ISSN: 0973-7510
E-ISSN: 2581-690X
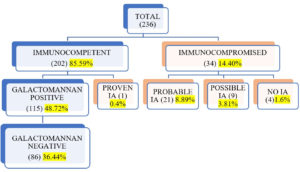
Invasive Aspergillosis (IA) is a severe and fatal infection, especially in immunocompromised patients. Galactomannan is a polysaccharide antigen present in the cell wall of Aspergillus species, which is secreted into the blood and other body fluids during hyphal growth. Therefore, detecting galactomannan antigen is very useful in diagnosing IA, along with clinical features and radiological findings. The study period was one year (January 2022 to December 2022). The data was collected retrospectively from the medical records and case sheets of all clinically suspected invasive aspergillosis patients. Galactomannan antigen assay was performed using an FDA-approved Platelia Aspergillus EIA test kit, and results were interpreted according to the manufacturer’s instructions (Cut off > 0.5). A total of 236 clinically suspected Invasive Aspergillosis cases were enrolled in the study. Galactomannan positivity was predominantly seen in patients aged 40 – 60 years, with male preponderance. Of 236 patients, 14.40% were immunocompromised, and 85.59% were immunocompetent. According to EORTC/MSG definitions, we got one proven IA case, 21 probable cases (8.89%), and nine (3.81%) possible cases. In immunocompetent individuals also, we observed 48.72% (115/236) galactomannan positivity, especially in old Pulmonary Tuberculosis (PTB) patients. Galactomannan positivity was higher in Broncho alveolar lavage (BAL) samples (n=70, 85.36%) than in serum samples (n=77, 46.67%). We found culture positivity of 14.06%, with Aspergillus fumigatus being the commonest isolate, followed by Aspergillus flavus. There is increased positivity of galactomannan in BAL samples compared to serum; hence BAL is a better specimen for diagnosis of Invasive Pulmonary Aspergillosis (IPA).
BAL, Galactomannan, Invasive Aspergillosis
Aspergillus is a ubiquitous saprophytic fungus causing a variety of clinical syndromes. The spectrum ranges from invasive aspergillosis (IA), chronic pulmonary aspergillosis (CPA), and aspergilloma to various allergic manifestations. The interaction between the pathogen and host immunity determines the spectrum of clinical presentation. IA is seen in patients with immune dysfunction, like solid organ and hematopoietic stem cell transplant recipients, and patients on cancer chemotherapy and immunosuppressants.1
In recent years, the prevalence of IA has also increased in immunocompetent hosts with previously damaged lungs, like chronic obstructive pulmonary disease (COPD), old pulmonary tuberculosis, and bronchiectasis.2 Early diagnosis of IA is difficult since the radiological and clinical features are often nonspecific and occur late in the course of the disease.3
Galactomannan (GM) is a cell wall polysaccharide antigen released by Aspergillus hyphae during growth.4 It is released during growth and invasion. GM can be detected in blood and other body fluids since it is water soluble and released even in the early stages of Aspergillus invasion.5 Recently, GM antigen detection has been included in the diagnostic criteria of IA by EORTC/MSG guidelines 2020.6
This study was conducted to determine the utility of galactomannan antigen in the diagnosis of IA.
This study was conducted from January 2022 to December 2022 at the Microbiology department of the tertiary care teaching hospital of Central India. Data was collected retrospectively from the medical records of the patients. All clinically suspected patients of invasive aspergillosis were included in the study. Patients already on mold-active antifungals at the time of specimen collection were excluded from the study.
The demographic, clinical, and radiological details were recorded for all enrolled patients. Also, the results of mycological investigations like culture and galactomannan antigen tests were recorded. A total of 236 clinically suspected Invasive Aspergillosis cases were enrolled in the study. Of this, 54.66% were males (n=129), and 45.34% were females (n=107). Patients range from neonates (10 days) to older people (83 years). Fungal culture of BAL specimens were done on Sabouraud dextrose agar and incubated at 25°C and 37°C for four weeks. Fungal culture was requested for 128 patients. Galactomannan antigen testing was requested for 165 serum and 82 BAL samples (for 11 patients, both BAL and serum samples were tested). Galactomannan antigen assay was performed using an FDA-approved Platelia Aspergillus EIA (Bio-Rad, Vienna, Austria) test kit, and results were interpreted according to the manufacturer’s instructions (Cut off > 0.5).
All the enrolled patients were categorized into proven IA, probable IA, and possible IA as per definitions of EORCT/MSG.6
Statistical analysis
All data collected were entered and analyzed using Microsoft Excel. Data were expressed as percentages. Sensitivity and specificity were calculated using OpenEpi software.
Galactomannan positivity was predominantly seen in patients from 41–60 years, followed by 21–40 years with male preponderance (Male-61% vs female-39%).
We have analyzed specificity and sensitivity only for serum samples in immuno-compromised patients since they were the only patients we could categorize as having proved, probable, or suspected IA according to EORTC/MSG definitions. In our study, sensitivity for serum galactomannan was 75%, and specificity was 100% against diagnosis by EORTC/MSG guidelines. We calculated only positivity for BAL galactomannan since we received very few BAL samples from immunocompromised hosts. We got 85% galactomannan positivity in BAL samples.
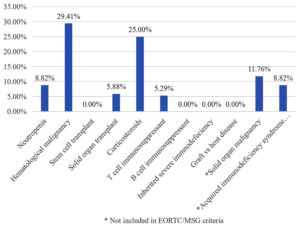
Of 236 patients, 14.40% were immunocompromised, and 85.59% were immunocompetent. According to EORTC/MSG definitions, one patient had IA, 21 probable IA (8.89%), and nine possible IA (3.81%) (Figure 1). The proven IA patient had undergone surgery for renal calculi. Immunocompromised patients in the study consisted of the majority (29.41%) of hematological malignancy, followed by corticosteroids use (25%), neutropenia (8.82%) and solid organ transplant (5.88%). We got some immunocompromised conditions not included in EORTC/MSG criteria, like solid organ malignancy (11.76%) and acquired immunodeficiency syndrome (8.82%) (Figure 2).
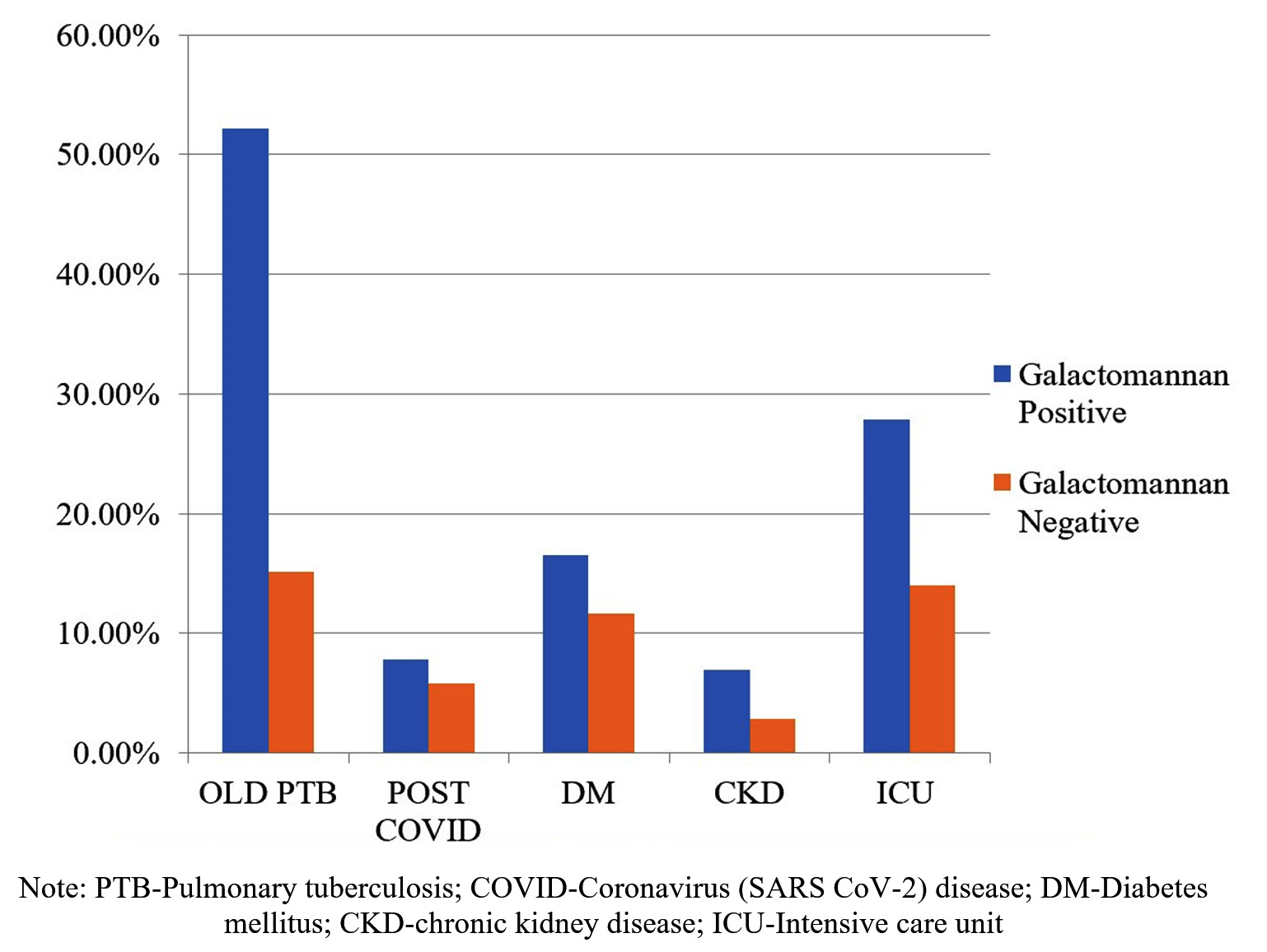
Among immunocompetent individuals, 48.72 % (115/236) and 36.44 % (86/236) patients were galactomannan positive and negative, respectively. The common risk factors in this group were old Pulmonary Tuberculosis (PTB), post-COVID, diabetes mellitus (DM), chronic kidney disease (CKD), and admission to the intensive care unit (ICU) (Figure 3). We observed that all these risk factors were more associated with galactomannan positivity.
Figure 3. Comparison of risk factors in galactomannan positive and negative immunocompetent patients
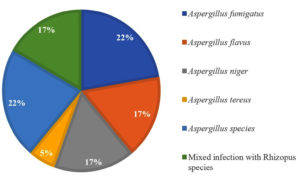
Radiological abnormalities on the CT chest were analyzed only for galactomannan-positive patients as shown in Table 1. Galactomannan antigen positivity was relatively more in BAL specimen (85.36%) as compared to serum specimen (46.67%) (Table 2). Fungal culture was requested for only 128 patients. We found culture positivity of 14.06%, with Aspergillus fumigatus being the commonest isolate, followed by Aspergillus flavus (Figure 4). A comparison of galactomannan antigen and culture positivity is shown in Table 3.
Table (1):
Radiological abnormalities on CT chest of galactomannan positive patients
Radiological Abnormality |
BAL Galactomannan positives (n = 71) |
Serum Galactomannan positives (n = 66) |
Total *(n = 137) |
|---|---|---|---|
Well Circumscribed lesions with or without a halo sign |
05 |
– |
5 (3.64%) |
Air crescent sign |
03 |
02 |
5 (3.64%) |
Consolidation |
09 |
07 |
16 (11.67%) |
Cavity |
22 |
05 |
27 (19.7 %) |
Cystic lesions |
05 |
04 |
9 (6.56%) |
Opacities |
06 |
09 |
15 (10.94%) |
CT not available / not done |
21 |
39 |
60 (43.79%) |
* Note: Radiological abnormalities were analyzed only in 137 galactomannan antigen positive patients
Table (2):
Comparison of galactomannan positivity in serum and BAL samples
| Galactomannan antigen tests results | ||
|---|---|---|
| Specimen | Positive (%) | Negative (%) |
| Serum (n=165) | 77 (46.67) | 88 (53.54) |
| BAL (n=82) | 70 (85.36) | 12 (14.63) |
Table (3):
Comparison of culture results and galactomannan positivity
| Culture | Galactomannan Ag Test | Total | |
|---|---|---|---|
| Positive | Negative | ||
| Positive | 15 (11.71%) | 3 (2.34%) | 18 |
| Negative | 76 (59.37%) | 34 (26.56%) | 110 |
| Total | 91 | 37 | 128 |
Invasive aspergillosis is a life-threatening infection in the immunocompromised as well as immunocompetent patients. Its diagnosis is challenging because of nonspecific clinical and radiological features and also due limitations of fungal culture. Galactomannan Antigen detection, a relatively rapid and noninvasive test, plays a vital role in diagnosis at an early stage of infection. Recently WHO released a priority list for fungal pathogens in which Aspergillus fumigatus is included in a critical priority group.7
Male preponderance was noted in the current study, similar to Malhotra et al.,8 and Khanna et al.9 Males who participate in outdoor activities more frequently are more likely to contract a fungus. There were 3.22%, 67.74%, and 29.03% of proven, probable, and possible IA cases, respectively. Mohindra et al. found a similar prevalence (Proven IA – 1.8%, Probable IA – 69.37%, and Possible IA – 2.83%).10 Khanna et al. observed a relatively higher percentage of proven IA cases (14.8%) and a lower prevalence of probable IA cases (48.2%).9
Amongst the immuno-compromised host, the maximum number of patients were of hematological malignancy (29.41%), followed by corticosteroids use (25%), neutropenia (8.82%) and solid organ transplant (5.88%). There were a few immunocompromised conditions not included in EORTC/MSG criteria, like solid organ malignancy (11.76%) and acquired immunodeficiency syndrome (8.82%). In a study by Singh et al., most patients had hematological malignancy (60.93%), followed by solid organ transplant (26.56%).11 The common risk factors observed by Vazquez JA et al.12 were the use of immunosuppressants (39%), solid organ transplant (33%), malignancy (22%), etc. A study from Pune reported 48.75% cases of hematological malignancy, followed by solid organ tumors (12.5%) and organ transplant recipients (2.5%).13 All these patients were neutropenic and immunocompromised due to chemotherapy and immunosuppressive drugs, hence more prone to Invasive Aspergillosis.
In our study, the most common radiological abnormality was cavitation (19.70%), followed by consolidation (11.67%), nodules (3.64%), and air crescent sign (3.64%). S. Raveendran et al. reported nodules in 38.7%, consolidation in 19.4%, and crescent sign followed by cavitations in 29% of IA cases.14 In the study by Tong et al., the radiological abnormalities commonly seen were consolidation (60.7%), nodules (50.4%), cavity (39.3%), opacity (17.1%), and the halo sign (12.0%).15 These radiological findings are nonspecific; galactomannan plays a crucial role in diagnosing IA. In the medical records of 43.79% of patients, either the chest CT scans were not performed or reports were not accessible. In 43.79% of patients, chest CT chest was either not done or reports were unavailable in medical records. Of these, 29.41% of patients were children with hematological malignancy without a CT scan.
Among immunocompetent patients, BAL and serum galactomannan positivity was 85.52% and 42.75%, respectively. Similarly, Zhou W et al. observed that among nonneutropenic patients, the sensitivity of galactomannan in BAL and serum samples was 75.68% and 37.84%, respectively.16 Among old PTB and post-COVID cases, galactomannan positivity was 52.17% and 55.56%, respectively. Yusuf et al. reported galactomannan positivity in BAL samples between 10% and 28% and in serum samples between 0.9% to 6.7% from post-COVID patients.17 In research by Zhou W et al., out of 37 IA cases (diagnosed by EORTC/MSG), nine (24.32%) had pulmonary tuberculosis.16 In a study by Sivasankari et al., among 80 PTB patients, 8 (10%) were IA cases and were diagnosed by culture and PCR.18 Invasive Aspergillosis cases are increasing in immunocompetent patients like old PTB and post-COVID patients due to previous lung damage. Any recent change in symptoms or radiological findings should not be ignored in such patients and can be supplemented with a galactomannan antigen test to diagnose IA.
We have analyzed specificity and sensitivity only in immunocompromised patients since they were the only patients we could categorize as having proved, probable, or suspected IA according to EORTC/MSG definitions. In our study, sensitivity for serum galactomannan was 75%, and specificity was 100% against diagnosis by EORTC/MSG guidelines. Singh et al.11 observed relatively high sensitivity and specificity (both 90%), and Zhou W et al.16 observed relatively low sensitivity (37.8%) and specificity (87.1%). We calculated only positivity for BAL galactomannan since we received very few BAL samples from immunocompromised hosts. We got 85% galactomannan positivity in BAL samples, similar to that of Singh et al. (90%).11
Galactomannan can be detected in serum, BAL & CSF in invasive aspergillosis. It is also successfully detected in sputum, endotracheal aspirate, plasma, urine, and fluid from abscesses. Nuh et al.19 performed a galactomannan antigen test on sputum samples, demonstrating its utility in diagnosing chronic pulmonary aspergillosis. Sputum GM showed sensitivity and specificity of 70% and 71%, respectively, compared to clinical diagnosis. When sputum GM was tested against Aspergillus culture/IgG, a sensitivity of 77% and specificity of 78% were discovered. Hence sputum can also be a suitable sample for GM testing in critically ill patients unable to tolerate bronchoscopy procedures.
Culture positivity (14.06%) observed in the present study is similar to that of Mohindra et al. (17.22%)10 and Kaur et al. (19.4%).20 However, Roohani et al. (31.11%)21 and Khanna et al. (59.25%)9 observed relatively more culture positivity. Our 2.34% patients were culture positive and galactomannan negative. This is probably due to colonization. Also, our 59.37% patients were culture negative and galactomannan positive. Sensitivity of fungal culture in diagnosing IA depends on the population tested and the prevalence of infection in that area, and thus it is higher among immunocompromised patients and in endemic areas.22 The most common isolated species in the present study was A. fumigatus, followed by A. flavus, similar to the findings of Roohani et al.21 However, many other researchers got A. flavus most common pathogen.9,10,20 A fungal culture is less sensitive and takes time, and there are also chances of contamination during processing. Here galactomannan has a significant role along with chest CT for diagnosing IA.
In the present study, there were 15 patients (10.94%) with galactomannan antigen positive, but they were not immunocompromised and had no radiological abnormality. These patients may be considered false positives. Of these 15 patients, nine were on piperacillin-tazobactam, and five were on amoxicillin-clavulanic acid. Previous researchers also found galactomannan false positivity with beta-lactam antibiotics like piperacillin–tazobactam and amoxicillin–clavulanic acid.23-25 Hence, physicians should cautiously interpret galactomannan results in such patients.
BAL samples are better for diagnosis of Invasive Pulmonary Aspergillosis. Galactomannan positivity was seen in both immunocompromised and immunocompetent individuals. PTB is the most common risk factor for GM positivity in immunocompetent patients. Hence, PTB may be considered for diagnosing IPA in TB-endemic regions. More prospective studies are required to evaluate the performance of the galactomannan antigen test for diagnosing IPA in immunocompetent hosts, especially in PTB patients. GM detection can prove an excellent diagnostic test for IPA, but a panel of tests should be implemented in patients suspected of pulmonary aspergillosis.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70(3):270-277.
Crossref - Sugui JA, Kwon-Chung KJ, Juvvadi PR, Latge JP, Steinbach WJ. Aspergillus fumigatus and related species. Cold Spring Harb Perspect Med. 2014;5(2):a019786.
Crossref - Penack O, Rempf P, Graf B, Blau IW, Thiel E. Aspergillus galactomannan testing in patients with long-term neutropenia: implications for clinical management. Ann Oncol. 2008;19(5):984-989.
Crossref - Ghosh I, Raina V, Kumar L, Sharma A, Bakhshi S, Iqbal S. Serum galactomannan assay for diagnosis of probable invasive Aspergillosis in acute leukemia and hematopoietic stem cell transplantation. Indian J Med Paediatr Oncol. 2013;34(2):74-79.
Crossref - Wheat LJ. Rapid diagnosis of invasive aspergillosis by antigen detection. Transpl Infect Dis. 2003;5(4):158-166.
Crossref - Donnelly JP, Chen SC, Kauffman CA, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71(6):1367-1376.
Crossref - WHO fungal priority pathogens list to guide research, development and public health action. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. http://apps.who.int/iris.
- Malhotra S, Kumari R, Chauhan AK, et al. Diagnostic value of galactomannan antigen test in serum and bronchoalveolar lavage fluid sample from suspected patients of invasive pulmonary aspergillosis. Indian J Pathol Microbiol. 2021;64(4):732-734.
Crossref - Khanna S, Oberoi JK, Datta S, Aggarwal S, Wattal C. Variables affecting the performance of galactomannan assay in high-risk patients at a tertiary care centre in India. Indian J Med Microbiol. 2013;31(1):34-39.
Crossref - Mohindra R, Capoor MR, Puri S, et al. Evaluation of serum galactomannan enzyme immunoassay at two different cut-offs for the diagnosis of invasive aspergillosis in patients with febrile neutropenia. Indian J Med Microbiol. 2017;35(2):237-242.
Crossref - Singh S, Kaur H, Choudhary H, et al. Evaluation of biomarkers: Galactomannan and 1,3-beta-D-glucan assay for the diagnosis of invasive fungal infections in immunocompromised patients from a tertiary care centre. Indian J Med Microbiol. 2018;36(4):557-563.
Crossref - Vazquez JA, Paula Tovar-Torres M, Hingwe A, Cheema F, Welch VL, Ford KD. The changing epidemiology of invasive aspergillosis in the non-traditional host: risk factors and outcomes. Pulm Crit Care Med. 2016;1(3):67-71.
Crossref - Kumar M, Mugunthan M. β-d-Glucan and Aspergillus Galactomannan assays in the diagnosis of invasive fungal infections. Med J Armed Forces India. 2019;75(4):357-360.
Crossref - Raveendran S, Lu Z. CT findings and differential diagnosis in adults with invasive pulmonary aspergillosis. Radiol Infect Dis. 2018;5(1):14-25.
Crossref - Tong X, Liu T, Jiang K, Wang D, Liu S, Wang Y, Fan H. Clinical Characteristics and Prognostic Risk Factors of Patients With Proven Invasive Pulmonary Aspergillosis: A Single-Institution Retrospective Study. Front Med (Lausanne). 2021;8:756237.
Crossref - Zhou W, Li H, Zhang Y, et al. Diagnostic Value of Galactomannan Antigen Test in Serum and Bronchoalveolar Lavage Fluid Samples from Patients with Nonneutropenic Invasive Pulmonary Aspergillosis. J Clin Microbiol. 2017;55(7):2153-2161.
Crossref - Yusuf E, Seghers L, Hoek RAS, van den Akker JPC, Bode LGM, Rijnders BJA. Aspergillus in Critically Ill COVID-19 Patients: A Scoping Review. J Clin Med. 2021;10(11):2469.
Crossref - Sivasankari S, Senthamarai S, Anitha C, et al. Prevalence of Invasive Aspergillosis Among (PTB) Patients in Kanchipuram, India. J Clin Diagn Res. 2014;8(3):22-23.
Crossref - Nuh A, Ramadan N, Shah A, Armstrong-James D. Sputum Galactomannan Has Utility in the Diagnosis of Chronic Pulmonary Aspergillosis. J Fungi. 2022;8(2):188.
Crossref - Kaur S, Gupta V, Chhina D K, Singh A, Sharma D. Mycological and serological study of invasive Aspergillosis in a tertiary care hospital. J Microbiol Infect Dis. 2018;8(1):8-12.
Crossref - Roohani AH, Fatima N, Shamim M, Khan HM, Akhtar A. Distribution of Galactomannan antigen in BAL and serum samples among Aspergillus isolates and its correlation with culture among Immunocompetent and Immunocompromised patients in Aligarh region, a north India town. Annals Pathol Lab Med. 2019;6(8):A414-420.
Crossref - Arvanitis M, Anagnostou T, Fuchs BB, Caliendo AM, Mylonakis E. Molecular and nonmolecular diagnostic methods for invasive fungal infections. Clin Microbiol Rev. 2014;27(3):490-526.
Crossref - Reiss E, Shadomy HJ, Lyon GM III. Aspergillosis. Fundamental Medical Mycology. New Jersey: Wiley-Blackwell Publication; 2012:357-94.
Crossref - Adam O, Auperin A, Wilquin F, Bourhis JH, Gachot B, Chachaty E. Treatment with piperacillin-tazobactam and false-positive Aspergillus galactomannan antigen test results for patients with hematological malignancies. Clin Infect Dis. 2004;38(6):917-20.
Crossref - Fortun J, Martin-Davila P, Alvarez ME, et al. False-positive results of Aspergillus galactomannan antigenemia in liver transplant recipients. Transplantation. 2009;87(2):256-260.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.