ISSN: 0973-7510
E-ISSN: 2581-690X
The present study was focusing on qualitative and quantification of bioactive compounds present in Aspergillus terreus and evaluating its anticancer activity and apoptosis detection against lung cancer. Methods: A. terreus was sequentially extracted using the Soxhlet extraction technique with hexane, ethyl acetate, methanol and distilled water. Detection of bioactive compounds was done using Standard biochemical tests and GC-MS analysis was performed with NIST database to identify the bioactive compounds. The toxicity and anticancer activity of crude extract was investigated using MTT assay on L929 cells and lung cancer A549 cells whereas apoptosis study was conducted through Flowcytometry-based surface marker study on the A549 cancer cell line. Results: secondary metabolites analysis showed the presence of phenols and terpenoids as major constituents in the methanol extract whereas other solvent extracts have shown the absence of major bioactive compounds. Quantification studies showed that methanol extract has shown the phenolic content 179 µg/g of Gallic acid equivalent. The GC-MS analysis showed the presence of 1-Flurodecane, Methyl palmitate, Ethyl palmitate, 9, 12-Octadecanopic acid, 10-Octadecanoic acid, Methyl stearate, Octadecadeoinoate, Ethyl 9-hexadecanoate and 1-Monoarachidin as major bioactive compounds. Further, MTT based toxicity study on the L929 cell line revealed that methanol extract at lower concentrations like 50µg, 100µg and150µg shown more than 50% of cell viability and at higher concentration between 200µg-250 µg it was showing toxic nature with 47.89±0.01% viability. In case of anticancer activity against lung cancer A549 cell line the methanol extract have shown the dose dependent activity i.e the percentage of cell viability was decreased with increase in the concentration of methanol extract at 250µg the cell viability was found to be 35.12±0.005%. Flow cytometry based apoptosis study revealed that methanol extract has shown the inducing apoptosis in treated lung cancer A549 cells with percentage of 10.84. Conclusion: overall the present study shown that A. terreus possess different class of bioactive compounds and it has higher phenolic content. Toxicity study showed that methanol extract exhibited toxic nature at higher concentration on tested cell line and Anticancer and Apoptosis study revealed that methanol extract has shown the prominent with inhibiting the growth of lung cancer A549 cells through inducing apoptosis. Further, A. terreus would be a promising natural microorganism that has to be further researched in order to discover and isolate potent drug to treat cancer. Future studies will be on study of in-vivo animal studies and study of molecular mechanism of drug action on particular with anticancer study.
A. terreus, Lung Cancer A549, MTT, Flow Cytometry, Apoptosis
Cancer has become the most hazardous issue related to health problems in everyone, regardless of age, gender, ethnicity, wealth, or socioeconomic status. As a result, it is without a doubt the biggest challenge facing the medical system and the scientific community in the twenty-first century. About 10 million people die from cancer each year, making it the second biggest cause of death in the world, and this number is continually rising.1 The main cancer treatment involves a number of therapeutic techniques, such as surgery, chemotherapy, and/or radiation.2 Lung carcinoma, often known as lung cancer, affects both men and women. It is recognized as the leading cause of death in the globe. Some of the treatment options for breast cancer include surgery, radiation therapy, hormone therapy, chemotherapy, and targeted therapy. These medications do have some serious negative effects, though.3 Traditional foundational elements of the healthcare system have been natural substances. Approximately 80% of the population has utilized alternative medicines for primary healthcare, according to research.4 The chemotherapeutic drugs used today to treat cancer give patient’s temporary relief and lengthen their lives, but they also have drawbacks like side effects, lack of selectivity, and high prices that not only lower patients’ quality of life but are also out of reach for millions of patients in developing countries.5 In the past, natural products have achieved tremendous advancements in the field of anti-cancer research; more than 60% of anti-cancer medications used in clinical trials now come from natural sources, such as plants and microorganisms.6
Natural products produced by microorganisms offer a wide range of potential novel medicinal compounds. Microbial metabolites can be utilized as targets for the discovery and development of novel medications, primarily anticancer and antibiotics, according to pertinent reviews.7,8 Some of the most amazing chemical factories currently known to science are found in filamentous fungi like Aspergillus, Penicillium, and Talaromyces. In light of this, a variety of bioactives, including mycotoxins, antifungal, and anticancer compounds, have been described in the literature over the past more than 100 years.9 A variety of anticancer chemicals have been produced by marine fungi, which are a significant source of bioactive molecules.10 The decomposition of substrates and animal remains, for example, is one way that marine fungus, which can be found in many habitats, contribute significantly to marine environments. Due to the evolution of fungal cell biology and feeding mechanisms, marine fungi are categorized as saprotrophs, parasites, or symbionts (epiphytic or endophytic).11-13
A typical fungus utilized extensively in the chemical and pharmaceutical sectors is Aspergillus terreus. It serves as the primary strain for making the significant chemical intermediary itaconic acid.14 Mevinolin, a key cholesterol-lowering medication first discovered from A. terreus, is still manufactured commercially by A. terreus through submerged fermentation.15 Additionally, it has been demonstrated that A. terreus from a particular eco-environment is a rich source of bioactive natural compounds.16 17 There are very less experimental reports were available on the anticancer studies on the A. terreus hence with this background in the present study A. terreus was undertaken to study qualitative and quantitative analysis bioactive compounds present in it along with the anticancer and apoptosis study against lung cancer A549 cell line using in-vitro assays.
Crude extraction
After the mass production the around 50 g dried biomass of Aspergillus terreus was subjected to the process of serial extraction using soxhlet apparatus for 6-12hrs by selecting different solvents system based on polarity i.e hexane, ethyl acetate, methanol and distilled water. After the extraction each extract was concentrated by rotary vacuum evaporator and evaporated to dryness.
Detection of secondary metabolites
In the present study the different solvent extracts of A. terreus were subjected for screening of secondary metabolites based on standard biochemical tests for tannins, flavonoids, steroids, anthocyanin, alkaloids, terpenoids, glycosides, quinones, cardiac glycosides, coumarins, phlobatannins, anthraquinone, or phenols.18, 19
Estimation of total phenolic content
Through the use of Spectrophotometry, the total phenolic content of the Aspergillus terreus methanol extract was determined.20 The crude methanol extract was made by combining 0.5 ml (with stock concentration of 1mg/ml) of dissolved with 2.5 ml of distilled water that had been dissolved in 10% FCR and 2.5 ml of 7.5 percent Na2CO3. Further test sample was incubated for 45min in dark conditions at room temperature. After the incubation the absorbance was measured at 730nm with use of spectrometer. Concurrently, 0.5 ml of methanol was used to prepare blank in a similar manner, but without extract. Gallic acid’s calibration curve was created in the 20–100mg/ml range. Finally, the amount of phenolics was reported as mg of gallic acid per gram of dry weight.
In-vitro toxicity and anticancer activity of methanol extract of A. terreus against L929 cell line and lung cancer A549 cell line
In the present study toxicity as well as anticancer activity was performed using the MTT standard technique. In the current investigation, Methanol extract of A. terreus was tested on normal l929 cell line and lung cancer A549 cell line at different concentrations (50, 100, 150, 200, and 250 µg/ml). Untreated cells are taken as a negative control, whereas cisplatin-treated cells are used as a positive control. The selected cell lines were plated for 24 hours; the cells were maintained in serum-free media for 24 hours. After the growth the spent media was removed and replaced with different concentrations of methanol extract of A. terreus with media were added and incubated for 24 hours. After incubation the treated cells were subjected to MTT treatment for 3 hours later medium was taken out and 200 µl of DMSO was added. Further, the amount of formazan produced at 595 nm was measured using an Elisa reader after the. Based on optical density (O.D) readings, the percentage mortality for each exposure level and control groups was calculated. The following equation was used to calculate the IC50 value based on the percentage of cells that survived.21
GC-MS profiling
The methanol extract of Aspergillus terreus was further subjected for the identification of probable compounds based on Gas Chromatography with Mass spectral analysis. In the present study GCMS of model GCMS-QP2010S was used for analysis. The methanol extract underwent for separation in fused silica type of closed column with helium gas as a mobile phase. The 1 µl aliquot of the extract was injected into the equipment with specifications of Linear Velocity, Pressure:65.0 kPa, Total Flow:24.0 mL/min, Column Flow:1.00 mL/min, Linear Velocity:36.8 cm/sec, Purge Flow:3.0 mL/min, and Final Temperature adjusted to 280°C and run for 6 min., the initial temperature of the column was set at 80°C, while the injector temperature was set at 260°C Based on a comparison of the components’ mass spectra with those in the NIST mass spectral database, the components were identified.22
Apoptosis study by Flowcytometry
The pulmonary lung cancer A549 cells were plated in a 6-well flat bottom microplate with cover slips and incubated in CO2 incubator at 37°C for 24 hours. After the attaining cells confluence the used media was replaced with fresh media with known concentration (204.16µg) of methanol extract of A. terreus and incubated for 24 hours. Cells were given two PBS washes after the incubation. 500 x g centrifuged for 5 minutes at 4°C. Re-suspend the cell pellets at 1 x 106 per mL in ice-cold 1X Binding Buffer after discarding the supernatant. Maintain tubes on ice. Then carefully mix in 2 L of PI and 5 L of AbFlour 488 Annexin V. Incubate tubes for 15 minutes in the dark while keeping them on ice. Gently stir in 400 L of ice-cold 1X binding buffer. Analyze cell preparations using flow cytometry within 30 minutes. FlowJo X 10.0.7 software was then used for analysis.23
Statistical analysis
In the current study, the experiments executed in triplicates (n=3) and after the performance and analysis the results were quoted as the mean ± standard deviation or standard error.
In 2020, there are projected to be 18.1 million cancer cases worldwide, based on the report of Global Cancer Statistics it showed that in case of men and women approximate 9.3 million and 8.8 million respectively cancer incidents were identified.24 In 2020, 12.5 percent and 12.2 percent of all new cases of cancer were diagnosed as breast and lung cancer, respectively. With 1.9 million new cases, colorectal cancer was the third most prevalent cancer in 2020, making up 10.7% of all new cases.25 In 2020, 15.4% of all new cases of malignancy in males will be lung cancer, making it the most incident cancer type in men worldwide. Resistance to chemotherapy and reoccurring illnesses are also significant hindrances. To improve the effectiveness of treatment, new therapeutic approaches or anticancer drugs may be considered. To create anticancer drugs, a variety of plants, marine life, and microbes are used as sources. It is well recognized that bacterial and fungal metabolites are important sources of bioactive substances in microorganisms. However, there are currently not many anticancer medications made from microbial metabolites.
According to research, phytochemicals have anticancer properties, and many of them are being employed to treat this well-known illness.26-28 Recently several research findings revealed that phytochemicals isolated from plant origin have been used as potent drugs to cure many disorders but at the same time it is associated with several practical difficulties working with plants such as slow growth, abnormal habitat with less percentage of reproducibility of targeted desired phytochemcials.29,30 Natural substances are a well-known significant source of potent anticancer medicines that help us fight cancer. Plants, microorganisms, and marine species make up the majority of the principal sources. A variety of substances that are successfully employed as chemotherapy drugs, including daunorubicin, doxorubicin, actinomycin D, bleomycin, and mitomycin C, are mostly derived from bacteria.31 Numerous fungal metabolites are still being developed and tested in human therapeutic trials. For instance, the anti-angiogenesis agent fumagillin made from A. fumigates.32 It was discovered that phenylahostin, isolated from A. ustus, is cytotoxic to a number of cancer cell lines, including those from the breast, colon, lung, ovary, and leukaemia.33 It has been demonstrated that the mycotoxin anguidine, a member of the trichothecenes family and generated by a number of Fusarium species, prevents leukaemia cells from proliferating.34
Due to the production of primary metabolites that have beneficial products like enzymes, such as -amylase, amyloglucosidase, hemicellulase, and glucose, as well as citric acid, Aspergillus species are important economically.35-38 Itaconic acid, a co-polymer used in the manufacturing of paint, is produced by A. terreus. A. terreus has the ability to produce secondary metabolites that can be exploited to produce therapeutically useful products, such as statins, a medication used to decrease cholesterol.34,37
According to the accepted practice for biochemical testing for secondary metabolites, the phytochemical analysis for A. terreus in the current study was carried out. The results of the phytochemical study indicate that, among the various solvent extracts, the Aspergillus terreus methanol extract has demonstrated the presence of phenols and terpenoids as key phytochemical constituents. The outcomes are displayed in Table 1. Phenols in general have drawn a lot of attention due to their crucial physiological functions (such as hormones, aliphatic membrane anchors, maintaining membrane structure), ecological functions (such as defence compounds, insect/animal attractants), and their numerous uses in pharmaceutical and industrial applications, ranging from flavors and fragrances to disinfectants and lubricants.38,39 Triterpenoids, which are these substances’ primary bioactive components, have analgesic and anticancer effects.40
Table (1):
Phytochemical analysis of different solvent extracts of Aspergillus terreus.
Tests |
Hexane Extract |
Ethyl Acetate Extract |
Methanol Extract |
Water Extract |
|---|---|---|---|---|
Alkaloids |
-ve |
-ve |
-ve |
-ve |
Flavonoids |
-ve |
-ve |
-ve |
-ve |
Glycosides |
-ve |
-ve |
-ve |
-ve |
Phenols |
-ve |
-ve |
+ve |
-ve |
Saponins |
-ve |
-ve |
-ve |
-ve |
Tannins |
-ve |
-ve |
-ve |
-ve |
Terpenoids |
-ve |
-ve |
+ve |
-ve |
Steroids |
-ve |
-ve |
-ve |
-ve |
+ve: Present; –ve: absent
Based on phytochemical findings, the total phenolic content of the methanol extract has been quantified using standard Gallic acid calibration curve (Y=0.001X+0.113) and it was found to be 176 µg/g. Phenols have the nature of antioxidant and possess several applications such as fight against ageing, cancer, and other diseases of the heart.41 Applications of phenolic to enhance the nutritional content and quality of food are proliferating quickly in the food business.42
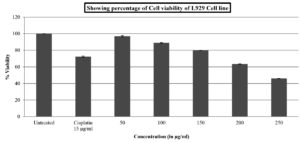
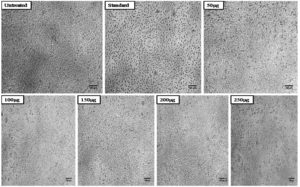
The methanol extract of A. terreus was tested for toxicity on the non-cancerous transformed fibroblast cell line L929 using the conventional MTT cell viability assay method before being examined for anticancer activities. In this test, the viability of the L929 cell line was assessed after exposure to methanol extract at various doses (50, 100, 150, 200, and 250µg/ml). The percentage of cell viability in the methanol extract-treated dropped as extract concentrations rose. Nearly higher concentrations of the extract have demonstrated its toxicity, with more than 50% of the cells being inhibited. Table 2 and Figure 1 present the findings. Figure 2 depicts the morphological impact of A. terreus methanol extract on the L929 cell line.
Table (2):
Cytotoxicity of methanol extract of A. terreus against non-cancerous L929 cell line.
Treatment |
Concentration in µg |
Percentage of cell Viability |
|---|---|---|
Methanol extract of A. terreus |
50 |
91.70±0.00 |
100 |
76.92±0.02 |
|
150 |
65.99±0.00 |
|
200 |
53.71±0.01 |
|
250 |
47.89±0.01 |
|
Standard drug |
15µg |
72.46±0.01 |
*Results are taken in triplicates and expressed as Mean±SE
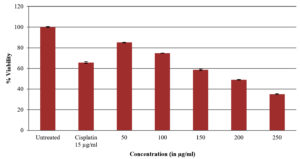
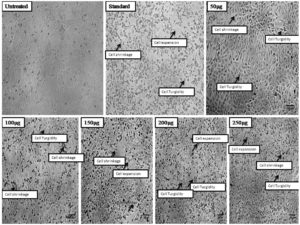
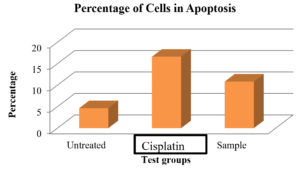
Despite considerable advancements in medical technology for its diagnosis and treatment, cancer is arguably the most deadly and progressing disease, posing a mortality danger to the entire planet. All cells are subject to oxidative stress; hence oxidation and free radicals generation leads to the incident of various tumor locations’ carcinogenesis.43 Both men and women can develop lung cancer, commonly known as lung carcinoma. It is regarded as the leading global cause of death. Lung cancer can be treated in a variety of ways, including surgery, radiation therapy, hormone therapy, chemotherapy, and targeted therapy, but each of these approaches has potentially severe adverse effects. Natural remedies have long been a cornerstone of medical care. Nearly 80% of the population, on average, uses natural medicine systems for primary healthcare, according to observations3. In the present study different concentrations of the methanol extract of A. terreus (50µg, 100µg, 150µg, 200µg and 250µg) were treated to the lung cancer A549 cell line. The cell viability results shown that as the concentration of test sample was increased there is gradual decrease in the percentage of cell viability. In the methanol extract treated A549 cells the percentage of viable cells in lung cancer was found to be 85.06% at 50µg concentration and at higher concentration i.e. 250µg it was found to be 35.12 and for standard drug cisplatin it was about 65.60 (Table 3 and Figure 3). It was evident from looking at the cell viability tested methanol extract shown appreciable results and demonstrated to be more efficient against lung cancer A549 cell lines. These findings even correlate with the morphological results and the MTT outcomes are correlated. Compared to untreated cells, the test samples of treated A549 have different cellular shape like elongated, ruptured and uneven shapes whereas untreated cells had a distinct spherical shape without any cellular spaces. Contrarily, in extract treated cells it was found dose dependant manner with formation of intra cellular spaces between the cells were seen (Figure 4) with several apoptotic characteristics such as cell expansion, cell shrinkage, membrane blabbing and cells turgidity. These were all characteristics of cells going through apoptosis. The test samples may be causing the lung cancer A549 cell line to undergo apoptosis, according to MTT and microscopic examination findings.
Table (3):
Percentage of cell viability in lung cancer A549 cells treated by methanol extract of Aspergillus terreus.
| Sample | Concentrations in µg | % of Cell viability | IC50 value in µg |
|---|---|---|---|
| Methanol extract of Aspergillus terreus | 50 | 85.06±0.0025 | 204.16 |
| 100 | 74.68±0.0015 | ||
| 150 | 58.70±0.011 | ||
| 200 | 48.98±0.0025 | ||
| 250 | 35.12±0.0055 | ||
| Standard drug Cisplatin | 15 | 65.60±0.014 | 11.43 |
The results are represented as mean± standard error
Figure 3. Percentage of cell viability of lung cancer A549 cells treated by methanol extract of Aspergillus terreus
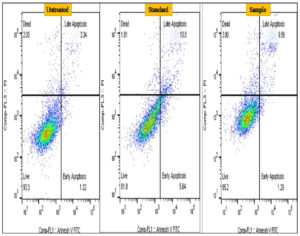
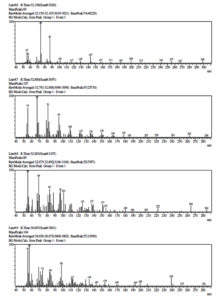
Apoptosis (planned cell death) is characterized by a number of different features such as release of protein phosphatidylserine through the plasma membrane, internucleosomal DNA breaking, membrane blabbing, chromatin condensation and fragmentation, and membrane blabbing.44 As a result, one of the best cancer treatment methods is triggering apoptosis.45 Phosphatidylserine (P.S.) on the plasma membranes outer layer functions as a marker site in causing the early stages of apoptosis.46,47 The membrane’s outer layer contains phosphatidyl-serine (P.S.) is specifically binds with the targeted protein like Annexin V.48 The process of phosphatidyl-serine translocation is ongoing. In the current work, A549 lung cancer cells that have undergone treatment were used to determine early and late apoptosis using flow cytometry. The findings of current work showed that untreated cells experienced a small percentage of apoptosis that may have been caused by natural mechanisms, whereas methanol extract treated A549 cells shown around 10.84% of cells with apoptosis and whereas for standard drug cisplatin it was found to be 16.64% (Figure 5 and Figure 6).
Figure 6. Detection of early and late apoptosis in lung cancer A549 cells treated by methanol extract of A. terreus
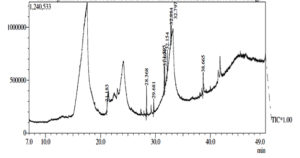
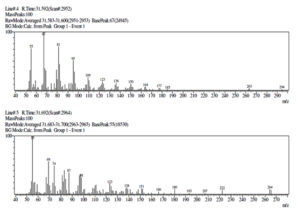
Gas chromatography (GC), one of the most used techniques in chromatography procedures, has emerged as one of the most crucial instruments for the separation of phytocompounds. Recently, GC-MS has solidified its position as a potent method for identifying secondary metabolites in both plant and non-plant species.49 In the current investigation, GC-MS analysis was used to determine the potential chemicals present in the methanol extract. The gas chromatogram has nine strong peaks, according to the GC-MS findings. To identify the substances, each peak will be put through a similarity search utilizing the NIST library database. 1-Flurodecane, Methyl palmitate, Ethyl palmitate, 9, 12-Octadecanopic acid, 10-Octadecanoic acid, Methyl stearate, Octadecadeoinoate, Ethyl 9-hexadecanoate, and 1-Monoarachidin have all been detected in a methanol extract of the A. terreus (Table 4 and Figure 7). These reported compounds are known to have a variety of uses, including use in the cosmetics sector, detergents, flavouring agents, and the pharmaceutical industry. According to Sermakkani and Thangapandian,50 9, 12-Octadecandionoic acid has anti-inflammatory, antimicrobial, hypocholesterolemic, and hepatoprotective effects.
Table (4):
GC-MS identified compounds present in Methanol extract of Aspergillus terreus.
Peak No |
Compound name |
Retention time |
Base m/z |
Nature |
Uses |
|---|---|---|---|---|---|
1 |
1-Flurodecane |
21.183 |
57.05 |
Aliphatic hydrocarbon |
Used in the organic synthesis |
2 |
Methyl palmitate |
28.368 |
74.05 |
Fatty acid methyl ester |
It was used in the soaps and detergents industry and also acts as anti-inflammatory and anti-fibrotic agent |
3 |
Ethyl palmitate |
29.681 |
88.05 |
Ethyl ester |
It is used to produce soaps, cosmetics, and industrial mold release agents. |
4 |
9, 12-Octadecanopic acid |
31.595 |
67.05 |
Fatty acid |
It is used for the treatment or prevention of cardiac arrhythmias. |
5 |
10-Octadecanoic acid |
31.694 |
55.05 |
Fatty acid |
It is used in hardening soaps, softening plastics and in making cosmetics, candles and plastics. |
6 |
Methyl stearate |
32.154 |
74.05 |
Fatty acid Methyl ester |
It is used as solubilizing agents and unfolding of proteins. |
7 |
Octadecadeoinoate |
32.797 |
67.05 |
Fatty acid |
Cosmetics |
8 |
Ethyl 9-hexadecanoate |
32.884 |
55.05 |
Ethyl ester |
It is used to produce soaps, cosmetics |
9 |
1-Monoarachidin |
38.665 |
57.10 |
Natural prenylated resveratrol |
Anticancer and Antioxidant |
Aspergillus terreus biomass was used in the current study, and it was extracted using various polar solvents to produce different extracts, which were then tested for the presence of secondary metabolites. Methanol extract contains phenols and terpenoids among the various solvent extracts. The presence of a greater phenolic content in the methanol extract is also confirmed by a quantification analysis. In-vitro toxicity research findings shown that methanol extract was proven to be less toxic at lower concentrations but at the range of the concentration of 200-250ug/ml it was showing toxicity on tested normal cell line L929. Anti-cancer research A549 lung cancer cell line MTT assay demonstrates that methanol extracts exhibits dose-dependent activity as the concentration is raised. Flow cytometry study clearly shows that methanol extract was inducing apoptosis in selected lung cancer A549 cell line. The GC-MS analysis showed the presence of probable bioactive compounds present in the methanol extract of A. terreus. Overall study concludes that in the future the A. terreus can be taken as natural microorganism for the isolating potent anticancer drug. Further, in future there is a need for additional research on the separation, purification, and structural elucidation of chemicals needed for a specific activity with a specific mechanism.
ACKNOWLEDGMENTS
The authors would like to thank P.G. Department of studies in Biotechnology and Microbiology, Karnataka University, Dharwad, Karnataka for providing facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Globocan. The global cancer observatory – all cancers. Int. Agent Res. Cancer – WHO. 2020;419:199-200.
- Subramaniam S, Selvaduray KR, Radhakrishnan AK. Bioactive compounds: natural defense against cancer? Biomolecules. 2019;9(12):758.
Crossref - Christopher GS, Jason WC, David HA, Jessie AS, White E. Lung Cancer and Hormone Replacement Therapy: Association in the Vitamins and Lifestyle Study. J Clin Oncol. 2010;28(9):1540-1546.
Crossref - Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177.
Crossref - Reis-Mendes A, Alves M, Valho FC, Remiao F, Bastos ML, Costa VM. Pixantrone, a new anticancer drug with the same old cardiac problems? An in vitro study with differentiated and non-differentiated H9c2 cells. Interdiscipl. Toxicol. 2018;11(1):13-21.
Crossref - Seelinger M, Popescu R, Giessrigl B, et al. Methanol extract of the ethnopharmaceutical remedy Smilax spinosa exhibits anti-neoplastic activity. Int J Oncol. 2012;41(3):1164-1172.
Crossref - Vederas JC, Li JWH. Drug discovery and natural products: end of an era oran endless frontier? Science. 2009;325(5937):161-165.
Crossref - Walsh CT, Fischbach MA. Natural products version 2.0: connecting genesto molecules. J Am Chem Soc. 2010;132(8):2469-2493.
Crossref - Frisvad JC, Smedsgaard J, Larsen TO, Samson RA. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Stud Mycol. 2004;49:201-241.
- Deshmukh SK, Prakash V, Ranjan N. Marine Fungi: A Source of Potential Anticancer Compounds. Front Microbiol. 2018;8:2536.
Crossref - Zuccaro A, Schoch CL, Spatafora JW, Kohlmeyer J, Draeger S, Mitchell JI. Detection and identification of fungi intimately associated with the brown seaweed Fucus serratus. Appl Environ Microbiol. 2008;74(4):931-941.
Crossref - Loque CP, Medeiros AO, Pellizzari FM, Oliveira EC, Rosa CA, Rosa LH. Fungal community associated with marine macroalgae from Antarctica. Polar Biol. 2010;33:641-648.
Crossref - Harvey JBJ, Goff LJ. Genetic covariation of the marine fungal symbiont Haloguignardia irritans (Ascomycota, Pezizomycotina) with its algal hosts Cystoseira and Halidrys (Phaeophyceae, Fucales) along the west coast of North America. Fungal Biol. 2010;114(1):82-95.
Crossref - Lai LST, Hung CS, Lo CC. Effects of lactose and glucose on production of itaconic acid and lovastatin by Aspergillus terreus ATCC 20542. J Biosci Bioeng. 2007;104(1):9-13.
Crossref - Lai LST, Pan CC, Tzeng BK. The influence of medium design on lovastatin production and pellet formation with a high-producing mutant of Aspergillus terreus in submerged cultures. Process Biochemistry. 2003;38(9):1317-1326.
Crossref - Ge HM, Peng H, Guo ZK, Cui JT, Song YC, Tan RX. Bioactive alkaloids from the plant endophytic fungus Aspergillus terreus. Planta Med. 2010;76(8):822-824.
Crossref - Gu W, Qiao C. Furandiones from an endophytic Aspergillus terreus residing in Malus halliana. Chem Pharm Bull. 2012;60(11):1474-1477.
Crossref - Harborne AJ. Phytochemical methods a guide to modern techniques of plant analysis. Springer science & business Media. 1998.
- Roghini R, Vijayalakshmi K. Phytochemical Screening, Quantitative Analysis of Flavonoids and Minerals in Ethanolic Extract of Citrus Paradisi. Int. J Pharm Sci Rev Res. 2018;11:4859-4864.
- Singleton VL, Orthofer R, Lamuela-Raventos R M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999;299:152-178.
Crossref - Van Meerloo J, Kaspers GJ, Cloos J. Cell sensitivity assays: the MTT assay. Methods Mol Biol. 2011;731:237-245.
Crossref - Masada Y. Analysis of essential oils by gas chromatography and mass spectrometry. John Wiley & Sons: Nashville. 1976.
- Moraes VW, Caires AC, Paredes-Gamero EJ, Rodrigues T. Organopalladium compound 7b targets mitochondrial thiols and induces caspase-dependent apoptosis in human myeloid leukemia cells. Cell Death Dis. 2013;4(6):e658.
Crossref - Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249.
Crossref - Pilleron S, Alqurini N, Ferlay J, et al. International trends in cancer incidence in middle-aged and older adults in 44 countries. J Geriatr Oncol. 2022;13(3):346-355.
- Patel S, Waghela B, Shah K, et al. Silibinin, A natural blend in polytherapy formulation for targeting Cd44v6 expressing colon cancer stem cells. Sci Rep. 2018;8(1):16985.
Crossref - Bhadresha KP, Jain NK, Rawal RM. Assessing the protective effect of Moringa Oleifera extract against bone metastasis: an in vitro simulated digestion approach. Nutr. Canc. 2022;74(3):1023-1036.
Crossref - Shah K, Mirza S, Desai U, Jain N, Rawal R. Synergism of curcumin and cytarabine in the down regulation of multi-drug resistance genes in acute myeloid leukemia. Anticancer Agents Med Chem. 2016;16(1):128-135.
Crossref - Kala CP, Dhyani PP, Sajwan BS. Developing the medicinal plants sector in northern India: challenges and opportunities. J Ethnobiol Ethnomed. 2006;2(1).
Crossref - Garcia-Oliveira P, Otero P, Pereira AG, et al. Status and challenges of plant anticancer compounds in cancer treatment. Pharmaceuticals. 2021;14(2):157.
Crossref - Demain AL, Vaishnav P. Natural products for cancer chemotherapy. Microb Biotechnol. 2011;4(6):687-99.
Crossref - Ingber D, Fujita T, Kishimoto S, et al. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumour growth. Nature. 1990;348(6301):555-557.
Crossref - Kornienko A, Evidente A, Vurro M, et al. Toward a cancer drug of fungal origin. Med Res Rev. 2015;35(5):937-967.
Crossref - Adler SS, Lowenbraun S, Birch B, Jarrell R, Garrard J. Anguidine: a broad phase II study of the Southeastern Cancer Study Group. Cancer Treat Rep. 1984;68:423-425.
- Deacon JW. Fungal biology. John Wiley & Sons. 2013.
- Junior DPL, Yamamoto ACA, Amadio JVRS, et al. Trichocomaceae: Biodiversity of Aspergillus spp and Penicillium spp Residing in Libraries. J Infect Dev. Ctries. 2012;6(10):734-743.
Crossref - Carlile MJ, Watkinson SC, Gooday GW. The Fungi. 2 ed. Academic Press, UK. P: 2001:462-510.
- Chase K, Zuodong J, Bell S, Chappell J. Metabolic Engineering of Higher Plants and Alage for Isoprenoid Production. Adv Biochem Eng Biotechnol. 2015;148:161-199.
Crossref - Schwab W, Davidovich-Rikanati R, Lewinsohn E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008;54(4):712-732.
Crossref - Ali SS, Kasoju N, Luthra A, et al. Indian medicinal herbs as sources of antioxidants. Food Res Int. 2008;41(1):1-15.
Crossref - Karimi E, Oskoueian E, Hendra R, Jaafar HZE. Evaluation of Crocus sativus L. Stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules. 2010;15(9):6244-6256.
Crossref - Aneta W, Jan O, Renate C. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105(3):940-949.
Crossref - Sies H. Oxidative stress: oxidants and antioxidants. Exp Physiol. 1997;82(2):291-295.
Crossref - Talib WH, Mahasneh AM. Antiproliferative activity of plant extracts used against cancer in traditional medicine. Sci Pharm. 2010;78(1):33-46.
Crossref - Reed JC. Apoptosis-based therapies. Nat Rev Drug Discov. 2002;1(2):111-121.
Crossref - Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol. 2007;9(12):767-776.
Crossref - Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148(7):2207-2216.
- Demchenko AP. Beyond annexin V: fluorescence response of cellular membranes to apoptosis. Cytotechnology. 2013;65(2):157-172.
Crossref - Sharma P, Vijayvergia R. In vitro a-amylase inhibitory activity and GC-MS analysis of Petrea volubilis. Int J Sci Res. 2015;4:190-194.
- Sermakkani M, Thangapandian V. GC-MS analysis of Сassia italica leaf methanol extract. Asian J Pharm Clin Res. 2012;5:90-94.
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.