread of the disease; however, for an effective assessment, it is important to use reliable diagnostics. Currently, there is no evidence to suggest how long the post-infection immunity will remain for COVID-19. Studies on the SARS outbreak indicated that antibodies are present in the blood for years after recovery. Both COVID-19 and SARS are caused by coronaviruses; however, it is too early to state that COVID-19 will elicit a similar immune response. Countries should ensure that the transmission of the virus is under control; establish public health measures such as detection, testing, isolation, treatment, contact tracing, and hotspot risk-minimization; and, finally, create awareness among the public, to handle the situation while lifting a lockdown. The epidemic curve may peak and flatten but humanity will be at its apex if we succeed in holding on to the lessons learned during the pandemic.
ACKNOWLEDGMENTS
All the listed author(s) are thankful to their representative universities/institutes for providing the related support to compile this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All the listed author(s) have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
Not applicable.
- Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. The Lancet. 2020;395(10223):470-473.
- COVID-19 Coronavirus pandemic. https://www.worldometers.info/coronavirus/(2020). Accessed 29 April 2020. Accessed 29 April 2020.
- Drosten C, Chiu LL, Panning M, et al. Evaluation of advanced reverse transcription-PCR assays and an alternative PCR target region for detection of severe acute respiratory syndrome-associated coronavirus. Journal of Clinical Microbiology. 2004;42(5):2043-2047.
- Coronavirus (SARS-CoV-2): Test Kits to Detect the Causative Agent of COVID-19. https://www.rapidmicrobiology.com [Accessed 25April 2020]
- Tang YW, Schmitz, JE, Persing DH,Stratton CW. The laboratory diagnosis of COVID-19 infection: current issues and challenges. Journal of Clinical Microbiology, 2020.DOI: 10.1128/JCM.00512-20
- Peeri NC, Shrestha N, Rahman MS, Zaki R, et al. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? International Journal of Epidemiology. 2020. DOI: 10.1093/ije/dyaa033
- Psarras S, Papadopoulos NG, Johnston SL. Diagnosis of viral respiratory illness: Practical applications. Kendig’s Disorders of the Respiratory Tract in Children. 2006;388-403. DOI: 10.1016/B978-0-7216-3695-5.50026-2. Epub 2009. PMCID: PMC7150358.
- Bentley TG, Catanzaro A, Ganiats TG. Implications of the impact of prevalence on test thresholds and outcomes: lessons from tuberculosis. BMC Research Notes. 2012;5:563. DOI: 10.1186/1756-0500-5-563. PMID: 23050607; PMCID: PMC3503683.
- Wang X, Yao H, Xu X, et al. Limits of detection of six approved RT-PCR kits for the novel SARS-coronavirus-2 (SARS-CoV-2). Clinical Chemistry. 2020. DOI: 10.1093/clinchem/hvaa099. Epub ahead of print. PMID: 32282874; PMCID: PMC7184447.
- Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clinical Infectious Diseases. 2020. DOI: 10.1093/cid/ciaa344. Epub ahead of print. PMID: 32221519; PMCID: PMC7184337.
- Udugama B, Kadhiresan P, Kozlowski HN, et al. Diagnosing COVID-19: The disease and tools for detection. ACS Nano. 2020;14(4):3822-3835. DOI: 10.1021/acsnano.0c02624. Epub 2020 Mar 30. PMID: 32223179; PMCID: PMC7144809.
- Weaver C. Questions About Accuracy of Coronavirus Tests Sow Worry. The Wall Street Journal. April 2, 2020. https://www.wsj.com [ Accessed 25 April 2020]
- Maxim LD, Niebo R, Utell MJ. Screening tests: a review with examples. InhalationToxicology. 2014;26(13):811-828. DOI: 10.3109/08958378.2014.955932. Epub 2014 Sep 29. Erratum in: Inhalation Toxicology. 2019; 31(7): 298. PMID: 25264934; PMCID: PMC4389712.
- Informed Health.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-. Benefits and risks of screening tests. 2013; [Updated 2019 Dec 17]. https://www.ncbi.nlm.nih.gov/books/NBK279418/. Accessed 25 April 2020.
- Caruso D, Zerunian M, Polici M, et al. Chest CT features of COVID-19 in Rome, Italy. Radiology. 2020;3:201237. DOI: 10.1148/radiol.2020201237
- Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: A report of 1014 cases. Radiology. 2020:200642. DOI: 10.1148/radiol.2020200642
- Hope MD, Raptis CA, Shah A, Hammer MM, Henry TS. A role for CT in COVID-19? What data really tell us so far. Lancet (London, England). 2020.
- Report of the WHO–China Joint Mission on Coronavirus Disease 2019 (COVID-19) [February 16-24, 2020]
- Singh BR, Gandharva R, Karthikeyan R, et al. Epidemiological determinants of acute respiratory syndrome coronavirus-2 disease pandemic and the role of the Bacille-Calmette-Guerin vaccine in reducing morbidity and mortality. Journal of Pure Applied Microbiology. May 2020; 14(SplEdn.).
- Singh BR, Gandharva R. Are BCG Vaccination, Population Density, Median Age and Poverty Important Determinants of COVID-19 Pandemic Spread, Morbidity and Mortality?DOI:10.13140/RG.2.2.21116.49282.2020. https://www.researchgate.net/publication/340443017_Are_BCG_Vaccination_Population_Density_Median_Age_and_Poverty_Important_Determinants_of_COVID19_Pandemic_Spread_Morbidity_and_Mortality 2020
- Global surveillance for COVID-19 caused by human infection with COVID-19 virus: interim guidance (No. WHO/2019 nCoV/SurveillanceGuidance/2020.6). World Health Organization.20 March 2020.
- Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance (No. WHO/COVID-19/laboratory/2020.4). World Health Organization.2 March 2020.
- Lassauniere R, Frische A, Harboe ZB, et al. Evaluation of nine commercial SARS-CoV-2 immunoassays. medRxiv. 2020. DOI: https://doi.org/10.1101/2020.04.09.20056325
- Coronavirus disease (COVID-19) technical guidance: Laboratory testing for 2019-nCoV in humans. Geneva, Switzerland. World Health Organization, 2020.
- McCloskey B, Heymann DL. SARS to novel coronavirus – old lessons and new lessons. Epidemiology & Infection. 2020;148:e22. DOI: 10.1017/S0950268820000254. PMID: 32019614; PMCID: PMC7026896.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239-1242.
- Falush D. Isolate, isolate, isolate: China’s approach to Covid-19 quarantine could be the most effective. Apr 20,2020. https://www.telegraph.co.uk [ Accessed 25 April 2020 ]
- Tay JY, Lim PL, Marimuthu K, et al. De-isolating COVID-19 Suspect Cases: A Continuing Challenge. Clinical Infectious Diseases. 2020.
- Lee VJ, Chiew CJ, Khong WX. Interrupting transmission of COVID-19: lessons from containment efforts in Singapore. J Travel Med. 2020. DOI: 10.1093/jtm/taaa039. Epub ahead of print. PMID: 32167146; PMCID: PMC7107552.
- Ministry of Health Singapore. Risk assessment raised to DORSCON Orange. 2020. https://www.moh.gov.sg [ Accessed 25 April 2020]
- Wong J, Goh QY, Tan Z, et al. Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Canadian Journal of Anaesthesia. 2020;11:1-14. DOI: 10.1007/s12630-020-01620-9. Epub ahead of print. PMID: 32162212; PMCID: PMC7090449.
- Wong JEL, Leo YS, Tan CC. COVID-19 in Singapore—Current experience: Critical global issues that require attention and action. JAMA. 2020;323(13):1243-1244. DOI:10.1001/jama.2020.2467
- Fleming S. South Korea’s Foreign Minister explains how the country contained COVID-19, 2020. https://www.weforum.org [ Accessed 25 April 2020]
- Shim E, Tariq A, Choi W, Lee Y, Chowell G. Transmission potential and severity of COVID-19 in South Korea. Int J Infect Dis. 2020;93:339-344. DOI: 10.1016/j.ijid.2020.03.031. Epub ahead of print. PMID: 32198088; PMCID: PMC7118661.
- McClean D. How South Korea is suppressing COVID-19. United Nations Office for Disaster Risk Reduction, 2020. https://www.undrr.org [Accessed 25 April 2020]
- Ferrier K. South Korea ramps-up exports of COVID-19 testing kits. The Diplomat. 2020. https://thediplomat.com [ Accessed 25 April 2020]
- Kwon KT, Ko JH, Shin H, Sung M, Kim JY. Drive-through screening center for COVID-19: a safe and efficient screening system against massive community outbreak. Journal of Korean Medical Science,. 2020;35(11):e123. DOI: 10.3346/jkms.2020.35.e123. PMID: 32193904; PMCID: PMC7086085.
- Cohen J, Kupferschmidt K. Countries test tactics in‘war’ against COVID-19. Science (New York, NY). 2020;367(6484):1287.
- Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Eurosurveillance. 2020;25(3):2000045.
- Rauwald C, Loh T. New Virus Test Shortens Wait to 2.5 Hours from Two Days, 2020. https://www.bloombergquint.com [ Accessed 27 April 2020]
- Stafford N. Covid-19: Why Germany’s case fatality rate seems so low. BMJ, 2020; 369. DOI: https://doi.org/10.1136/bmj.m1395
- Whitworth J. COVID-19: a fast evolving pandemic. Trans R Soc Trop Med Hyg. 2020;114(4):241-248. DOI: 10.1093/trstmh/traa025. PMID: 32198918; PMCID: PMC7184420.
- Nkengasong JN, Mankoula W. Looming threat of COVID-19 infection in Africa: act collectively, and fast. Lancet. 2020;395(10227):841-842. DOI: 10.1016/S0140-6736(20)30464-5. Epub 2020 Feb 27. PMID: 32113508; PMCID: PMC7124371.
- Hopman J, Allegranzi B, Mehtar S. Managing COVID-19 in low-and middle-income countries. JAMA. 2020;323(16):1549-1550. DOI:10.1001/jama.2020.4169
- Di Caro B. COVID-19 in Africa: insights from our 23 April WHO media briefing, 2020. Available on https://www.weforum.org [ Accessed 27 April 2020]
- Machicao JC. Monitoring the Covid-19 pandemic in Peru with highly uncertain data.
- Oxford Analytica. COVID-19 will have wide-ranging impacts in Peru. Emerald Expert Briefings (oxan-db).
- Saglietto A, D’Ascenzo F, Zoccai GB, De Ferrari GM. COVID-19 in Europe: the Italian lesson. Lancet. 2020;395(10230):1110-1111. DOI: 10.1016/S0140-6736(20)30690-5. Epub 2020 Mar 24. PMID: 32220279; PMCID: PMC7118630.
- Gentile S, Strollo F, Ceriello A. COVID-19 Infection in Italian people with diabetes: lessons learned for our future (an experience to be used). Diabetes Research and Clinical Practice. 2020. DOI:https://doi.org/10.1016/j.diabres.2020.108137
- Barbieri C, Darnis JP. Technology: An Exit Strategy for COVID-19? Istituto Affar iInternazionali, IAI Commentaries, 2020; 1-4.
- Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323(16):1545-1546. DOI:10.1001/jama.2020.4031
- Alleva G, Arbia G, Falorsi PD, Zuliani A. A sample approach to the estimation of the critical parameters of the SARS-CoV-2 epidemics: an operational design with a focus on the Italian health system. arXiv preprint arXiv. 2004.06068. 2020.
- Criteria to Guide Evaluation and Laboratory Testing for COVID-19.April 27, 2020.https://www.cdc.gov. Accessed 29 April 2020.
- Burke RM. Active monitoring of persons exposed to patients with confirmed COVID-19—United States, January-February 2020. MMWR. Morbidity and Mortality Weekly Report. 2020;69.
- Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, Zhang C, Boyle C, Smith M, Phillips JP. Fair allocation of scarce medical resources in the time of Covid-19. 2020. DOI: 10.1056/NEJMsb2005114
- COVID Tracking Project. https://covidtracking.com/data.
- COVID-19 | 6 members of Delhi patient’s family test positive for coronavirus. The Hindu, 2020. Accessed 27 April 2020.
- Soutik B. Is India prepared for a coronavirus outbreak? BBC News. Archived from the original on March 7, 2020. Accessed 27 April 2020.
- Yadav PD, Potdar VA, Choudhary ML, et al. Full-genome sequences of the first two SARS-CoV-2 viruses from India. The Indian Journal of Medical Research. 2020;151(2):200-209 DOI: 10.4103/ijmr.IJMR_663_20
- We need to ramp up testing facilities. The Times of India (print edition). March 17, 2020. Accessed 27 April 2020.
- Indian Council of Medical Research. COVID-19.https://www.icmr.gov.in [ Accessed 28 April 2020]
- Government of India, Ministry of Science & Technology. Rapid response framework for COVID-19 to deal with applications for development of vaccines, diagnostics, prophylactics and therapeutics – reg., 2020. https://cdsco.gov.in [ Accessed 28 April 2020]
- Mylab receives approval for Covid-19 test kit. March 24,2020. https://www.medicaldevice-network.com
[ Accessed 28 April 2020] - DST funded startup develops kits for testing asymptomatic COVID-19 infections & gears up for vaccine production. https://dst.gov.in/. Accessed 28 April 2020.
- Sohrabi C, Alsafi Z, O’Neill N, Khan M, Kerwan A, Al-Jabir A, Iosifidis C, Agha R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int J Surg., 2020; 76: 71-76. https://doi.org/10.1016/j.ijsu.2020.02.034
- Advice on the use of point-of-care immunodiagnostic tests for COVID-19: scientific brief. April 8,2020 (No. WHO/2019 nCoV/Sci_Brief/POC_immunodiagnostics/2020.1). World Health Organization.2020.
- Lokuge K, Banks E, Davis S, Roberts L, Street T, O’Donovan D, Caleo G, Glass K. Exit strategies: optimising feasible surveillance for detection, elimination and ongoing prevention of COVID-19 community transmission. medRxiv. 2020. DOI: https://doi.org/10.1101/2020.04.19.20071217.
- Zhang F, Abudayyeh OO, Gootenberg JS. A protocol for detection of COVID-19 using CRISPR diagnostics. A protocol for detection of COVID-19 using CRISPR diagnostics. 2020; 8.
- Carbohydrate-based Diagnostics: A New Approach to COVID-19 Testing?, 2020. https://www.technologynetworks.com [ Accessed 29 April 2020]
- Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. European Radiology. 2020;1-9. DOI: 10.1007/s00330-020-06801-0. Epub ahead of print. PMID: 32193638; PMCID: PMC7088323.
- Zhang J, Wang S, Xue Y. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. Journal of Medical Virology. 2020;92(6):680-82. https://doi.org/10.1002/jmv.25742.
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.