ISSN: 0973-7510
E-ISSN: 2581-690X
Food preservation and safety is drawing more attention globally due to the increasing prevalence of food-borne diseases. The natural methods of food preservation are considered safer compared to methods using synthetic preservatives. The essential oils with natural preservative properties could be useful for food safety and preservation. The objective of this study was to analyze the chemical composition of commercially available Allium sativum and Trigonella foenum-graecum essential oils by gas chromatography-mass spectroscopy (GC-MS). The antimicrobial activities of Allium sativum and Trigonella foenum-graecum essential oils were determined by agar well diffusion technique. The GC-MS analysis of Garlic essential oil (GEO) revealed that, Allyl methyl trisulfide (13.10%), Di-allyl sulfide (9.47%) and Di-allyl tetrasulfide (4.38%) were the major components, while methanolic extract of Fenugreek essential oil (FEO) showed limonene (12.92%), Silane trimethylphenyl (10.71%), carvone (4.57%) and Trigolline (0.38%) as major components. The results of our study showed a significant antimicrobial activity of GEO and FEO against the tested microbial strains, which indicates the presence of broad-spectrum antimicrobial constituents in GEO and FEO. However, further studies are needed for individual bioactive components and safety aspects for their application in food preservation.
Bio-active components, Essential oils, GC-MS; Agar well assay; Antimicrobial activity.
The role of essential oils (EOs) in food preservation has been extensively studied due to the undesirable effects of synthetic preservatives used in food industries1. Furthermore, rise in food-borne diseases worldwide put people at the risk of health hazards, calling for more effective, safe and natural source of food preservation. To improve the food safety, one of the options in modern time is to study and investigate the antimicrobial activities of the bioactive compounds and use them in food industry. Antimicrobial properties of naturally occurring bioactive components restrict the use of chemical antimicrobial agents, which may possess a potential human health hazard2. The main bioactive components of EOs are mono and sesquiterpene, which are believed to be responsible for their biological activity; therefore, the identification of these bioactive components from various plant sources has become meaningful task. Gas chromatography (GC) or gas chromatography-mass spectrometry (GC-MS) is used exclusively for the qualitative analysis of the volatiles compounds3. Additionally, the antimicrobial properties of volatile components are known for decades and because of their potential microbial growth inhibition activity, these volatile components are being investigated as a substitute to synthetic chemical preservatives in food industries4. Since ancient times, herbs and spices are added in food and food products, not only as seasoning agent but also as a method of preservation5. Plant materials such as Garlic essential oil (GEO) and Fenugreek essential oil (FEO) with antibacterial properties could have a possible application in food preservation, for example, several studies have proved that garlic (Allium sativum) possesses a significant antimicrobial activity. Antimicrobial activity of garlic extracts has been reported against bacteria and fungi6. GEO have been reported to contain antibiotic, immunomodulatory, antioxidant, anti-inflammatory, cardiovascular-protecting and hypoglycemic effects7, 8. Garlic can be used as an effective source for food preservation and also as a natural herbal antibiotic9. Moreover, fenugreek seed (Trigonella foenum) oil has been known for their antimicrobial properties against food borne pathogens, thus it could be potentially useful in increasing shelf life of food products. FEO has been reported to have antimicrobial, anti-diabetic, anti-cancer, anti-fertility, and anti-parasitic activity10.
Thus the growing demand of natural ingredients like essential oils in food preservation appears as a viable and healthy alternative to synthetic preservatives. Based upon our literature survey, we did not find any reports on GC-MS analysis as well as antimicrobial activity of commercially available essential oils. Therefore, this work was designed to evaluate the chemical composition and antimicrobial activity of commercial sample (GEO and FEO) against various microbial pathogens.
Sample Collection
Garlic essential oil (GEO) and fenugreek essential oil (FEO) were procured from the local market of Ha’il, Kingdom of Saudi Arabia in December 2018. Selected samples were chosen based upon literature survey and their possible application in food industry. Quality of the oils was ascertained to be more than 98% pure.
Gas chromatography mass spectrometry (GC-MS)
GC-MS (Thermo Scientific, Triple quadropole MS, TSQ 8000) analysis were performed for the GEO and FEO using two fused silica capillary column TG-5MS, (30 m x 0.25 mm x 0.25µm). Moreover, temperature for detector and injector were fixed at 250°C as well as 220°C and helium (1 mL/min) as a carrier gas was employed for this study. Samples (1µL) dissolved in methanol were introduced into column which was initially fixed at 50°C for 1 min and concurrently raised to 280°C by slowly raising temperature of 5°C/min. Both the samples were run for 30 minutes and analysis of obtained chromatograms was performed. Identification and characterization of various compounds were made by comparing relative retention time (RT) and mass spectra of samples with reference standards by using National Institute of Standards and Technology (NIST) library database5.
Test Organisms
Micro-organism used in this study was procured from ATCC (American Type Culture Collection from LGC Promochem, Banglore INDIA as well as MTCC strains were procured from Institute of Microbial Technology (IMTECH), Chandigarh, INDIA. Fungal strains used in this study was Aspergillus niger (MTCC 2196), Penicillium pinophilium (MTCC 2192), Candida albicans (ATCC 10231), Aspergillus flavus (MTCC 2798), and Saccharomyces cerevisiae (MTCC 786). However, bacterial strains selected for this study was Rhodococus equi (ATCC 6939), Listeria innocua (ATCC 33090), Listeria monocytogenes (ATCC 19111), Vibrio parahaemolyticus (ATCC 17802), Enteococcus hirae (ATCC10541), Escherichia coli (ATCC 15597), Cronobacter sakazakii (ATCC 29544), Listeria ivanovii (ATCC 19119), Bacillus cereus (MTCC 430), Shigella (MTCC 1457), Enteococcus faecalis (MTCC 439), Salmonella enterica (MTCC 733), Staphylococcus aureus (MTCC 96), Clostridium perfringens (MTCC 450), Vibrio cholera MTCC (3906), Enterobacter aerogenes (MTCC 111), Salmonella typhi (MTCC 733), Klebsiella pneumonia (MTCC 109), Micrococcus luteus (MTCC 2470), Pseudomonas aeruginosa (MTCC 741) and Citrobacter freundii (MTCC 1658).
Culture medium and inoculum preparation
Pure test organisms of all the selected micro-organism (Bacteria and fungus) were sub-cultured onto fresh plates of Mueller-Hinton agar (Hi Media laboratories) for 24 h and Saboraud dextrose agar (Hi Media laboratories) for 5-7 days at 37°C for bacteria and fungi, respectively. All the test organisms were incubated as specified for each organism for a period of 18- 24 h11.
Agar well diffusion assay
Antimicrobial activity of GEO and FEO were analyzed by using agar well diffusion assay techniques5. Muller-Hinton Agar and Sabouraud Dextrose Agar plates were used for antibacterial and antifungal activity, respectively. 100µL standard bacterial and fungal inoculums were spread over the sterile plates and subsequently 8mm diameter wells were borer over the respective agar plates. Afterwards, 100µL GEO and FEO were filled into the Muller-Hinton agar and Sabouraud Dextrose agar plate wells and kept at room temperature for 1 hour for proper diffusion and incubated for 37C for 24 hours and 30 C for 3–5 days respectively12. All the samples were prepared in triplicates, essential oils having antimicrobial activity inhibited the microbial growth and the clear zones were formed. The zones of inhibition were measured in millimeters13.
Statistical analysis
All the experimental results were carried out in triplicates and expressed as mean ± SEM (Standard Error of Means) of three independent experiments (n = 3).
Characterization of chemical constituents using Gas Chromatography-Mass Spectrometry
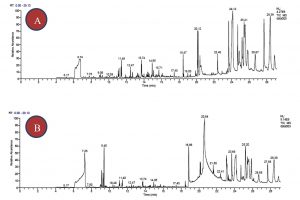
Identification of various chemical components present in GEO and FEO were determined by GC-MS. The full scan GC-MS chromatograms are presented in Fig. 1A and 1B. Based upon the GC-MS chromatogram analysis, major components identified are presented in table 1. The identification of bioactive components are determined by comparing the chromatogram peak obtained in our samples with reference peaks as mentioned in National Institute of Standards and Technology (NIST) Library5. Furthermore, reference peaks were directly compared with the retention time and mass spectral data obtained in GEO and FEO sample chromatogram. According to GEO chromatogram (figure 1A), nineteen distinctive peaks were analyzed by GC-MS. Moreover, the major bioactive components analyzed by GC-MS were found to be Allyl methyl trisulfide (13.10%), Diallyl sulfide (9.47%), c-Sitosterol (6.15%), Diallyl tetrasulfide (4.38%) and Allyl methyl disulfide (3.40).. The results of the current study were comparable to previous studies14-16. Previously, allicin degradation has been proved responsible for the presence of sulphide compounds in oils. Moreover, formation of allicin in garlic occurs due to release of allinase enzyme after crushing garlic bulb. Subsequently, allicin is converted into alliin and because allicin is very unstable compound, it suddenly undergo reactions to form sulphur derivative components17. The GC-MS analysis of Chinese commercial GEO sample, which reports diallyl disulphide (45.1–63.2%) as highest components compared to other diallyl sulfide (4.5–11.4%), and diallyl tetrasulfide (6.3–10.5%)18. However, our results showed that GEO sample had highest amount of Allyl methyl trisulphide followed by diallyl sulphide and diallyl tetra sulphide. The differences obtained in sulphide content could be due the geographical as well as the process of distillation to obtain the essential oils.
Fig. 1. GC-MS chromatogram of the bioactive compounds present in (A) Garlic essential oil and (B) Fenugreek essential oil.
On the other hand, FEO chromatogram showed seventeen distinctive peaks analyzed by GC-MS and all the major components identified are presented in table 1. The major components present in the fenugreek EO were identified as limonene (12.92%), c-Sitosterol (9.58%), Carvone (4.57%), Campesterol (3.60%), Stigmasterol (2.83%), Cedrane-8-propoxy (1.50%) and Trigolline (0.38%). The previous scientific reports revealed that, stolones- furanones are the principle volatile compounds present in fenugreek oils19. The bioactive component sigmasterol present in FO have been reported to decrease blood cholesterol level20.
Table (1):
Garlic and fenugreek essential oil compositionobtained bygas chromatography mass spectrophotometry.
| RT | Compound Name | Molecular Formula | Area % | Identification |
|---|---|---|---|---|
| Garlic Essential Oil (GEO) | ||||
| 7.25 | Benzyl alcohol | C7H8O | 5.79 | MS, RI |
| 9.13 | l-Menthone | C10H18O | 1.82 | MS, RI |
| 9.29 | p-Menthan-3-one | C10H18O | 1.63 | MS, RI |
| 9.67 | a-Terpineol | C10H18O | 0.19 | MS, RI |
| 10.40 | Pulegone | C10H16O | 0.26 | MS, RI |
| 28.38 | c-Sitosterol | C29H50O | 6.15 | MS, RI |
| 9.40 | Diallyl tetrasulfide | C10H20O | 4.38 | MS, RI |
| 11.12 | Menthyl acetate | C12H22O2 | 1.05 | MS, RI |
| 11.45 | 1-Triethylsilyloxyheptadecane | C23H50OSi | 0.99 | MS, RI |
| 12.70 | 2,4,7,9-Tetramethyl5decyn4,7diol | C14H26O2 | 0.19 | MS, RI |
| 15.71 | 1-Monolinoleoylglycerol Tri-methylsilyl ether | C27H54O4
Si2 |
0.33 | MS, RI |
| 18.47 | Hexadecanoic acid, methylester | C17H34O2 | 0.43 | MS, RI |
| 18.86 | l-(+)-Ascorbic acid 2,6-dihexadecanoate | C38H68O8 | 5.66 | MS, RI |
| 20.60 | Allyl methyl tri-sulfide | C18H32O2 | 13.10 | MS, RI |
| 23.09 | Oleic acid, 3-hydroxypropylester | C21H40O3 | 5.61 | MS, RI |
| 23.66 | Allyl methyl disulfide | C19H38O4 | 3.40 | MS, RI |
| 24.05 | 2,6-Bis(3,4methylenedioxyphenyl)-3,7-dioxabicyclo (3.3.0)octane | C20H18O6 | 5.16 | MS, RI |
| 24.13 | 2,6-Bis(3,4-methylenedioxyphenyl)-3,7-dioxabicyclo(3.3.0)octane | C20H18O6 | 4.12 | MS, RI |
| 25.20 | Diallyl sulfide | C21H40O4 | 9.47 | MS, RI |
| Fenugreek Essential Oil (FEO) | ||||
| 12.70 | 2,4,7,9-Tetramethyl-5-decyn-4,7-diol | C14H26O2 | 0.35 | MS, RI |
| 13.27 | Dimethyl phthalate | C10H10O4 | 0.44 | MS, RI |
| 13.43 | n-Cetyl alcohol | C16H34O | 0.33 | MS, RI |
| 18.47 | Hexadecanoic acid, methyl ester | C17H34O2 | 1.19 | MS, RI |
| 19.29 | 5,8,11-Heptadecatriynoic acid, methyl ester | C18H24O2 | 0.28 | MS, RI |
| 19.88 | Trigolline | C28H58O9 | 0.38 | MS, RI |
| 20.39 | Methyl stearate | C19H38O2 | 1.64 | MS, RI |
| 20.49 | Cedryl propyl ether | C18H32O | 1.50 | MS, RI |
| 22.90 | 1-Monolinoleoylglycerol trimethylsilyl ether | C27H54O4 Si2 | 0.29 | MS, RI |
| 23.66 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | C19H38O4 | 4.04 | MS, RI |
| 24.12 | Limonene | C20H18O6 | 12.92 | MS, RI |
| 24.68 | Nonaethylene glycol | C18H38O10 | 3.57 | MS, RI |
| 25.83 | Campesterol | C28H48O | 3.60 | MS, RI |
| 26.67 | Stigmasterol | C29H48O | 2.83 | MS, RI |
| 27.68 | Carvone | C18H38O10 | 4.57 | MS, RI |
| 28.39 | c-Sitosterol | C29H50O | 9.58 | MS, RI |
Antimicrobial activity of essential oils
Both Garlic and Fenugreek essentials oils were investigated for in-vitro antimicrobial activity and both GEO and FEO showed positive antibacterial activity against Gram-negative, Gram-positive bacteria and few fungal strains tested in this study21. Antimicrobial activities of tested microorganisms are presented in Table 2 & Table 3. GEO showed antimicrobial activity against all the tested strains. Among the fungal strains, Aspergillus flavus showed the maximum inhibition zone (18.3 ± 0.29) followed by Candida albicans (18.1 ± 0.41). Saccharomyces cerevisiae showed least zone of inhibition (12.5 ± 0.29). Clotrimazole (50µg/ml) were tested against the stated fungal strains and their inhibition zone found to be in the range of 26.4 ± 0.42 – 35.2 ± 0.47. Micrococcus luteus showed the highest sensitivity (20.8 ± 0.28) followed by Escherichia coli, Bacillus cereus, Enteococcus hirae and Listeria monocytogenes were the least sensitive (5.2 ± 0.21) to GEO. Tetracycline (50µg/ml) antibacterial activity varied from (9.6 ± 0.48 – 31.1 ± 0.38). Clotrimazole and tetracycline antimicrobial standards were compared with GEO, which showed t GEO had a broad antimicrobial potential. The antibacterial activity of GEO has been attributed to the presence of allicin21. Additionally, allicin contains thiosulfnate group (-S(O)-S- group) found in the GEO extract is proved to possess antimicrobial properties22. One of the previous studies have revealed that, SH group of cellular proteins react with -S(O)-S- group, for the production of mixed disulfides23. Furthermore, the antimicrobial activity of GEO has been reported mainly because of the presence of organosulfur compounds such as allicin, ajoene and diallyl sulfides24. In addition, Mousumi & Prabir also reported that, garlic extracts possesses a very strong antibacterial activity against Staphylococcus aureus, Escherichia coli 25. In addition to that, garlic juice is also reported to shown antibacterial activity against, Escherichia coli and Staphylococcus aureus26,27. Yin et al., reported that, Salmonella typhimurium growth in ground beef were effectively inhibited by GEO derived organosulfur compounds28. Antimicrobial activity of FEO showed poor results against the tested microorganism, when compared with standard clotrimazole and tetracyclines (50µ/ml). Zone of inhibition for both the standards varied from (26.4 ± 0.42- 35.2±0.47) and (9.6 ± 0.48 – 31.1 ± 0.38) respectively. Among the fungal strains, Aspergillus flavus had maximum zone of inhibition (6.1± 0.62). Moreover, least zone of inhibition was found for Penicillium pinophilium (3.1 ± 0.17). Bacterial strains had similar results varying from (1.5 ± 0.06 – 6.8 ± 0.26). The antimicrobial activity of GEO and FEO could be due to the collective effect of tannins, phenolic compounds, flavonoids, alkaloids, and terpenoids present in oil29.
Table (2):
Antifungal activity of essential oils using Agar diffusion method.
| Micro-organism | Zone of Inhibition in diameter (mm) | ||
|---|---|---|---|
| GEO | FEO | Clotrimazole (50 µg/ml) | |
| Aspergillus niger | 16.1 ± 0.43 | 4.9 ± 0.26 | 28.2 ± 0.37 |
| Aspergillus flavus | 18.3 ± 0.29 | 6 .1± 0.62 | 26.4 ± 0.42 |
| Candida albicans | 18.1 ± 0.41 | 8.1 ± 0.56 | 27.1 ± 0.33 |
| Penicillium pinophilium | 14.6 ± 0.13 | 3.1 ± 0.17 | 37.5 ± 0.19 |
| Saccharomyces cerevisiae | 12.5 ± 0.29 | 5.2 ± 0.08 | 35.2 ± 0.47 |
Values are expressed as Mean ± SEM (Standard Error of Means)
Table (3):
Antibacterial activity of essential oils using Agar diffusion method.
| Micro-organism | Zone of Inhibition in diameter (mm) | ||
|---|---|---|---|
| GEO | FEO | Tetracycline (50 µg/ml) | |
| Rhodococusequi | 13.2 ± 0.18 | 3.0 ± 0.41 | 22.3 ± 0.31 |
| Bacillus cereus | 18.1 ± 0.31 | 6.8 ± 0.26 | 31.1 ± 0.38 |
| Enteococcus faecalis | 9.7 ± 0.53 | 5.2 ± 0.13 | 24.7 ± 0.27 |
| Staphylococcus aureus | 15.4 ± 0.19 | 4.2 ± 0.08 | 27.1 ± 0.32 |
| Listeria monocytogenes | 5.2 ± 0.21 | 1.5 ± 0.06 | 19.8 ± 0.22 |
| Escherichia coli | 18.5 ± 0.28 | 5.1 ± 0.24 | 21.6 ± 0.39 |
| Enterobacter aerogenes | 17.3 ± 0.41 | 4.0 ± 0.08 | 25.2 ± 0.41 |
| Cronobactersakazakii | 10.3 ± 0.61 | 3.6 ± 0.23 | 22.6 ± 0.37 |
| Klebsiella pneumonia | 8.7 ± 0.46 | 3.7± 0.0 | 18.8 ± 0.26 |
| Pseudomonas aeruginosa | 13.5 ± 0.39 | 3.7 ± 0.14 | 19.4 ± 0.32 |
| Citrobacter freundii | 11.7 ± 0.32 | 4.0 ± 0.00 | 23.4 ± 0.41 |
| Clostridium perfringens | 4.5 ± 0.35 | 4.0 ± 0.45 | 20.7 ± 0.26 |
| Micrococcus luteus | 20.8 ± 0.28 | 5.0 ± 0.37 | 29.5 ± 0.53 |
| Salmonella typhi | 14.6 ± 0.29 | 3.5 ± 0.36 | 18.3 ± 0.28 |
| Vibrio parahaemolyticus | 5.1 ± 0.25 | 3.9 ± 0.09 | NT |
| Vibrio cholerae | 5.7 ± 0.24 | 6.1 ± 0.34 | NT |
| Salmonella enterica | 12.7 ± 0.23 | 2.5 ± 0.08 | 18.2 ± 0.31 |
| Shigella | 10.6 ± 0.67 | 2.0 ± 0.00 | 9.6 ± 0.48 |
| Enteococcushirae | 18.5 ± 0.41 | 4.3 ± 0.19 | 19.8 ± 0.36 |
| Listeria ivanovii | 5.6 ± 0.35 | 1.6 ± 0.29 | NT |
| Listeria innocua | 6.3 ± 0.24 | 1.8 ± 0.08 | NT |
Values are expressed as Mean ± SEM (Standard Error of Means), NT; Not Tested
The antimicrobial activity against the broad range of tested microorganism indicates the presence of wide spectrum of antimicrobial compounds in Garlic and Fenugreek essential oils. Additionally, antimicrobial components showed by GC-MS/MS analysis could be a good source of food preservation rendering the growth of microbes. In conclusion, it is suggested that these plant-derived products could be valuable to find out natural bioactive compounds. More importantly, these can be incorporated in the list of food preservation system due to their antimicrobial activity and lesser side effects. Hence, essential oils and their chemical components can be recommended for food preservation.
ACKNOWLEDGMENTS
We are grateful to the Department of Clinical Nutrition, College of Applied Medical Sciences, Hail University and Department of Food technology, Hamdard University, New Delhi, and for encouragement and providing facilities to carrying out the present study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript and/or the Supplementary Files.
- Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front. Microbiol., 2012; 25(3): 1-24.
Crossref - Djenane D, Yanguela J, Roncales P, Aider M. Use of essential oils as natural food Preservatives: Effect on the growth of Salmonella Enteritidis in liquid whole eggs stored under abuse refrigerated conditions. J. Food. Res., 2013; 2(3): 65-78.
Crossref - Zhao CX, Liang YZ, Fang HZ, Li XN. Temperature-programmed retention indices for gas chromatography-mass spectroscopy analysis of plant essential oils. J. Chromatogr. A., 2005; 25; 1096(1-2): 76-85.
Crossref - Ebrahimi M, Khosravi-Darani K. Essential oils as natural food preservatives: Antimicrobial and antioxidant applications.7 Antimicrobials from Nature: Effective Control Agents for Drug Resistant Pathogens, Edition: first, Chapter: 1. Publisher: Transworld Research Network, Editors: James Hamuel Doughari, 2013; p.15-37.
- Ashraf SA, Al-Shammari E, Hussain T, Tajuddin S, Panda BP. In-vitro antimicrobial activity and identification of bioactive components using GC-MS of commercially available essential oils in Saudi Arabia. J. Food Sci. Technol., 2017, 54: 3948–3958.
- Dash BK, Sultana S, Sultana N. Antibacterial activities of methanol and acetone extracts of Fenugreek (Trigonella foenum) and Coriander (Coriandrum sativum). Life Sciences and Medicine Research, 2011; 27: 1-8.
- Wallock-Richards D, Doherty CJ, Doherty L, Clarke DJ, Place M. Garlic Revisited: Antimicrobial Activity of Allicin- Containing Garlic Extracts against Burkholderia cepacia Complex. PLoS ONE, 2014; 9(12): e112726.
Crossref - Anees AM, Ravi S, Ghogare P. Studies on antimicrobial activity of spices and effect of temperature and pH on its antimicrobial properties. IOSR Journal of Pharmacy and Biological Sciences, 2015; 10(1): 99-102.
- Iwalokun BA, Ogunledun A, Ogbolu DO, Bamiro SB, Jimi-Omojola J. In-vitro antimicrobial properties of aqueous garlic extracts against multidrug-resistant bacteria and candida Species from Nigeria. J. Med. Food., 2004; 7(3): 327–333.
Crossref - El-Nour MEM, Ali MAA, Saeed BEAE. Antimicrobial activities and phytochemical screening of callus and seeds extracts of Fenugreek (Trigonella foenum-graecum). Int. J. Curr. Microbiol. App. Sci., 2015; 4(2): 147-157.
- Ramya P, Sudisha J, Devi NL, Aradhya SM, Antibacterial and anti-oxidant activities of fenugreek (Trigonella foenum graceum L.) leaves. Res. J. Med. Plant., 2011; 5(6): 695-705.
Crossref - Balouiri M Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal., 2016; 6: 71–79. https://doi.org/10.1016/j.jpha.2015.11.005
- Burt S. Essential oils: their antibacterial properties and potential applications in foods –a review. Int. J. Food Microbiol., 2004; 1; 94(3): 223–253.
Crossref - Reeves DS. Antibiotic assays. Hawkey PM, Lewis DA(Eds.) Medical bacteriology, A Practical approach. IRL Press, Oxford. 1989; 195-221.
- Raid AA, Yazeed AS, Ayesha M, Rabbani SK, Janardhan C, Gupta VC. Evaluation of antibacterial activity of crude protein extracts from seeds of six different medical plants against standard bacterial strains. Saudi. J. Biol. Sci., 2014; 21(2): 147–151.
Crossref - Kim JW, Huh JE, Kyung KH. Antimicrobial activity of alk(en)yl sulfides found in essential oils of garlic and onion. Food Sci. Biotechnol., 2004; 13(2): 235–239.
- Banerjee S, Mukherjee K, Maulik S. Garlic as an antioxidant: The good, the bad and the ugly. Phytother. Res., 2003; 17(2): 97–106.
Crossref - Satyal P, Craft JD, Dosoky NS, Setzer WN. The chemical compositions of the volatile oils of Garlic (Allium sativum) and Wild Garlic (Allium vineale). Foods, 2017; 6(63).
Crossref - Gupta R, Sharma A, Maina P, Shukla RN. Study of chemical composition of garlic oil and comparative analysis of co-trimazole in response to in-vitro antibacterial activity. Int. Res. J. Pharm. 2014; 5(2): 97-101.
Crossref - Bano DH, Tabassum A, Ahmad A, Mabood, Ahamd IZ. The medicinal significance of the bioactive compounds of trigonella foenum-graceum: A review. Int. J. Res. Ayurvadic Pharm., 2016; 7(4): 84-91.
Crossref - Sohrevardi N, Sohrevardi F. Essential oil composition and antioxidant activity of Trigonella foenum graecum L. plant. Int. J. Agri. Crop Sci., 2012; 4(12): 793-797.
- Babu JA, Sundari AR, Indumathi J, Srujan RVN, Sravanthi M. Study on the antimicrobial activity and minimum inhibitory concentration of essential oils of spices. Vet. World., 2011; 4(7): 311-316.
Crossref - Leontiev R, Hohaus, N, Jacob C, Martin CH, Gruhlke, Alan J, Slusarenko. A comparison of the antibacterial and antifungal activities of thiosulfnate analogues of allicin. Sci. Rep., 2018; 8: 6763.
Crossref - Kyung KH. Antimicrobial properties of allium species. Curr. Opin. Biotechnol., 2012; 23(2): 142–147.
Crossref - Chekki RZ, Ahmad S, Imen H, Nabiha B. Chemical composition, antibacterial and antioxidant activities of Tunisian garlic (Allium sativum) essential oil and ethanol extract. Mediterr. J. Chem., 2014; 3(4): 947-956.
Crossref - Mousumi B, Sarkar PK. Inhibitory effect of garlic on bacterial pathogens from spices. World J. Microbiol. Biotechnol., 2003; 19(6): 565-569.
Crossref - Al-Waili NS, Saloom KY, Akmal M, Al-waili TN, Al-Waili AN, Al-Waili H, Ali A, A-Sahlani K. Effects of heating, storage and ultraviolet exposure on antimicrobial activity of garlic juice. J. Medicinal Food, 2007; 10(1): 208-212.
Crossref - Alluri N, Majumdar M. Phytochemical analysis and in vitro antimicrobial activity of Calotropis gigantea, Lawsonia inermis and Trigonella foecum-graecum. Int. J. Pharm. Pharm. Sci., 2014. 6(4): 524-527.
- Moniruzzaman, Shahinuzzaman, Haque A, Khatun R, Yaakob Z. Gas chromatography- mass spectrometry analysis and in vitro antibacterial activity of essential oil from Trigonella foenum-graecum. Asian Pac. J. Trop. Biomed., 2015; 5(12): 1033–1036.
Crossref
© The Author(s) 2019. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.