ISSN: 0973-7510
E-ISSN: 2581-690X
The demand for cellulose is accelerating in the paper making industry. Alternate sources of cellulose has to be traced in order to reduce the demand for plant cellulose. Hence, in this study bacterial cellulose has been chosen as an option. In this study, the potential of soil bacteria Lactococcus lactis to produce cellulose has been assessed. The results obtained indicate that the inocula size of the bacteria had a vital role in altering the quantity of cellulose produced. Among the inocula size, 100 µl of broth culture exhibited highest production of cellulose. The cellulose produced was characterised spectrally and its microarchitectural study reveal its crystalline nature. FTIR spectra of the bacterial cellulose produced depict the signature peak of bacterial cellulose. Based on these observations, it could be concluded that Lactococcus lactis produce cellulose. Further studies has to be carried out to optimize the bacterial cellulose production.
Lactococcus lactis, cellulose, glucose, FTIR, SEM.
Cellulose, the major polymer produced in the biosphere serves as the raw material in the high fidelity acoustic speakers, high quality paper and dessert foods, wound dressings, artificial skin, dental implants, membrane dialysis, drug carrier for controlled release, wet-end additive for paper making process1-5. Cellulose is synthesized by plants, animals (Tunicates), bacteria, algae and plankton3, 6.
Bacterial cellulose is preferred over plant cellulose due to his high purity, high degree of polymerization, crystallinity index, high tensile strength and water holding capacity3. One of the major bottleneck in BC application is industry is its low productivity. Microorganisms, production methods, carbon and nitrogen sources, temperature, pH and reactor type influence the bacterial cellulose production7,4,8. Production of cellulose by bacteria like Gluconacetobacter xylinum9,10, Sarcina, Agrobacterium, Rhizobium, Acetobacter11 Sucrofermentans BPR200112, Enterobacter sp. RVII, Pseudumonas sp., RVI4, Gloconacetobacter sp.13, have been documented.
Many researchers have evaluated the use of low cost natural carbon sources like coconut water, fruit juices, corn steep liquor, date syrup, dates molasses, sugarcane juice14,15,16,12,17,18,19, 2, 20 and synthetic carbon sources like glucose, ethanol etc.,21,15,22,12,23,24,13,25,26. Bacterial cellulose has been produced under static condition 27,22,28,23 and agitated condition21. Some researchers have suggested that static condition is suitable for bacterial cellulose production 14,29,16,12,30. The accelerating demand for cellulose based products may shrink the forest cover. Alternative sources of cellulose could be a sustainable option to minimize the pressure on plant cellulose. Hence, the present study was designed to tap the potential of soil bacteria to produce cellulose and also to characterize cellulose through FTIR and SEM images.
Collection of soil and isolation of bacteria
Soil was collected from garden in a bottle aseptically. 1g of soil was dissolved in 100ml sterile distilled water and serially diluted. 1µl of dilutions of 10-3, 10-5 and 10-7 were inoculated on nutrient agar plates and incubated at 37 ºC for 48 hours. The bacterial colonies were isolated and identified according to the methods mentioned in Bergeys Manual of Determinative Bacteriology 31. Among the bacterial isolates, dominant bacteria Lactococcus lactis was evaluated for its potential to produce cellulose.
Inoculation of Lactococcus lactis in HS medium32
HS medium (2% w/v D-glucose, 0.5 % w/v peptone, 0.5 % w/v yeast extract, 0.27 % w/v di- sodium hydrogen Phosphate (Na2H PO4) and 0.115 % w/v citric acid) was taken in a 250ml conical flask and 100 µl, 200 µl and 300 µl of Lactococcus lactis broth culture of 24 hours was inoculated. The experiment was conducted in triplicates. The culture was incubated at 37 ºC in agitated condition in a orbital shaker at 100 rpm for a period of 15 days. Wet BC pellicles produced were pre- heated and weighed. The wet BC pellicles produced was filtered from the media and washed with running water and immersed in 2% w/v sodium hydroxide and boiled for 30 minutes and dried it in the oven at 70 ºC for 6 hours 15.
Evaluation of bacterial cellulose properties:
Weight (g) of cellulose was measured in the analytical balance. The moisture content (% w/w) of bacterial cellulose was determined based on the weight loss of bacterial cellulose when dried at 75 °C14.Bacterial cellulose production was determined by the method of Hongmel Lu et al.,33.
Observation of bacterial cellulose film under Scanning Electron Microscope (SEM)
BC dry films produced were observed under SEM to study morphology and microstructure of cellulose fibres. Prior to examining, the sample were gently fixed on an Aluminium stab with two side adhesive tape and coated with 15 – 20mm thick layer of gold. The samples were then examined under scanning Electron Microscope (Spectrum 2).
FTIR Spectroscopy
FTIR spectra of bacterial cellulose samples were recorded with a BIO-RAD spectrometer (model FTS 40A) using the KBr (Potassium bromide) disc technique (1 mg of BC powder / 300mg KBr) in the range of 4000 – 400cm-1. The FT-IR spectra were recorded at a resolution of 2 cm-1 and at an accumulation of 32 scans.
The biochemical test reveals that the isolated bacteria is Lactococcus lactis (Table 1). Highest BC moisture content was recorded in bacterial cellulose produced by 100 µl of Lactococcus lactis broth culture (4.6%). Highest quantity of BC was produced at 100 µl of Lactococcus lactis broth culture (41.4 g/L) (table 2).
Table (1):
Identification of bacteria by biochemical analysis.
S. No |
Test |
Result |
|---|---|---|
1 |
Gram staining |
+ve |
2 |
Oxidase |
-ve |
3 |
Catalese |
-ve |
4 |
Indole |
-ve |
5 |
MR |
+ve |
6 |
VP |
+ve |
7 |
Citrate |
-ve |
8 |
Urease |
-ve |
9 |
TSI slant/butt |
AK/A |
10 |
Glucose |
+ve |
11 |
Sucrose |
+ve |
12 |
Lactose |
+ve |
13 |
Fructose |
+ve |
Table (2):
Moisture content and the quantity of cellulose produced by Lactococcus lactis.
Inocula size (µl of Lactococcus lactis broth culture) |
Moisture content(%) |
Bacterial cellulose produced (g/ L) |
|---|---|---|
100 |
4.60 |
41.4 |
200 |
0.20 |
1.70 |
30 |
0.49 |
4.25 |
The signature peak of bacterial cellulose produced by Lactococcus lactis reveal the presence of –OH (3368 cm-1), ½(CH2) (2925 cm-1), ½(CH2) (2865 cm-1), C = O (1745 cm-1), COOH (1655 cm-1), Amide II (1556 cm-1), CH (1408 cm-1), CH (1382 cm-1), (1256 cm-1), C- O – C (1151 cm-1), C- O – C (1073 cm-1), (1037 cm-1) and CH (858 cm-1) at inoculum density of 100 µL. (Fig. 1) (Table 3). The SEM Image reveals the BC structure. It is crystalline in nature (fig 2).
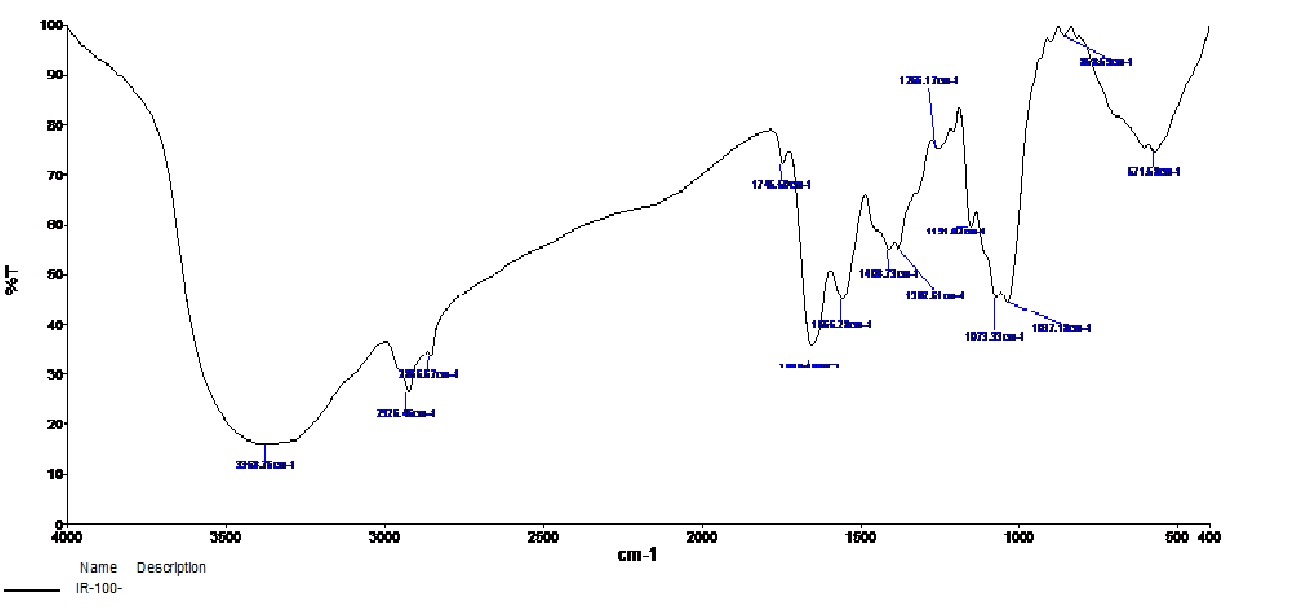
Fig. 1: FTIR Spectra of BC produced by Lactococcuslactis (100 µL)
Table (3):
Band Assignment of FTIR spectra of Cellulose produced by Lactococcus lactis (100 µl / ml).
Wave Number (cm-1) |
Intensity |
Functional Group |
References |
|---|---|---|---|
3368 |
s |
OH |
23, 24, 34,35,36,37, 38,39,40, 41, 42,43, 44,45 |
2925 |
s |
ⱱ(CH)2 |
2, 24,25, 35,36, 38, 39, 40,41, 43, 44,46,47,48,49,50 |
2865 |
s |
ⱱ(CH)2 |
35, 39, 41, 46, 47 |
1745 |
m |
C=O |
23, 47, 51 |
1655 |
m |
COOH |
2,24, 25,37, 40, |
1556 |
S |
Amide II absorption |
23, 47 |
1408 |
s |
CH |
25, 35, 38,39, 45 |
1382 |
s |
Planar CH |
24, 36,39, 45,47 |
1256 |
m |
O-C |
51 |
1151 |
m |
C-O-C |
25, 38,39, 42, 44,47,54,55,56 |
1073 |
s |
C-O-C |
2, 24, 41,56 |
1037 |
s |
C-O-C |
25, 35, 51 |
858 |
w |
CH out of plane bending vibrations |
43,56 |
s – strong, m – medium, w – weak

Fig. 2: SEM image of bacterial cellulose produced by Lactococcus lactis (100 µl / ml)
The present finding agrees with that of Faridah et al.,27 who have reported that cellulose produced by A. xylinum under different sugar concentrations (7.5 % and 10 %) does not alter the functional groups but causes changes in the intensity of absorption peak in the FTIR spectra of cellulose and have concluded that the concentration of sugar does not alter the microstucture of the cellulose. Similarly, Hestrin and Schramm medium containing different carbon sources influence the yield of cellulose by Gluconactobacter xylinusstrain ATCC 53524 but doesnot affect the molecular and microscopic features of cellulose21. Hungund et al.,15have also observed that highest cellulose yield in combination with fructose and sucrose (1:1) in Hestrin and Schramm medium by Gluconactobacter persimmonis. Yodsuwan et al.,22 also have reported that mannitol and fructose enhanced the cellulose yield of Acetobacter xylinum strain TISTR 975.Gluconoacetobacter hansenii yielded cellulose in the range of 0.81g/ L to 0.84 g/ L in standard HS medium after a period of 14 days39.
The present result partially agrees with that of Ragaswamy et al.,13 who have stated that Gluconacetobacter sp., RV28 produced 4.7 g/ L of cellulose at optimum growth conditions of temperature (30 ºC), pH (6.0), sucrose (2%), peptone (0.5 %) and inoculum density (5 %) under static condition. Auta et al., 24 have reported that Gluconacetobacter xylinus produced an average dry yield of 1.4 ± 0.09 g/ L cellulose after 9 days by using glucose as a carbon source under static condition at 30 ºC. This finding is in consistent with the present observation.
The present observation gains support from the findings of Gayathri and Gopalaswamy2 who have reported that Acetobacter xylinum produced 11g/L bacterial cellulose in HS medium after 14 daysof fermentation period. Castro et al.,23 have demonstrated that Gluconacetobacter medellensis produced optimum cellulose in HS medium modified with glucose ( 4.2 g/ L) followed by sucrose and fructose under static condition at 28 ºC. These findings are in conformity with the observations of Barbara Surma- Slusarska et al.,28who have reported that Acetobacter xylinum yielded highest bacterial cellulose using glucose and mannitol when compared to other carbon sources (arabinose, mannose, galactose and xylose) at 30 ºC after 7 days under static condition. Alaa Raheem Kazim19 have reported that dates molasses enhances the production of cellulose by Pseudomonas sp., when compared to other carbon sources (glucose, fructose, maltose, ethanol) and have attributed it to the nutrient content of dates. These observations are in harmony with the findings of Masaoka et al.,26 who have reported that bacterial cellulose production by Acetobacter xylinum was enhanced when glucose was used as a carbon source at 30 ºC statically.
The crystalline nature of bacterial cellulose observed in te SEM image is similar to our previous findings35. We have observed the crystalline nature of cellulose produced by Actinomycetes sp. and Pseudomonas sp. The crystalline nature of cellulose produced by bacteria using glucose as a carbon source have been reported (Gluconacetobacter sp.13, Acetobacter xylinum sub sp. Sucrofermentans BPR200112, Acetobacter xylinum30 ,Acetobacter xylinum2, Achromobacter sp.,41, Acetobacter aceti57, Acetobacter xylinum29, Gluconacetobacter23,Gluconacetobacter xylinus strain ATCC53524 21. The results obtained indicate that Lactoccocus lactis could be used to produce cellulose but further investigations have to be carried out to optimise the bacterial cellulose production.
- Shoda M., Sugano Y. Recent advances in bacterial cellulose production. Biotechnology and Bioprocess Engineering. 2005; 10(1): 1.
- Gayathry G., Gopalaswamy G. Production and Characterisation of microbial cellulose fibre from Acetobacter xylinum. Indian Journal of fibre and Textile Research , 2014; 39: 93-96.
- Raghunathan D. Production of microbial cellulose from the new bacterial strain isolated from temple wash waters. Int. J. Curr. Microbiol. App. Sci., 2013; 2(12): 275-290.
- Chawla P. R., Bajaj I. B., Survase S. A and Singhal R. S. Microbial Cellulose; Fermentative Production and applications. Food Technol. Biotechnol., 2009; 47:107-124.
- Brown E. E., Laborie M. P. G. Bioengineering bacterial cellulose/poly (ethylene oxide) nanocomposites. Biomacromolecules., 2007; 8(10) : 3074-3081.
- Keshik M. S. Bacterial cellulose production and its industrial applications. Bioprocessing and Biotechniques, 2014; 4(2): 100150.
- Bielecki S., Krystynowicz A., Kalinowska H. Bacterial cellulose. In A. Steinbuchel, Biotechnology of Polymer: From synthesis to patents. Munster, Germany: Wiley – VCH, verlag Gmbh ; 2005: 381 – 434.
- Huang H.C.,Chen L.C.,Lin S.B., Hsu C., Panel Chen H.H. In situ modification of bacterial cellulose network structure by adding interfering substances during fermentation. Bioresource Technology, 2010; 101(15) : 6084 – 6091.
- Ross P., Mayer R., Benziman M. Cellulose biosynthesis and function in bacteria. Microbiol., Rev., 1991; 55: 35-58.
- Tanaka M., Murakami S., Aoki R. Genetic characteristics of Cellulose –forming acetic acid bacteria identified phenotypically as Gluconacetobacter xylinus Biosci. Biotechnol Biochem., 2000; 64: 757- 760.
- Deinema H. M., Zevenhuizen L.P. Formation of Cellulose fibrils by Gram-negative bacteria and their role in bacterial flocculation. Arch. Mikrobiol., 1971;78: 42- 51.
- Watanabe K., Tabuchi M., Morinaga Y., Yoshiinaga F. Structure features and properties of bacterial cellulose produced in agitated culture. Cellulose, 1998; 5: 187-200.
- Rangaswamy B.E., Vanitha K.P., Basavaraj S.H. Microbial cellulose production from bacteria isolated from rotten fruit. International journal of polymer science, 2015 ; http://dx.doi.org/10.1155/2015/280784.
- Afreen S.S., Lokeshappa B. Production of Bacterial Cellulose from Acetobacter xylinum using fruits wastes as substrate. The International Journal of Science and Technoledge, 2014 : 2 (8) : 57 – 63.
- Hungund B., Prabhu S., Shetty C., Acharya S., Prabhu V. Production of Bacterial Cellulose from Gluconacetobacter persimmonis GH-2 using dual and cheaper carbon sources. J. Microb. Biochem. Technol., 2013; 5: 031-033.
- Hiroshi Toyosaki., Takaaki Naritomi., Akira Seto., Masanobu Matsuoka ., Takayasu Tsuchida ., Fumihiro Yoshinaga . Screening of Bacterial Cellulose – producing Acetobacter strains suitable for agitated culture. Bioscience , Biotechnology and Biochemistry, 1995 ; 59(8) :1498 – 1502.
- Abu Hassan Bin Mohd Nazir. Optimization of Bacterial cellulose production in apple juice medium by using response surface methodology (RSM). Bachelor of Chemical Engineering (Biotechnology) Thesis, Faculty of Chemical and Natural Resources Engineering , Universiti Malaysia Pahang . January 2012.
- Moosavi-Nasab M., Yousefi A. R. Investigation of physicochemical properties of the bacterial cellulose produced by Gluconacetobacter xylinus from date syrup. World Academy of Science, Engineering and Technology, 2010; 44: 1258-1263.
- Alaa Raheem Kazim . Production , optimization and characterization of cellulose produced from Pseudomonas spp . World Journal of Experimental Biosciences, 2015; 3(2) : 89 – 93.
- Kurosumi A., Sasaki C., Yamashita Y., Nakamura Y. Utilization of various fruit juices as carbon source for production of bacterial cellulose by Acetobacter xylinum NBRC 13693. Carbohydrate Polymers, 2009; 76: 333 – 335.
- Deirdre Mikkelsen ., Bernadine Mary Flanagan ., Dykes G A ., Mike Gidley . Influence of different carbon sources on bacterial cellulose production by Gluconacetobacter xylinus strain ATCC 53524. Journal of Applied Microbiology, 2009; 107 : 576-583.
- Yodsuwan N., Owatworakit A., Ngaokla A., Tawichai N., Soykeabkaew N. Effect of carbon and nitrogen sources on bacterial cellulose production for bionanocomposite materials. In Ist Mae Fah Luang University International Conference; 2012 :1- 6.
- Castro C., Zuluaga R., Álvarez C., Putaux J. L., Caro G., Rojas O. J., Gañán P. Bacterial cellulose produced by a new acid-resistant strain of Gluconacetobacter genus. Carbohydrate polymers, 2012; 89(4): 1033-1037.
- Auta R., Adamus G., Kwiecien M., Radecka I., Hooley P. Production and characterization of bacterial cellulose before and after enzymatic hydrolysis. African Journal of Biotechnology, 2017; 16(10) : 470-482.
- Mohammadkazemi F., Doosthoseini K., Azin M. Effect of ethanol and medium on bacterial cellulose (BC) production by Gluconacetobacter xylinus (PTCC 1734). Cellulose chemistry and technology, 2015; (49) : 5-6.
- Masaoka S., Ohe T and Sakota N. Production of cellulose from glucose by Acetobacter xylinum. J. Ferment. Bioeng., 1993; 75 : 18 – 22.
- Faridah., Selvie Diana., Helmi., Sami M., Mudliana. Effect of sugar concentrations on bacterial cellulose production as cellulose membrane in mixture liquid medium and material properties analysis. Prosiding Seminar Internasional AASIC ( Asean Academic Society International Conference) November 2013.
- Barbara Surma – Slusarska., Sebastian Presler ., Dariusz Danielewicz. Characteristics of bacterial cellulose obtained from Acetobacter xylinum culture for application in Papermaking. Fibres and Textiles in Eastern Europe, 2008; 16(4) : 108 – 111.
- Krystynowicz A., Czaja W., Wiktorowska-Jezierska., Goncalves – Miskiewicz M., Turkiewicz M., Bieleck S. Factors affecting the yield and properties of bacterial cellulose. Journal of Industrial Microbiology and Biotechnology., 2002; 29: 189-185.
- Wojciech Czaja ., Dwight Romanovicz ., Malcolm Brown R Jr. Structural investigations of microbial cellulose produced in stationary and agitated culture. Cellulose, 2004; 11: 403-411.
- Sneath P. H. K., Mair S. N., Elisabeth Sharp M ., Holt G. J. Bergy’s Manual of systematic Bacteriology , Williams aailkines, Baltimore, USA.,1994.
- Hestrin S., Schramm M . Synthesis of cellulose by Acetobacter xylinum. 2 . Preparation of freeze- dried cells capable of polymerizing glucose to cellulose. Biochem J ., 1954; 58: 345 – 352.
- Hongmei Lu ., Qinghui Jia ., Li Chen ., Liping Zhang. Effect of organic acids on bacterial cellulose produced by Acetobacter xylinum. Research and Reviews: Journal of Microbiology and Biotechnology, 2016; 5(2): 1-6.
- Gomes P. F., Silva H. C. S. N., Trovatti E., Serafim L. S., Duarte F. M., Silvestre J. D. A., Neto C. P., Freire S. R. C. Production of bacterial cellulose by Gluconacetobacter sacchari using dry olive mill residue. Biomass and Bioenergy, 2013; 55: 205-211.
- Sepperumal U., Selvanajagam S ., Murali M. Characterisation of Cellulose produced by Pseudomonas sp. and Actinomycetes sp. European Journal of Zoological Research, 2014; 3(4) :13- 18.
- Oliveira R. L., Vieira J. G., Barud H. S., Assunção R. R., Filho G., Ribeiro S. J., Messadeqq Y. Synthesis and characterization of methylcellulose produced from bacterial cellulose under heterogeneous condition. Journal of the Brazilian Chemical Society, 2015; 26(9) :1861-1870.
- Moharram M. A., Mahmoud O. M. FTIR spectroscopic study of the effect of microwave heating on the transformation of cellulose I into cellulose II during mercerization. Journal of Applied Polymer Science, 2008; 107(1) : 30-36.
- Halib N., Amin M. C. I. M., Ahmad, I. Physicochemical properties and characterization of nata de coco from local food industries as a source of cellulose (Sifat fizikokimia dan pencirian nata de coco daripada industri makanan tempatan sebagai sumber selulosa). Sains Malaysiana, 2012; 41(2): 205-211.
- Gündüz G., A_ik N., Aydemir D., Kiliç A. Bakteriyel Selüloz Üretimi ve Karakterizasyonu. Ormancilik dergisi; 2015; 10(2): 1-10.
- Marzieh M., Ali R. Investigation of physicochemical properties of the bacterial cellulose by Gluconacetobacter xylinum from date syrup. World Academy of Science Engineering and Technology, 2010; 68: 1248 – 1253.
- Farag S., Asker M. S. M., Mohmoud G. M., Ibrahim H., Ahmed amr. Comparative study of bacterial cellulose production using Egyptian Achromobacter sp. Research Journal of Pharmaceutical, Biological, and Chemical sciences, 2016; 7(6): 954 – 959.
- Pandey M., Abeer M. M., Amin M. C. I. Dissolution study of bacterial cellulose (nata de coco) from local food industry: solubility behaviour and structural changes. Int. J. Pharm. Pharmaceut. Sci., 2014; 6 : 89-93.
- Dayal M. S., Goswami N., Sahai A., Jain V., Mathur G., Mathur A. Effect of media components on cell growth and bacterial cellulose production from Acetobacter aceti MTCC 2623. Carbohydrate polymers, 2013; 94(1) : 12-16.
- Costa L. M. M., de Olyveira G. M., Basmaji P., Lauro Filho X. Bacterial cellulose towards functional green composites materials. Journal of Bionanoscience, 2011; 5(2) : 167-172.
- Gao W H., Chen K F., Yang R D., Yang , Fand Han W J. Properties of bacterial cellulose and its influence on the physical properties of paper. Bioresources, 2010; 6(1) : 144-153.
- Oh S. Y., Yoo D. I., Shin Y., Kim H. C., Kim H. Y. Chung Y. S.. Crystalline structure analysis of cellulose treated with sodium hydroxide and carbon dioxide by means of X-ray diffraction and FTIR spectroscopy. Carbohydrate Research, 2005; 340(15) : 2376–2391.
- Movasaghi Z., Rehman S., Rehman D. I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Applied Spectroscopy Reviews, 2008; 43(2): 134-179.
- He J., Kunitake T., Nakao A. Facile in situ synthesis of noble metal nanoparticles in porous cellulose fibers. Chemistry of Materials, 2003;15: 4401–4406.
- Shezad O., Khan S., Khan T., Park J. K. Physicochemical and mechanical characterization of bacterial cellulose produced with an excellent productivity in static conditions using a simple fed-batch cultivation strategy. Carbohydrate Polymers, 2010; 82(1) :173-180.
- Qin Z., Ji L., Yin X., Zhu L., Lin Q., Qin J. Synthesis and characterization of bacterial cellulose sulfates using a SO 3/pyridine complex in DMAc/LiCl. Carbohydrate polymers, 2014; 101: 947-953.
- Silverstein R.M, Bassler G.C, Morril T.C. Spectrometric Identification of Organic Compounds, 4th edn. New York: John Wiley; 1990.
- Nelson M, O’Connor R. Relation of certain infra-red bands to cellulose crystallinity. J. Appl. Polym. Sci., 1964; 8:1325.
- Yu. R. Mitrofanov, Budaeva V. V., Sakovich G. V. Preparation and Properties of bacterial cellulose gel films. Chemistry for Sustainable Development, 2010; 18 : 503 – 508.
- Zhou L. L., Sun D. P., Hu L. Y., Li Y. W., Yang J. Z. Effect of addition of sodium alginate on bacterial cellulose production by Acetobacter xylinum. Journal of industrial Microbiology and Biotechnology, 2007; 34 (7) : 483.
- Phisalaphong M., Jatupaiboon N. Biosynthesis and characterization of bacteria Cellulose – chitosan film. Carbohydr.polym., 2008; 74: 482-488.
- Kacurakova M., Smith A. C., Gidley M. J., Wilson R. H. Molecular interactions in bacterial cellulose composites studied by 1D FT-IR and dynamic 2D FT-IR spectroscopy. Carbohydrate Research, 2002; 337: 1145-1153.
- Yamanaka S., Sugiyama J. Structural modification of bacterial cellulose. Cellulose, 2000; 7(3) : 213-225.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


