Tomatoes, a vital global crop valued for their nutritional benefits and culinary versatility, are under significant threat from various pathogens, particularly Phytophthora infestans, which causes Late Blight (LB). Originally from South America, tomatoes have become a staple crop worldwide. However, diseases, such as LB, can devastate yields by as much as 80%, reminiscent of its role in the historic Irish Potato Famine. Symptoms on tomato foliage and fruits characterized by rapid infection and destruction under humid conditions with visible white sporulation. Survival between crops occurs through infected tomato fruit, producing airborne sporangia that infect healthy foliage. Environmental factors like temperature (15-20 °C) and humidity strongly influence disease progression. Cloudy weather supports late blight due to reduced UV radiation, crucial for sporangia viability. Integrated Disease Management (IDM) presents the most sustainable approach to controlling LB. The objective of this review article was to overview research achievements of tomato late blight management, identifies gaps and suggests future research directions in the area of tomato late blight management research and development. It combines cultural practices, fungicide applications, and the use of resistant varieties. Non-pesticide management options such as cultural and host resistance against the test pathogen did not reach the smallholder vegetable farmers due to limited effort made by the research-extension system. Cultural methods like crop rotation and sanitation are pivotal in reducing pathogen reservoirs, while resistant varieties offer primary defense against Phytophthora infestans. Biological control methods, such as using biocontrol agents and plant extracts, hold promise for environmentally friendly disease suppression. Nonetheless, optimizing their effectiveness under severe disease pressure remains a challenge. Chemical control through fungicides like ridomil remains crucial for immediate disease suppression, underscoring the ongoing necessity for balanced, integrated strategies to mitigate LB’s impact on global tomato production. In this review use of various management options are important to reduce epidemiology of late blight. Future research should focus on developing an IDM with no or minimum input of chemical pesticides. Continuous research and application of these strategies are critical for sustaining tomato yields and ensuring food security amidst evolving environmental and pathogenic challenges.
Late Blight, Tomato, Epidemiology, Integrated Disease Management (IDM), Oomycete, Phytophthora Infestans
Tomato (Solanum lycopersicum L.), a vital vegetable crop, belongs to the Solanaceae family, also known as the nightshade family. It is primarily cultivated for its nutritional value, being a rich source of vitamin C and the beneficial phytochemical lycopene. This herbaceous plant typically grows between 1 to 3 meters in length and 10 cm in diameter, maturing over approximately three months from germination to harvest. Initially green during germination, the tomato fruit transforms into shades of red or pink as it ripens.1 Tomatoes are versatile in culinary applications, commonly consumed raw in salads, cooked into sauces, or integrated into various dishes. Industrially, they are processed into products such as canned tomatoes, purees, juices, ketchup, and dehydrated pulp, making them one of the most utilized fruits globally due to their nutritional versatility.2
Originating from Peru in South America, tomatoes derive their name from the Aztec word “Tomatl”. Introduced to Europe, the first recorded instance was in Italy in 1544, after which its cultivation spread rapidly worldwide. Today, tomatoes are the second most widely cultivated vegetable globally after potatoes, with significant production occurring in countries like Brazil, China, India, and the United States.3 Despite its popularity and economic significance, tomatoes are susceptible to various pathogens including fungi, bacteria, viruses, and nematodes. These pathogens can spread through infected seeds, transplants, equipment, insects, water runoff, and aerosols, posing significant challenges to tomato cultivation worldwide.4
Fungal diseases such as late blight, Alternaria stem canker, and powdery mildew, along with bacterial diseases like bacterial wilt and bacterial stem and fruit rot, are among the most damaging. Viruses such as tomato mosaic and tomato yellow leaf curl also contribute to yield losses.5,6 These diseases of tomato cause yield reductions about 10% to 80%, depends on the severity and conditions of environment.7 Late blight, caused by Phytophthora infestans, is particularly notorious for its devastating effects on tomato crops. Originating in the 19th century, it triggered significant historical events such as the Irish potato famine, leading to mass starvation and migration. P. infestans is a water mold that spreads rapidly in cool, moist conditions, producing spores that can travel through wind or water, infecting plant tissues and causing rapid decay.8,9
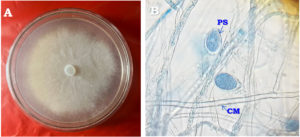
The life-cycle of Phytophthora infestans involves the production of papillate sporangia (Figure 1), which release motile zoospores capable of chemotaxis towards plant tissues.10 This enables the pathogen to propagate quickly under favorable environmental conditions, contributing to its epidemic potential.11 P. infestans produces sporangia that are dispersed by wind, which can either germinate directly or release zoospores through cytoplasmic cleavage upon landing on a suitable host plant. Zoospores exhibit active swimming behavior on water surfaces found on plant tissues and within soil, allowing the pathogen to effectively locate and select its infection site.12 Symptoms of late blight on tomato plants include asymmetrical, water-soaked lesions on young leaves, often surrounded by a lighter halo. During periods of high humidity, white cottony growth may appear on the of leaves underside, progressing to darkened, shrivelled foliage that eventually dies off. The disease also affects tomato fruits, causing firm, oily patches that turn leathery and brown, leading to substantial crop losses if not managed effectively.13
Figure 1. Morphological structures of Phytophthora species: (A) Colony morphology on carrot agar medium; (B) Coenocytic mycelium (CM) and papillate lemoniform sporangia (PS) with branched sporangiophore
Historical significance of late blight
Late blight (LB) is one of the most destructive plant diseases ever documented, particularly affecting tomatoes and potatoes. Without protection, a tomato crop can suffer total yield losses of up to 100% due to LB infection.14 The pathogen responsible, Phytophthora infestans-meaning “plant destroyer” in Greek-originates from the Andean region, which is also where tomatoes and potatoes are believed to have originated.15,16 This common origin for both the host plants and the pathogen was initially proposed in the 19th century, shortly after the Irish potato famine.17 This hypothesis has since been corroborated through isozyme and DNA analyses, which reveal similarities in Pathogenicity among Peruvian, U.S., and European isolates of P. infestans.14-16
The pathogen first caused potato late blight to be recorded in Philadelphia and New York City in the U.S. in 1843. Due to favorable weather conditions, the pathogen’s sporangia were dispersed by the wind, rapidly spreading the disease across neighbouring states. By 1845, late blight had reached areas from Illinois to Nova Scotia and from Virginia to Ontario. The disease was then transported across the Atlantic Ocean to Europe in 1845 via infested seeds. When P. infestans arrived in Ireland, it led to a catastrophic failure of the potato crop. This resulted in the death of approximately one million people and the displacement of another million, many of whom emigrated to the United States. The continued spread of P. infestans throughout the following years led to its global distribution by the early 20th century, causing extensive damage to potato and tomato crops worldwide. Today, the pathogen still poses a significant threat, with the ability to devastate unprotected crops in fields, greenhouses, or under plastic covers within just seven to ten days.15 The economic impact includes reduced yields, lower fruit quality (such as decreased specific gravity), reduced storability, and increased costs for fungicide treatments.14
Molecular techniques for Identification of Phytophthora infestans
For molecular analysis, pure mycelium from potato slices was cultured on oat agar medium supplemented with rifampicin and subcultured every 3-4 weeks. DNA extraction followed a modified method where mycelia were ground in liquid nitrogen, mixed with an extraction buffer, and treated with nuclei lysis buffer and SDS. Chloroform alcohol extraction was performed, and DNA was precipitated with sodium acetate and ethanol, and then re-suspended in distilled water. PCR amplification targeted the b-tubulin gene using specific primers (TUBUR1: CCT GGT ACT GCT GGT ACT CAG and TUBUF2: CGG TAA CAA CTG GGC CAA GG), producing a 990 bp fragment. This PCR product was sequenced, and the sequences were compared using DNAMAN software. Analysis of six Jordanian isolates revealed nucleotide identity percentages ranging from 79% to 98% among themselves. However, when compared with P. infestans isolates from other countries, the Jordanian isolates showed lower nucleotide identity, ranging from 79% to 85%. Specifically, isolates 2T5, 2T18, and 2T22 had nucleotide identities of 78%-79% with world isolates, while 2P5 and 3P10 had identities ranging from 84% to 85%. The isolate H4 showed 81%-82% identity with global isolates. The study concluded that PCR is a reliable method for detecting P. infestans in infected plants and that isolates from tomato and potato tissues showed close genetic relationships within their respective hosts, although some isolates exhibited significant variability.18
Morphological and serological examinations were performed, with microscopic analysis revealing characteristic lemon-shaped sporangia (Figure 1) and a positive serological response from immunostrip assays. Although serological tests identified the presence of Phytophthora, the intensity of bands suggested a low pathogen concentration, leading to inconclusive results due to possible similarities with closely related species. To confirm species identification, genomic DNA was extracted from the mycelia of the isolates using a phenol extraction method. Polymerase chain reaction (PCR) was then conducted with specific primer pairs (Table 1) to amplify target DNA regions. Electrophoresis of the PCR products revealed that only three of the isolates matched P. infestans, indicating that the remaining isolates were likely other closely related Phytophthora species. This approach demonstrated that PCR, despite some variability in DNA purity and concentration, provided accurate and reliable identification, affirming its utility in distinguishing P. infestans from other Phytophthora species.19
Table (1):
List of primer pairs used for molecular validation of Phytophthora infestans
| Primer ID | Primer Sequence (5’-3’) | Amplicon size (bp) | Temp. (°C) | Ref. |
|---|---|---|---|---|
| AE-7-1 | GCC GCC GAC ATA TTG AAT | 171 | 50 | 20 |
| AE-7-2 | CAA ATC TGC GAA CGA GAC AT | |||
| O-8-1 | AAG ATG ATG TTG GAT GAT TG | 245 | 58 | – |
| O-8-2 | TGC CTG ATT TCT ACC TTC T | |||
| INF-F | TGG GCG AGC CCT ATC AAA A | 613 | 50 | 21 |
| INF-R | CCG ATT CAA ATG CCA AGC TAA | |||
| ITS3 | GCA TCG ATG AAG AAC GCA GC | 612 | 50 | 22 |
| ITS4 | TCC TCC GCT TAT TGA TAT GC |
Disease cycle
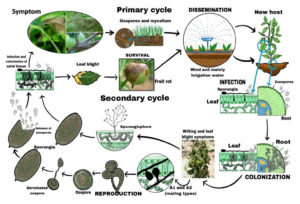
The fiscal and social impacts of the pathogen Phytophthora infestans, which causes late blight (LB), have spurred significant scientific research into its biology. This research, particularly focused on potatoes, has also illuminated the understanding of LB in tomatoes. P. infestans is particularly destructive in regions where both potatoes and tomatoes are cultivated year-round, such as the highland tropics of Africa, the Americas, Asia, and Europe.14 The success of P. infestans as a pathogen is due to its effective reproduction methods, both asexual and sexual. Asexual reproduction is the primary driver of seasonal epidemics. In this mode, the pathogen produces numerous sporangia per lesion on sporangiophores, which are structures that facilitate the dispersal of sporangia through wind and rain. The disease cycle starts when sporangia land on host plant tissue that is covered with a film of water. This water is crucial for the motile, germinated spores to move toward a penetration site.23 Sporangia can germinate directly through germ tubes or via zoosporogenesis, the latter being favored in cooler, moist conditions and allowing infections to occur over a wider range of weather conditions.24
Direct germination happens at temperatures above 21 °C, ideally around 25 °C, within 8 to 48 hours. In cooler temperatures (below 21 °C), sporangia release biflagellate zoospores, which, at an optimum of 12 °C, form germ tubes after encysting.25 These tubes develop into appressoria, penetrating the host leaf cuticle or occasionally the stomata. The optimal temperature for germ tube differentiation is between 21 and 24 °C. Inside the host, intercellular hyphae form and use haustoria to establish a biotrophic feeding relationship. Rapid colonization occurs at temperatures between 22 and 24 °C, and LB symptoms typically appear within 5 to 10 days after inoculation. Sporulation produces new sporangia, which release zoospores to spread the disease further. Disease development halts above 35 °C, but the pathogen can persist in living tissue and resume its spread when conditions improve.26 Sporangia contain organelles not found in hyphae, such as vesicles, kinetosomes, and flagella. Zoosporogenesis involves the cleavage of the sporangial cytoplasm and formation of flagella, leading to the release of uninucleate zoospores. Sporangia can remain viable for up to a week under favorable conditions, although their germination-related mRNA levels generally decline over time.14,23,24
The sexual life cycle of P. infestans (Figure 2) requires mating between individuals of opposite mating types, A1 and A2,27,28 which are compatibility types determined by mating hormones.24 Interaction between these types leads to gametangial formation and sexual reproduction. During this cycle, diploid mycelia undergo meiosis to form haploid antheridia and oogonia. Fertilization produces thick-walled oospore that can survive harsh conditions outside the living host plant, serving as a long-term inoculum source.14,29 Oospores can germinate when conditions are favorable, leading to new, potentially more aggressive progeny.25,30,31 LB remains a major threat due to P. infestans’ rapid reproduction and destruction capabilities. The asexual cycle of penetration, colonization, sporulation, and dispersal can complete in less than five days, with each lesion producing up to 300,000 sporangia daily.32 Early disease stages are hard to detect, making timely fungicide application challenging. The migration of the A2 mating type outside Mexico in the 1980s introduced sexual reproduction and genetic recombination, resulting in more aggressive isolates and complicating disease management.30,31
Pathogenesis of Phytophthora infestans
Phytophthora infestans, the causative agent of late blight, exhibits a complex life cycle involving multiple specialized cell types adapted for various stages of infection and reproduction.33 Its life cycle includes both asexual and sexual reproduction, as well as stages of pathogen dispersal, spore germination, and interaction with host plants.14 The infection process begins with a biotrophic phase, where the pathogen extracts nutrients from living plant cells. This involves the formation of a penetration peg that breaches the plant cuticle and enters an epidermal cell, creating an infection vesicle. The pathogen then produces haustoria that extend into the plant cell wall, facilitating nutrient uptake while remaining outside the plant cell membrane.23,34
As the infection progresses, the pathogen shifts to a necrotrophic phase, during which it feeds on dead and dying plant tissue. This phase is characterized by extensive tissue necrosis and sporulation.35 The pathogen develops sporangiophores, which grow through plant stomata and release numerous asexual sporangia. Sporulation is favored under dark conditions, as continuous light has been shown to inhibit sporangia formation. The lack of evidence for a light-regulated clock in P. infestans suggests that plant-derived signals related to the light-dark cycle may influence sporulation.36 At the plant surface, P. infestans zoospores position themselves with their ventral side facing the plant, shedding their flagella and secreting proteins from cortical vesicles to form cysts. These cysts germinate, allowing the pathogen to grow hyphae that penetrate the plant tissues. Mature sporangia can also initiate infections directly under favorable conditions, pre-synthesizing the proteins needed for zoosporogenesis and encystment.14,23
Symptoms
Tomato plants are vulnerable to late blight, a disease that also affects potatoes. The symptoms on tomato foliage resemble those seen on potatoes, with rapid infection and destruction possible when attacked by P. infestans, especially in humid conditions where white sporulation (sporangia and sporangiophores) can be observed. The pathogen can spread within tomato stems and through infected tomato transplants. The pathogen’s transmission through contaminated tomato seedlings was blamed for a major outbreak that occurred in the US in 2009.37 Late blight on tomato fruit appears as hard, dark brown lesions that can enlarge and eventually kill the fruit altogether. This can frequently result in soft rot and disintegration that resembles potato tubers. If sporulation and aerial dispersal can place, discarded infected fruits may become a possible source of inoculum.38
Epidemiology
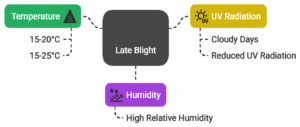
The disease triangle model, which takes host, pathogen, and environment interactions into account, captures the influence of environmental factors on disease progression.39 Numerous factors impact late blight’s survival, germination, penetration, and sporulation (Figure 3). It is a disease that is especially sensitive to transient environmental changes. High relative humidity along with temperatures between 15 and 20 °C are ideal for the onset of late blight.40 The impact of temperature on infection varies depending on the genotype, with temperatures between 15 °C and 25 °C identified as optimal for infection.41 Although late blight can reproduce at temperatures as high as 30 °C, temperatures above this range are generally unsuitable for its reproduction, although survival may still occur in some stages.42 Cloudy days are particularly conducive to late blight due to reduced UV radiation, which can significantly diminish sporangia viability.41
Integrated Disease Management (IDM)
Implementing an integrated approach that combines cultural techniques, fungicide spraying, and the use of cultivars with broad-spectrum genetic resistance against LB is the most sustainable method of managing tomato LB.43
Cultural control
The main objectives of using this practice are: Reducing inoculum buildup of LB, preventing inoculum from tomato transplants, lowering infection rates of LB, and creating an environment that is not conducive to the growth and spread of the P. infestans, Particular cultural customs that are typically used to restrict LB include.44 Every two to three years, crops should be rotated to non-host crops in order to control late blight. The Solanaceae family includes nightshades among other weeds and ornamental plants that are known to be susceptible to late blight, in addition to potatoes and tomatoes. Rotation schedules may need to be modified to account for this extra inoculum source if oospore production proliferates. Oospores can remain in soil for a very long time, even while the virus survives in infected tubers, which break down rather quickly.9 Cultural techniques can mitigate the incidence of late blight on potatoes to a certain degree. The plant components, leaves, and tubers that are infected are the main source of late blight infections. Because they can survive in soil and develop inside plant tissue, oospores are important soil-borne inoculants. Crop rotation is directly correlated with the early development of late blight disease infections. Instances of infections usually start early in fields without crop rotation. In farms where there had been a crop rotation of three or more years between potato crops, the reduction in early infections was particularly noticeable.45,46 The important role that crop rotation has in lowering the incidence of P. infestans-caused soil-borne infections is one reason for the decreased inoculum survival in non-rotated fields when compared to rotated ones. The risk of late blight outbreaks can be effectively mitigated by planting potatoes on the appropriate day. On average, late blight epidemics were less severe when plants were planted in the final ten days of September.47
Minimize use of nitrogen and use moderate fertilization to postpone the onset of late blight. It has been demonstrated that higher potassium and phosphorus levels increase yield in years when late blight is likely to occur.48 To mitigate foliar blight, further preventive steps include selecting potato cultivars resistant to late blight, making sure fields are well-ventilated, pre-sprouting tubers before to planting, and starting early planting.49 Effective measures to combat tuber blight include planting potatoes on big, steep ridges, timing mechanical weeding and harvesting precisely, and avoiding long-distance transfers or quick movements of harvested tubers.50
When polyethylene shelter was used in conjunction with sanitation treatments, the quantity of sick leaves and the severity of the disease were both reduced. When tomatoes were cultivated in early shelters together with sanitation, flower and fruit production was far higher than when sanitation was used alone. The growth and productivity of tomatoes as well as late blight were not significantly impacted by an increase in planting density. When compared to the fungicide treatment and control, there were significantly (P <0.001) less sick leaves produced by sanitation. The study found no statistically significant differences across treatments or experiments. However, there was a significant interaction between treatments and experiments, which could be explained by the relatively low levels of damaged leaves in some sanitation treatments.51 It has been found that an epidemic can be prevented or postponed by three to six weeks by successfully eradicating all first infections that resulted from early potatoes. Studies show that late blight outbreaks are typically caused by diseased plants discovered in abandoned piles. In order to reduce the major cause of the disease, it is imperative to cover these piles with black plastic sheets for the duration of the season and make sure the seed tubers do not become infected.52,53
The 2010 results demonstrated a significant (P = 0.002) increase in the severity of late blight caused by Phytophthora infestans. The mean number of late blight infections varied from 1.8 to 30.8 in experimental plots in an open field among cultivars, whereas in high tunnels for these years, it was only 0 to 6.5. In addition, compared to high tunnels, the open field saw more hours of leaf wetness annually (857 versus 1,060 in 2010, 598 versus 998 in 2011, and 885 versus 923 in 2012). In high tunnels, cultivar sensitivity to late blight could not be differentiated due to low disease pressure. However, all five cultivars showed evidence of vulnerability in the open field, consistently displaying the highest number of lesions.54
Planting tomatoes in sequence or in multiple crops throughout time will reduce the chance that late blight may wipe out every tomato at once.55 Tighter intercrops inhibited tomato growth and production, but soybean (Glycine max) or sesame (Sesamum indicum) intercropping, when combined with sanitation, limited the development of late blight.56 In western Uttar Pradesh, specifically in Meerut, research has explored mixed cropping and barrier crops as effective strategies to mitigate the severity of potato late blight. Findings indicated that planting resistant and susceptible potato cultivars alternately delayed the onset of disease by 7 days. Similarly, using oat as a barrier crop also resulted in a 4-days delay in disease spread of potato late blight. These methods demonstrate promising potential in managing and slowing down the progression of late blight, offering practical approaches for farmers to enhance crop health and yield.57 Strip cropping with potatoes planted in a perpendicular arrangement to the prevailing wind and adjacent to grass clover substantially decreased the impact of late blight in organic farming.58
Use of resistant varieties
Host resistance is thought to be the most efficient and ecologically benign method of managing late blight resistance.59 A major factor in the management of this disease has been the creation of resistant cultivars and efficient screening techniques.60-65 Prominent cultivars such as Brandywise, Stellar, and Iron Lady show excellent resistance (Ph-2 + Ph-3) against US-11, US-23, and US-24 late blight strains. Some cultivars, such Mountain Magic and Merit, have strong tolerance to several strains. While certain heritage cultivars, like Aunt Ruby’s German Green and Brandywine, exhibit variable degrees of resistance, others, like Stupice and Black Plum, are unproductive against specific strains. The study highlights the different resistance levels seen in lab and field settings, which helps choose appropriate tomato cultivars for managing late blight.65,66 According to Lal et al., these hybrids show variable levels of foliage resistance under various circumstances, suggesting that there may not always be a correlation between foliage and tuber resistance to late blight.67 The emergence of Kufri Mohan, a late blight-resistant variety, recently highlights the continuous endeavors to develop plants with resistance against diseases.68
Internationally, cultivars like Payette Russet have been developed in the USA, offering dual resistance against LB in both foliage and tubers, along with high resistance to potato virus Y.69 Particularly in the sub-tropical plains, somatic hybrids like P4, P8, and P10 have been created in India by combining desired features like high tuber dry matter concentration and resistance to late blight by conventional breeding procedures.70,71 These advancements highlight ongoing global efforts to enhance potato cultivars with durable resistance to late blight, addressing both agricultural and environmental concerns effectively (Table 2).
Table (2):
List of some important resistant varieties of tomato used against late blight disease
No. |
Name of variety |
Country of development |
Developer/References |
|---|---|---|---|
1. |
Swarna Lalima |
India |
72 |
2. |
Arka Rakshak |
India |
73 |
3. |
Arka Samrat |
India |
74 |
4. |
H-88-78-1 |
India |
75 |
5. |
Palam Pink |
India |
76 |
6. |
Arka Vikas |
India |
77 |
7. |
Hisar Lalit |
India |
78 |
8. |
Punjab Chhuhara |
India |
79 |
9. |
Pant T-3 |
India |
80 |
10. |
Atka Meghali |
India |
81 |
11. |
Mountain Merit |
USA |
82 |
12. |
Defiant PhR |
USA |
83 |
13. |
Iron lady |
USA |
84 |
14. |
Jasper |
USA |
– |
15. |
Lizzano |
USA |
– |
16. |
Rose de Berne |
USA |
– |
17. |
Plum Regal |
USA |
85 |
18. |
Magic Mountain |
USA |
– |
19. |
Matt’s Wild Cherry |
USA |
86 |
20. |
Mountain Fresh Plus |
USA |
87 |
21. |
Legend |
USA |
88 |
22. |
Crimson Crush |
UK |
89 |
23. |
Lossetto |
UK |
– |
24. |
Mountain Gold |
USA |
90 |
25. |
Koralik |
Germany |
91 |
26. |
Sakura |
Japan |
92 |
Biological control
Managing late blight through eco-friendly methods poses significant challenges, especially under high disease pressure and favorable environmental conditions.29 Yet, the growing awareness of chemical impacts on the environment and human health underscores the increasing importance of eco-friendly approaches. In the late 20th century, European and American countries began exploring botanicals as eco-friendly alternatives for controlling late blight.93,94 Many studies have been conducted on the biological management of P. infestans, the oomycete that causes tomato late blight. Despite the fact that there has been a large body of scientific research into microorganisms with potential for the biological control of late blight disease, relatively few commercial biocontrol agents, licensed to control late blight, exist.95 Some important bacterial and fungal biocontrol agents are enlisted in Table 3.
Table (3):
List of biocontrol agents (BCAs) and their antagonistic activities against Phytophthora infestans
| Type of BCA | Genus species and strains | Activity antagonistic to the target pathogen | Ref. |
|---|---|---|---|
| Bacteria | Bacillus amyloliquefaciens 17A-B3 | 1; 2; 3 | 102 |
| B. subtilis 30B-B6 | 1; 2; 3 | 102 | |
| B. velezensis G341 | 4 | 103 | |
| Pseudomonas brenneri 43R-P1 | 1; 2; 3 | 102 | |
| P. chlororaphis R47 | 1; 5; 6; 7 | 100,104-108 | |
| P. fluorescens LBUM636, R76, S35, S49 | 1; 5; 6; 7 | 104-109 | |
| P. frederikbergensis S04, S19 | 1; 5; 6; 7 | 100,104-108 | |
| P. jessenii S34 | 1; 5; 6 | 100,104-108 | |
| P. koreensis 2.74 | 8 | 101 | |
| P. marginalis R84 | 1; 5; 6; 7 | 100,104, 105,107, 108 | |
| P. protegens 44R-P8 | 1; 2; 3 | 102 | |
| P. putida R32 | 1; 5; 7 | 100,104,105,107 | |
| Fungi | Chaetomium aureum | 1; 9 | 110 |
| C. cochliodes | 1; 9 | 110 | |
| C. globosum Cg-6, F0142 | 1; 9 | 111,112 | |
| Rhizopus irregularis MUCL41833 | 6 | 113 | |
| Yeasts | Aureobasidium pullulans L1, L8 | 1; 7; 11 | 114 |
| Curvibasidium pallidicorallinum strain 46 | 12 | 115 | |
| Metschnikowia pulcherrima | 12 | 115 |
Activity antagonistic to the target pathogen: 1-Mycelial growth inhibition; 2-cellulase and protease enzymatic activities; 3-production of siderophores and biosurfactants; 4-Mycelial growth inhibition with diffusible and volatile antimicrobials; 5-inhibition of sporangia germination, zoospore release, and germ tube elongation; 6-root colonization (epiphytic and endophytic); 7-mission of VOCsa with inhibitory activity against mycelial growth and sporangial germination; 8-Production of biosurfactant with inhibitory activity against mycelial growth; 9-production of antibiotics; 10-glucanase enzymatic activity; 11-induction of ISRb; and 12-Reduction of leaf lesion size
Bacillus spp.
Research has demonstrated that Bacillus species effectively suppress P. infestans. For example, in a two-year field trial, B. subtilis and B. pumilus were able to considerably reduce late blight.96 In a different investigation, B. subtilis, in the form of the biocontrol-formulated product Serenade, was treated in conjunction with the pathogen and simultaneously produced both protective effects and a decrease in disease pressure.97 However, it was discovered that liquid formulation of the treatment was vital to the biocontrol process, highlighting the significance of secondary metabolites.98,99
Pseudomonas spp.
Pseudomonas species are excellent makers of volatiles, biosurfactants, diffusible antibiotics, HCN, and siderophores, among other secondary metabolites with potent anti-oomycete activity.100 Pseudomonas koreensis strain 2.74’s cyclic lipopeptide (CLP) lokisin, in particular, shown remarkable control action against potato blight at low doses.101 It was discovered that the CLP lokisin mechanism involves lysis that follows a breakdown of zoospore integrity. Furthermore, no phytotoxicity was seen, even at ten times the efficient control concentration, indicating the environmental soundness of CLP lokisin.29
Arbuscular mycorrhizal fungi
The genus Glomeromycota comprises arbuscular mycorrhizal fungi (AMF), which are helpful organisms that aid in the encouragement of plant growth. Nearly 80% of vascular plants, including tomato and pepper, naturally host AMFs.116 Their introduction as biocontrol agents is therefore well-known, and research on their effectiveness is required. A BCA called Chaetomium globosum (Kunze ex Fr.) is known to oppose a variety of plant diseases, including multiple Phytophthora species.117 The commercial biofungicide KetomiumVR was registered and is currently being used globally. Studies on its effectiveness against the solanaceous crop-infecting Phytophthora spp., however, are scarce. According to a number of studies, C. globosum has antagonistic effect against P. infestans in tomato and potato plants.111,112 Fungal metabolites (chaetomins and chaetoviridins) and glucanolytic activity are linked to biocontrol effects. In vitro and in vivo studies by Park et al.111 showed the direct biocontrol action of chaetoviridin A isolated from C. globosum culture. There are other strains in the genus Chaetomium that may be used as biocontrol agents against P. infestans. Chaetomium cochliodes, Chaetomium aureum, Chaetomium nozdrenkoae, and Chaetomium elatum have all been reported to exhibit in vitro inhibition of P. infestans mycelial growth and sporangium germination, and C. aureum has shown complete inhibition.110
Yeasts
Due to the extensive history of research on P. infestans, all BCAs-including ones that aren’t employed against other Phytophthora diseases such yeast-like organisms-have been suggested for usage. The yeast-like fungus Aureobasidium pullulans, sometimes known as De Bary, is known to suppress a number of postharvest diseases, but little is known about how well it functions prior to harvest.118,119 Francesco et al.114 were the first to identify its possible antagonistic action against tomato-growing P. infestans. By stimulating plant defense enzymes and producing antagonistic metabolites, A. pullulans demonstrated both therapeutic and protective qualities. Metabolites that were both volatile and diffusible significantly inhibited pathogen growth. Hadwiger et al.115 have also reported on the biocontrol capability of two additional yeasts against P. infestans: Curvibasidium pallidicorallinum and Metschnikowia pulcherrima.
Use of plant extracts
In ecosystem plants are surrounded by various enemies which defend themselves by producing secondary metabolites like terpenes, phenols and nitrogen and sulphur compounds. A new approach to control the pathogens which hampers quality food production has been implemented by the application of plant extract.120 Indeed, several studies have shown that plant extracts can have strong antifungal properties, often being more effective or comparable to synthetic fungicides.121 The mycelial growth of Phytophthora infestans was significantly inhibited by leaf extracts of onions, garlic, Reynoutria japonica, onions, and Rheum coreanum, out of 100 species evaluated across 54 plant families. Extracts from Malus toringo in particular showed significant inhibition and worked effectively to treat late blight.122 The Plectranthus barbatus, Lantana camara and Sphaeranthus suaveolens plant extracts were effective as a commercial synthetic pesticide in reducing the growth of Phytophthora infestans and hence can be used alone as an alternative to chemical fungicide.123 The clove extract was considered as the best plant extract used against Phytophthora infestans causing late blight.124 Syzygium cumini leaves extract has a great potential as an alternative of chemical fungicides to control the late blight disease of potato in eco-friendly way.125 Leaf extracts of Podophyllum hexandrum were found more effective in minimizing the incidence of late blight disease caused by and produced better tuber yield under natural field conditions.126
The effectiveness of ethanol extracts derived from 20 different plant species against late blight (Phytophthora infestans) on tomato leaves was evaluated. Paeonia suffruticosa extracts inhibited mycelium growth and zoospore release of P. infestans and P. cubensis. Bioautography identified multiple antifungal zones in H. helix extracts and one in P. suffruticosa. Overall, higher extract concentrations were more effective against late blight.127 Yusuf128 evaluated antifungal activities of Xanthium strumarium, Laurisnobilis, Salvia officinalis and Styrax officinalis which were the most active against mycelial growth of P. infestans.
The use of Allium sativum and Azadirachta indica aqueous plant extracts at a concentration of 30% was found to be the most promising and effective measure against the late blight.129 Phytoextracts from Azadirachta indica and Allium sativum are known to significantly hinder fungal growth and spore germination.130 Many secondary metabolites, including flavonoids, scopolamine, quinones, terpenoids, polyphenols, and allicin, are thought to be responsible for this impact. These compounds disrupt the mitochondria, cell membrane, and cell wall of pathogens, thereby inhibiting their growth. Different extraction technologies are employed to obtain these phytoextracts, which act as natural bio-fungicides. Further research is essential to explore their potential in managing fungal infections and their impact on human health.131 For the P. infestans inoculated plants, the Mexican marigold and ginger treatments yielded the highest fruit weights, while the outcomes were not significantly different. There were no discernible differences between the tomato plants treated with Ridomil Gold® and those sprayed with essential oils made from garlic and Mexican marigold.132
Chemical control
The best defence against late blight is the application of fungicide. Both commercial growers and home gardeners can use protective fungicides against late blight such as Mancozeb (Manzate) and chlorothalonil (Bravo, Echo, Equus, or Daconil).133 According to Lewis,56 organic gardeners can prevent late blight infections by using fixed copper products like Kocide. According to Tumwine et al., an experiment conducted to combat tomato late blight (Phytophthora infestans) revealed that plants treated with fungicides kept the greatest amount of blossoms and connected fruits and produced the highest yields.51 It was discovered that combining resistant cultivars with foliar sprays of Ridomil was an efficient way to decrease tomato late blight outbreaks and boost fruit production. It is therefore advised to employ this spray frequency as, in comparison to other treatments and the control, it provided the best protection against late blight and the greatest financial advantage.134
Three resistant varieties and three distinct fungicides (Meru, Cal-J and Tanya, Ivory 72 WP, Volar MZ 690 WP, and Topsin-M 70 WP, with intervals of 14-, 10-, and 7-days, respectively) were applied in Morogoro, Tanzania. The outcome was variety. Tomato late blight disease was resistant in Meru, but very sensitive in Tanya and Cal-J cultivars. Despite this, Meru produced the fewest fruits per plant, which resulted in the lowest yield. Fungicides Ivory 72 WP and Volar MZ 690 WP demonstrated greater field efficacy against P. infestans. Compared to Topsin-M 70 WP, these two fungicides dramatically decreased disease intensity to the lowest level. The major tactic employed to halt the spread of late blight was the worldwide spraying of fungicides.135 Azoxystrobin, fluazinam, mandipropamid, metalaxyl, and other compounds that target specific metabolic pathways are among the most commonly employed site-specific agents to combat late blight. Because of their unique toxicity, they are less dangerous for people and the environment, but they also raise the possibility that P. infestans could become resistant to them with a single mutation, which could take some time and result in decreased viability. Nonetheless, these fungicides are still frequently used and regarded as being sufficiently effective.136 There are two main types of fungicides used to treat late blight: penetrant and protectant. Fungicides can prevent or minimize the development of new symptoms when applied promptly, but they are unable to get rid of existing late blight symptoms.137
Use of a prophylactic spray, such as one contains copper, chlorothalonil, or mancozeb, ideally before any symptoms appear. Alternate single sprays of a contact treatment with double sprays of a systemic agent when using Metalaxyl, cymoxanil, dimethomorph, or strobilurin. Remember that in some nations the preharvest interval is 5 days; therefore Fruit is not treated with mancozeb if producers harvest twice or more each week (i.e., the time between the last spray and harvest). Phosphorous acid should also be checked because it affects oomycetes both directly and indirectly. Its capacity to activate plants’ natural defense response against pathogen assaults accounts for the indirect effect.138
Some new fungicides are promising against late blight pathogen.139 Iprovalicarb is a protective, curative and antisporulant fungicide with translaminar and acropetal mode of action. It gets distributed evenly in plants. It is an inhibitor of phospholipid biosynthesis and cell wall synthesis. Propineb is a non-specific, multi-site fungicide with protective action against germinating conidia. It works as a good curative and anti-sporulant on disease causing pathogens. The excellent residual activity of famoxadone, combined with the strong curative attributes of cymoxanil is likely to contribute to the high level of performance if both of these fungicides are used together in the field.140 Dimethomorph is moderate amount of translaminar and acropetal systemicity and disrupts all stages of asexual life cycle of P. infestans.139
Forecasting models
Tomato late blight (Phytophthora infestans) forecasting models are essential instruments for controlling and reducing this harmful plant disease.25 Several important models that include different environmental and disease parameters have been created to forecast the risk of late blight (Table 4). One well-known model is BlightCast, which predicts the likelihood of late blight by taking into account meteorological factors including temperature, humidity, and leaf wetness. Based on these factors, this model aids in calculating the probability of infection.141 BlightPro, web-based Decision Support System (DSS) for managing potato and tomato late blight uses weather data, crop, and management information to predict disease dynamics and recommend fungicide applications. By integrating weather forecasts and crop-specific data, the DSS provides location-specific management advice, optimizing fungicide use. It includes an alert system for critical thresholds and serves as a tool for growers, consultants, and educators. Field and simulation experiments showed that DSS-guided schedules enhance fungicide efficiency and disease suppression, adjusting applications based on weather conditions to optimize crop protection strategies.142 To estimate infection risk, the TOMCAST model-which was created especially for tomatoes-combines historical disease trends and meteorological data.143 In a similar vein, the Phytophthora Decision Support System (P-DSS) incorporates disease dynamics and environmental factors into its forecasts to estimate the frequency and severity of late blight outbreaks.144 The Fungal Forecast model is another useful tool that can be used to manage late blight. It uses information from meteorological stations and field observations to generate timely alerts and recommendations.145 Forecasting systems are widely used to predict the application of fungicides for managing late blight. However, airborne inoculum has rarely been included in these forecasting systems. Monitoring the sporangia in crop environments may offer an opportunity to improve late blight forecast systems by integrating pathogen pressure.146 Finally, to effectively manage late blight, an Integrated Pest Management (IPM) method integrates several forecasting models with broader pest management strategies. This all-encompassing strategy combines different forecasting technologies with other pest management techniques.147
Table (4):
Development of Forecasting Models to predict the risk of late blight outbreaks
Forecasting Model |
Developed by |
Year |
Input variables |
Reference |
|---|---|---|---|---|
Blitecast |
Dr. William E. Fry and his team at Cornell University, New York, USA |
1970s |
One of the earliest and most widely used models for predicting late blight. It calculates a “severity value” (SV) based on temperature, humidity, and leaf wetness duration to predict the risk of late blight outbreaks. |
148,149 |
Smith Period Model |
Dr. L.P. Smith in the United Kingdom |
1950s |
A rule-based model that identifies periods favorable for late blight infection. A Smith period is defined by two consecutive days with a minimum of 10°C temperature and at least 11 hours each day with relative humidity above 90%. |
150 |
NegFry Model |
Dr. Henrik Nielsen from Denmark (Danish Institute of Agricultural Sciences) and Dr. William E. Fry from Cornell University, USA |
1983 |
A decision-support model that predicts late blight risk based on temperature, relative humidity, and rainfall. It calculates daily infection risks and incorporates fungicide degradation over time. |
151 |
SimCast Model |
Dr. William E. Fry and his colleagues at Cornell University, USA |
1990s |
A simulation-based model that predicts late blight development by integrating weather data and fungicide application schedules. It uses temperature and leaf wetness data to simulate the growth of the pathogen. |
152 |
ProPhy Model |
Dr. David A. H. Brown and his team |
Early 2000s |
The model was designed to provide decision support for managing late blight in potatoes and tomatoes by integrating weather data and disease forecasting. |
153 |
Plant-Plus Model |
Dr. Arne Hermansen and colleagues at the Norwegian Institute of Bioeconomy Research (NIBIO), Norway |
Early 2000s |
A sophisticated model that integrates field-specific information with weather data to forecast late blight risk. It incorporates various factors like host resistance, planting date, and fungicide efficacy. |
154 |
Phytophthora DSS (Decision Support System) |
Primarily led by teams from the Wageningen University & Research (WUR) in the Netherlands and the Danish Institute of Agricultural Sciences (DIAS). |
Early 2000s |
A comprehensive decision support system developed in Europe, combining several models to forecast late blight risk. It uses local weather data and integrates other factors like soil moisture, crop growth stage, and spore dispersal. |
155 |
TOMCAST (Tomato Disease Forecasting System) |
Dr. William E. Fry and his colleagues at Cornell University, USA. |
Early 1990s |
This model calculates disease severity values (DSVs) using temperature and leaf wetness duration. It helps predict the timing of disease outbreaks and fungicide applications. |
156 |
Wallin’s Model |
Gustav Wallin |
1960s |
An early model that combines temperature, humidity, and rainfall data to predict the development of late blight. It emphasizes the importance of continuous periods of high humidity for disease onset. |
157 |
Lateblight DSS |
Dr. Arne Hermansen and his team at the Norwegian Institute of Bioeconomy Research (NIBIO), Norway |
2002 |
A decision support system designed for late blight management in potatoes. It uses a combination of weather data, crop growth stage, and fungicide application records to predict disease risk. |
158 |
PHYTEB |
Dr. Bert van den Bosch and his team at Wageningen University & Research, Netherlands. |
2004 |
Integrates weather data to predict the risk of late blight outbreaks.Provides recommendations for fungicide application and other management practices based on forecasted disease risk. |
159 |
Late blight affects potato and tomato crops worldwide, with severe economic impacts on farmers and the agricultural industry. Vegetable seed production, particularly of tomato, has become an attractive business enterprise in many parts of the country. Growing of tomato all through the season has created conducive conditions for the buildup of many diseases demanding comprehensive research outputs for their effective and sustainable management. Controlling late blight is challenging due to the pathogen’s rapid life cycle, high reproductive potential, and ability to spread through airborne spores over long distances. Its ability to evolve and develop resistance to fungicides further complicates management efforts. There is a need of effective and sustainable disease management methods. The bulk of research activities conducted on tomato disease management so far dealt with pesticide chemicals. It is well known that repeated use of synthetic pesticides alone may create resistance within population of various pathogens. Moreover, since tomato are frequently treated with pesticides, there is a greater likelihood of direct human exposure and pesticide residue on fresh tomatoes, thereby adversely affecting both domestic and export tomato market.
This review clearly shown that there are critical research gaps to be filled for management of tomato late blight. Effective management requires an integrated approach, combining resistant cultivars, timely fungicide applications, cultural practices (e.g., crop rotation, removal of infected debris), and continuous monitoring. Accurate forecasts enable farmers to take timely preventive actions, minimizing disease impact and improving sustainability. Advancements in molecular biology, genomics, and remote sensing are enhancing our understanding of Phytophthora infestans and its interaction with host plants. This knowledge can lead to the development of more effective resistant varieties and targeted control strategies. Early detection, accurate forecasting, and timely intervention are critical to mitigating the impact of this devastating disease.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Fatima F, Javed MU. Seedling and Germination Stages of Tomatoes (Solanum lycopersicum) in Pakistan under Home Remedies, Packing and Transported to Other Countries with the Proper Identification of Pest and Diseases. Acta Scientific Agriculture. 2019;3(9):57-66
- Bai Y, Lindhout Y. Domestication and breeding of tomatoes: What have we gained and what have we gain in the future? Annals of Bot. 2007;100(5):1085-1094.
Crossref - Food and Agriculture Organization of the United Nations (FAO). World tomato production and productivity areas, Food and Agriculture Organization of the United Nations. FAO Data base. 2017. Accessed on December 27, 2023
- Ramya Bharathi SA, Meena B, Raguchander T. Induction of chitinase and b-1,3- glucanase PR proteins in tomato through liquid formulated Bacillus subtilis EPCO 16 against Fusarium wilt. J Today’s Biol Sci Res Rev. 2012;1(1):50-60
- Accotto GP, Nervo G, Acciarri N, et al. Field evaluation of tomato hybrids engineered with tomato spotted wilt virus sequences for virus resistance, agronomic performance, and pollen-mediated transgene flow. Phytopathol. 2005;95(7):800-807.
Crossref - Marissa S. Late blight of tomato and potato, University of Minnesota Extension discovers science, quick fact sheet. 2021.
- Sandeep KGM, Sriram S, Laxman RH, Harshita KN. Tomato late blight yield loss assessment and risk aversion with resistant hybrid. J Hortic Sci. 2022;17(2):411–416.
Crossref - Hampson CM. History, biology and control of potato late blight disease in Canada. Semantic Scholar. 1992;15(4):223.
Crossref - Ristaino JB, Fry WE, Birch PRJ, et al. Five reasons to consider Phytophthora infestans a re-emerging pathogen. Phytopathol. 2015;105(7):66-81.
Crossref - Perrine-Walker F. Phytophthora palmivora-Cocoa Interaction. J Fungi. 2020;6(3):167.
Crossref - Martin FN, Blair JE, Cofey MD. A combined mitochondrial and nuclear multilocus phylogeny of the genus Phytophthora. Fungal Genet Biol. 2014;66:19-32.
Crossref - Boevink PC, Birch PRJ, Turnbull D, Whisson SC. Devastating intimacy: the cell biology of plant-Phytophthora interactions. New Phytol. 2020;228(2):445-458.
Crossref - Meadows I, Quesada-Ocampo L. Tomato Late Blight. Vegetable Pathology, Factsheets, NC State Extension. Retrieved from https://content.ces.ncsu.edu/tomato-late-blight. (Accessed on 23 March 2024, 5:01 PM).
- Nowicki M, Foolad MR, Nowakowska M, Kozik, EU. Potato and tomato late blight caused by Phytophthora infestans: An overview of pathology and resistance breeding. Plant Dis. 2012;96(1):4-17.
Crossref - Foolad MR, Merk HL, Ashrafi, H. Genetics, genomics and breeding of late blight and early blight resistance in tomato. Crit Rev Plant Sci. 2008;27(2):75-107.
Crossref - Vleeshouwers VGAA, Raffaele S, Vossen JH, et al. Understanding and exploiting late blight resistance in the age of effectors. Annual Rev. Phytopathol. 2011;49:507-531.
Crossref - de Bary A. Researches into the nature of the potato fungus (Phytophthora infestans). J Royal Agr Soc Series. 1876;2:239-269.
- Shahrour WG, Shatnawi MA, Abubaker S, et al. Identification of Phytophthora infestans from infected potato and tomato plants using molecular techniques. J Food Agri Environ. 2023;11(3-4):1216-1221
- Khalid H, Grover A. Dwivedi SK. PCR-based Methods for identification and detection of Phytophthora infestans in infected leaves of tomato. Defence Life Sci J. 2018;3(1):41-44.
Crossref - Judelson HS, Tooley PW. Enhanced polymerase chain reaction methods for detecting and quantifying Phytophthora infestans in plants. Phytopathol. 2000;90(10):1112-1119.
Crossref - Hussain S, Lees AK, Duncan JM, Cooke DEL. Development of a species-specific and sensitive detection assay for Phytophthora infestans and its application for monitoring of inoculums in tubers and soil. Plant Pathol. 2005;54(3):373-382.
Crossref - Ristaino JB, Madritch M, Trout CL, Parra G. PCR amplification of ribosomal DNA for species identification in the plant pathogen genus Phytophthora. App Env Microbiol. 1998;64(3):948-954.
Crossref - Hardham AR, Blackman LM. Molecular cytology of Phytophthora-plant interactions. Aus Plant Pathol. 2010;39(1):29-35.
Crossref - Judelson HS. The genetics and biology of Phytophthora infestans: Modern approaches to a historical challenge. Fungal Genet Biol. 1997;2(2)2:65-76.
Crossref - Ivanov AA, Ukladov EO, Golubeva TS. Phytophthora infestans: An overview of methods and attempts to combat late blight. J Fungi. 2021;7(12):1071.
Crossref - Scott JW, Garnder RG. Breeding for resistance to fungal pathogens. Genetic improvement of Solanaceous crops: Tomato (eds. M.K. Razdan, A.K. Matoo). Science Publishers, Enfield (NH) USA. 2007:421-456.
Crossref - Gallegly ME, Galindo, J. Mating types and oospores of Phytophthora infestans in nature in mexico. Phytopathol. 1958;48:274-277.
- Gisi U, Walder F, Resheat-Eini Z, Edel D, Sierotzki H. Changes of genotype, sensitivity and aggressiveness in Phytophthora infestans isolates collected in European countries in 1997, 2006 and 2007. J. Phytopathol. 2011;159(4):233-232.
Crossref - Volynchikova E, Kim KD. Biological control of oomycete soilborne diseases caused by Phytophthora capsici, Phytophthora infestans, and Phytophthora nicotianae in Solanaceous crops. Mycobiol. 2022;50(5):269-293.
Crossref - Fry WE, Goodwin SB. Re-emergence of potato and tomato late blight in the United States. Plant Dis. 1997;81(12):1349-1357.
Crossref - Fry WE. Phytophthora infestans: The plant (and R gene) destroyer. Mol Plant Pathol. 2008;9(3):385-402.
Crossref - Whisson SC, Boevink PC, Wang S, Birch PR. The cell biology of late blight disease. Curr Opin Microbiol. 2016;34:127-135.
Crossref - Dong SM, Zhou SQ. Potato late blight caused by Phytophthora infestans: From molecular interactions to integrated management strategies. J Integr Agric. 2022;21(12):3456-3466.
Crossref - Wang S, Welsh L, Thorpe P, Whisson SC, Boevink PC, Birch PRJ. The Phytophthora infestans haustorium is a site for secretion of diverse classes of infection-associated proteins. mBio. 2018;9(4):e01216-18.
Crossref - Midgley KA, van den Berg N, Swart V. Unraveling plant cell death during Phytophthora infection. Microorg. 2022;10(6):1139.
Crossref - Raffaele S, Win J, Can L, Kamoun S. Analyses of genome architecture and gene expression reveal novel candidate virulence factors in the secretome of Phytophthora infestans. BMC Genomics. 2010;11:637.
Crossref - Hu CH, Perez FG, Donahoo R, et al. Recent genotypes of Phytophthora infestans in eastern USA reveal clonal populations and reappearance of mefenoxam sensitivity. Plant Dis. 2012;96(9):1323-1330.
Crossref - Ristaino J, Schumann GL, D’Arcy CJ. Late blight of potato and tomato. The Plant Health Instructor. 2001;1(1):1-12.
Crossref - Van der Plank JE. Disease Resistance in Plants. Academic Press, New York. 1968:187.
- Watterson JC. Diseases. Atherton JG, Rudich J. (Eds.). The Tomato Crop: A Scientific Basis for Improvement. Chapman and Hall, London 1986:461-462.
Crossref - Mizubuti ESG, Aylor DE, Fry WE. Survival of Phytophthora infestans sporangia exposed to solar radiation. Phytopathol. 2000;90(1):78-84.
Crossref - Agrios GN. Plant Pathology. 5th Edition. Academic Press, London, New York. 2005:421-427.
- Nowicki M, Kozik EU, Foolad MR. Late blight of Tomato. Translational Genomics for Crop Breeding, Biotic Stress. 2013;1:241-265.
Crossref - Anthony PK, Marjan K, James HB, Williamson J. Tomato Diseases & Disorders. Home and Garden Information Center, Factsheet, HGIC 2217. 2021. https://hgic.clemson.edu/factsheet/tomato-diseases-disorders. Accessed January 22, 2024.
- Bodker L, Pedersen H, Kristensen K, Moller L, Lehtinen A, Hannukkala A. Influence of crop history of potato on early occurrence and disease severity of potato late blight caused by Phytophthora infestans. Westerdijk CE, Schepers HTAM, editors. Proceedings of the 9th workshop of an European network for development of an integrated control strategy of potato late blight. PPO Special Report No. 11, 2006; 53-56.
- Hannukkala AO, Kaukoranta T, Lehtinen A, Rahkonen A. Late blight epidemics on potato in Finland 1933-2002;increased and earlier occurrence of epidemics associated with climate change and lack of rotation. Plant Pathol. 2007;56(1):167-176.
Crossref - Sekhon PS, Sokhi SS. Effect of date of planting on late blight development in Punjab. Indian Phytopathol. 1999;52(3):267-269.
- Arora RK, Sharma S, Singh BP. Late blight disease of potato and its management. Potato J. 2014;41(1):16-40.
- Roy SK, Sharma RC, Trehan SP. Integrated nutrient management by using farmyard manure and fertilizers in potato-sunflower-paddy rice rotation in the Punjab. J Agricultural Sci. 2001;137(3):271-278.
Crossref - Meinck S, Kolbe H. Control of leaf and tuber blight in ecological potato cultivation. Kartoffelbau. 1999;50:172-175.
- Tumwine J, Frinking HD, Jeger MJ. Integrating cultural control methods for tomato late blight (Phytophthora infestans) in Uganda. Ann Appl Biol. 2002;141(2):225-236.
Crossref - Zwankhuizen MJ, Govers F, Zadoks JC. Inoculum sources and genotypic diversity of Phytophthora infestans in southern Flevoland, the Netherlands. Eur J Plant Pathol. 2000;106:667-680.
Crossref - Cooke LR, Schepers HTAM, Hermansen A, et al. Epidemiology and integrated control of potato late blight in Europe. Potato Res. 2011;54:183-122.
- Powell M, Gundersen B, Cowan J, Miles CA. Inglis DA. The effect of open-ended high tunnels in western Washington on late blight and physiological leaf roll among five tomato cultivars. Plant Dis. 2014;98(12):1639-1647.
Crossref - Hong YH, Meng J, He XL, Zhang YY, Luan YS. Overexpression of MiR482c in tomato induces enhanced susceptibility to late blight. Cells. 2019;8(8):822.
Crossref - Lewis WJ. Understanding Late Blight of Tomatoes, West Virginia University Extension Service Agriculture and Natural Resources. 2012:175.
- CPRI. Annual Progress Report. Central Potato Research Institute, Shimla, India. 2004-2005.
- Bounes H, Finckh MR. Effects of strip intercropping of potatoes with non-hosts on late blight severity and tuber yield in organic production. Plant Pathol. 2008;57(5):916-927.
Crossref - Paluchowska P, Sliwka J, Yin Z. Late blight resistance genes in potato breeding. Planta. 2022;255(6):127.
Crossref - Bhardwaj V, Kaushik SK, Singh PH, Singh BP. Tuber and foliage resistance to late blight in advanced potato hybrids. Potato J. 2005;32:131-132.
- Bhardwaj V, Kaushik SK, Chakrabarti SK, et al. Combining resistance to late blight and PVY in potato. Potato J. 2007;34:41-42.
- Kaushik SK, Bhardwaj V, Singh PH, Singh BP. Evaluation of potato germplasm for adaptability and resistance to late blight. Potato J. 2007;34:43-44.
- Joseph TA, Kaushik SK, Singh BP, et al. Kufri Himalini: A high yielding, late blight resistant potato variety suitable for cultivation in Indian hills. Potato J. 2003;34(3-4):168-173.
- Joseph TA, Singh BP, Kaushik SK, et al. Kufri Girdhari: A medium maturing, late blight resistant potato variety for cultivation in Indian hills. Potato J. 2011;38(1):26-31.
- Bhardwaj V, Srivastava AK, Sharma S, Kumar V, Kaushik SK, Singh BP. Efficiency of different potato (Solanum tuberosum L) cross combinations in late blight resistance breeding. Inter J Horticulture Agri. 2013;2(1):63-69.
- McGrath MT. Late Blight Management in Tomato with Resistant Varieties. 2022 Update:Current Disease Situation in the USA. eOrganic. Oregon State University. News Letter. 2022. Accessed on July 10th 2024 https://eorganic.org/node/33858
- Lal M, Luthra SK, Singh BP, Yadav S. Screening of potato genotypes for late blight. Potato J. 2013;40(1):80-83.
- Luthra SK, Gupta VK, Lal M, Rawal S, Kumar V, Singh BP. Kufri Mohan-a new high yielding table potato variety. Potato J. 2017;44(1):65-73.
- Novy RG, Whitworth JL, Stark JC, et al. Payette russet: A dual-purpose potato cultivar with cold sweetening resistance, low acrylamide formation and resistance to late blight and potato virus Y. Am J Potato Res. 2017;94:38-53.
Crossref - Tiwari JK. Poonam, Kumar V, et al. Evaluation of potato somatic hybrids of dihaploid S. tuberosum (+) S. pinnatisectum for late blight resistance. Potato J. 2013;40:176-179.
- Luthra SK, Tiwari JK, Lal M, Chandel P, Kumar V. Breeding potential of potato somatic hybrids: Evaluations for adaptability, tuber traits, late blight resistance, keeping quality and backcross (BC1) progenies. Potato Res. 2016;59(4):1-17.
Crossref - Singh BP, Rai M, Pandey KK. Development and evaluation of late blight-resistant tomato varieties at ICAR-IIVR. ICAR-Indian Institute of Vegetable Research Annual Report. 2013:45-48.
- Dinesh MR, Prasanna KP. Development of Arka Rakshak, a high-yielding tomato hybrid resistant to multiple diseases. Indian Institute of Horticultural Research Annual Report. 2010:32-35.
- Dinesh MR, Prasanna KP, Krishna K. Arka Samrat: A new tomato hybrid with resistance to late blight, bacterial wilt, and early blight. Indian Institute of Horticultural Research Annual Report. 2012:50-52.
- Chahal GS, Singh SP, Sandhu AS. H-88-78-1: A late blight-tolerant tomato hybrid for cultivation in Punjab. Punjab Agricultural University Research Bulletin. 2004:15-18.
- Bhatia SS, Sharma JN. Development of Palam Pink: A late blight-resistant tomato variety for hilly regions. Dr. Y.S. Parmar University of Horticulture and Forestry Research Publications. 2008:22-25.
- Rao KVM, Bhattarai NK. Arka Vikas: A moderately late blight-resistant tomato variety. Indian Institute of Horticultural Research Research Papers. 1985:10-12.
- Yadav SK, Mehta DR. Hisar Lalit: A tomato variety resistant to late blight and bacterial wilt for the North Indian plains. CCS Haryana Agricultural University Research Reports. 2002:65-68.
- Chahal GS, Singh SP, Sidhu JS. Punjab Chhuhara: Development and performance of a late blight-tolerant tomato variety in Punjab. Punjab Agricultural University Bulletins. 2000:18-21.
- Gupta SK, Singh P. Pant T-3: A late blight-resistant tomato variety for plains and foothills. GB Pant University of Agriculture and Technology Research Publications. 1995:29-32.
- Singh SK, Subramanian S. Arka Meghali: A high-yielding tomato variety tolerant to late blight. Indian Institute of Horticultural Research Publications. 2006:37-39.
- Panthee DR. Gardner R.G. “Mountain Merit”: A late blight-resistant large-fruited tomato hybrid. Hort Sci. 2010;45(10):1547-1548.
Crossref - Holm DG. ‘Defiant PhR’:A Fresh Market Tomato Hybrid with Resistance to Late Blight. Hort Sci. 2011;46(12):1682-1684.
- Mazourek M, McCarthy K, Smart CD. Breeding Late Blight Resistant Tomato Varieties. Plant Breeding Rev. 2013;37:123-156.
Crossref - Panthee DR, Gardner RG, Ibrahem R. ‘Plum Regal’ and ‘Mountain Magic’: Late Blight-Resistant Tomato Hybrids for Fresh Market. Hort Sci. 2011;46(6):840-842
- Johnston SA. ‘Matt’s Wild Cherry’- A tomato variety with high resistance to late blight. Tomato Genetic Resour. 2008;1(2):101-105.
- Gardner RG. NC 109 tomato breeding line: ‘Mountain Fresh’ F-1 hybrid. Hort Science. 1999; 34:941-942.
- Arellano-Rodríguez LJ, Martínez-Ramírez JL, Rodríguez-Guzmán E, Ron-Parra J, Cruz Arriaga-Ruiz M. Tomato breeding resistance to late blight in western Mexico. Acta Horticulturae. 2011; 914: 433-436.
Crossref - Tiwari JK, Rai N, Reddy YS, Singh MK. Prospects of tomato breeding for processing in India. Indian Hortic. 2023; 68(2):62-64.
- Gardner RG, Panthee DR. NC 1 CELBR and NC 2 CELBR: Early blight and late blight resistant fresh market tomato breeding lines. Hort Sci. 2010;45(7):975-976.
Crossref - Seminis Seeds. Koralik F1: Late blight-resistant cherry tomato hybrid for fresh market production. Seminis Seeds Company Variety Release Bulletin. 2010:12-14.
- Takii Seed Co. Sakura F1:Early-maturing, high-yielding tomato hybrid with disease resistance for Asian markets. Takii Seed Co. Variety Introduction Report. 2015:22-25.
- Sas-Piotrowska B, Piotrowski W, Misiak M. The growth and development of potato pathogens on media with extracts from Polygonaceae plants. I. Pathogens causing dry leafspot disease. Phytopathologia Polonica. 1996;11:103-109. https://www.cabidigitallibrary.org/doi/full/10.5555/19971002390
- Blaeser P, Steiner U, Lyr H, Russell PE, Dehne HW, Sisler HD. Antifungal activity of plant extracts against potato late blight (Phytophthora infestans). In: Modern fungicides and antifungal compounds II. Proceedings of the 12th International Reinhardsbrunn Symposium, Friedrichroda, Thuringia, Germany, 24-29 May 1998. pp. 491–499.
- Hashemi M, Tabet D, Sandroni M, et al. The hunt for sustainable biocontrol of oomycete plant pathogens, a case study of Phytophthora infestans. Fungal Biol Rev. 2022;40:53-69.
Crossref - Gachango E, Kirk W, Schafer R, Wharton P. Evaluation and comparison of biocontrol and conventional fungicides for control of postharvest potato tuber diseases. Biol Control. 2012;63(2):115-120.
Crossref - Stephan D, Schmitt A, Martins Carvalho S, Seddon B, Koch E. Evaluation of biocontrol preparations and plant extracts for the control of Phytophthora infestans on potato leaves. Eur J Plant Pathol. 2005;112(3):235-246.
Crossref - Conrado R, Gomes TC, Roque GSC, De Souza AO. Overview of Bioactive Fungal Secondary Metabolites: Cytotoxic and Antimicrobial Compounds. Antibiotics (Basel). 2022;11(11):1604.
Crossref - Divekar PA, Narayana S, Divekar BA, et al. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int J Mol Sci. 2022;23(5):2690.
Crossref - de Vrieze M, Varadarajan AR, Schneeberger K, et al. Linking comparative genomics of nine potato-associated Pseudomonas isolates with their differing biocontrol potential against late blight. Front Microbiol. 2020;11:857.
Crossref - Hultberg M, Bengtsson T, Liljeroth E. Late blight on potato is suppressed by the biosurfactant-producing strain Pseudomonas koreensis 2.74 and its biosurfactant. Bio Control. 2010;55(4):543-550.
Crossref - Caulier S, Gillis A, Colau G, et al. Versatile antagonistic activities of soil-borne Bacillus spp. and Pseudomonas spp. against Phytophthora infestans and other potato pathogens. Front Microbiol. 2018;9(143):143.
Crossref - Lim SM, Yoon MY, Choi GJ, et al. Diffusible and volatile antifungal compounds produced by an antagonistic Bacillus velezensis G341 against various phytopathogenic fungi. Plant Pathol J. 2017;33(5):488-498.
Crossref - de Vrieze M, Germanier F, Vuille N, Weisskopf L. Combining different potato-associated Pseudomonas strains for improved biocontrol of Phytophthora infestans. Front Microbiol. 2018;9(2573):2573.
Crossref - de Vrieze M, Gloor R, Codina JM, et al. Biocontrol activity of three Pseudomonas in a newly assembled collection of Phytophthora infestans isolates. Phytopathol. 2019;109(9):1555-1565.
Crossref - de Vrieze M, Pandey P, Bucheli TD, Varadarajan AR, Ahrens CH, Weisskopf L. Volatile organic compounds from native potato associated Pseudomonas as potential anti-oomycete agents. Front Microbiol. 2015;6:1295.
Crossref - Guyer A, de Vrieze M, Bonisch D, et al. The anti-Phytophthora effect of selected potato-associated Pseudomonas strains: from the laboratory to the field. Front Microbiol. 2015;6:1309.
Crossref - Hunziker L, Bonisch D, Groenhagen U, et al. Pseudomonas strains naturally associated with potato plants produce volatiles with high potential for inhibition of Phytophthora infestans. Appl Environ Microbiol. 2015;81(3):821-830.
Crossref - Morrison CK, Arseneault T, Novinscak A, Filion M. Phenazine-1-carboxylic acid production by Pseudomonas fluorescens LBUM636 alters Phytophthora infestans growth and late blight development. Phytopathol. 2017;107(3):273-279.
Crossref - Linkies A, Jacob S, Zink P, Maschemer M, Maier W, Koch E. Characterization of cultural traits and fungicidal activity of strains belonging to the fungal genus Chaetomium. J Appl Microbiol. 2021;131(1):375-391.
Crossref - Park JH, Choi GJ, Jang KS, et al. Antifungal activity against plant pathogenic fungi of chaetoviridins isolated from Chaetomium globosum. FEMS Microbiol Lett. 2005;252(2):309-313.
Crossref - Shanthiyaa V, Saravanakumar D, Rajendran L, Karthikeyan G, Prabakar K, Raguchander T. Use of Chaetomium globosum for biocontrol of potato late blight disease. Crop Prot. 2013;52:33-38.
Crossref - Alaux PL, Cesar V, Naveau F, Cranenbrouck S, Declerck S. Impact of Rhizophagus irregularis MUCL 41833 on disease symptoms caused by Phytophthora infestans in potato grown under field conditions. Crop Prot. 2018;107:26-33.
Crossref - Di Francesco A, Milella F, Mari M, Roberti R. A preliminary investigation into Aureobasidium pullulans as a potential biocontrol agent against Phytophthora infestans of tomato. Biol Control. 2017;114:144-149.
Crossref - Hadwiger LA, McDonel H, Glawe D. Wild yeast strains as prospective candidates to induce resistance against potato late blight (Phytophthora infestans). Am J Potato Res. 2015;92(3):379-386.
Crossref - Afek U, Rinaldelli E, Menge JA, Johnson ELV, Pond E. Mycorrhizal species, root age, and position of mycorrhizal inoculum influence colonization of cotton, onion, and pepper seedlings. JASHS. 1990;115(6):938-942.
Crossref - Soytong K, Kanokmedhakul S, Kukongviriyapa V, Kanokmedhakul K. Application of Chaetomium species (Ketomium) as a new broad spectrum biological fungicide for plant disease control. Fungal Divers. 2001;7:1-15.
- Gostinear C, Ohm RA, Kogej T, et al. Genome sequencing of four Aureobasidium pullulans varieties: biotechnological potential, stress tolerance, and description of new species. BMC Genomics. 2014;15:549.
Crossref - Zhang X, Li B, Zhang Z, Chen Y, Tian S. Antagonistic Yeasts: A Promising Alternative to Chemical Fungicides for Controlling Postharvest Decay of Fruit. J Fungi. 2020;6(3):158.
Crossref - Choudhury D, Dobhal P, Srivastava S, Saha S, Kundu S. Role of botanical plant extracts to control plant pathogens – A review. Indian J Agric Res. 2018;52(4):341-346.
Crossref - Tamuli P, Das J, Boruah P. Antifungal Activity of Polygonum hydropiper and Solanum melongena against plant pathogenic fungi. Plant Arch. 2014;14(1):15-17.
- Paik SB. Screening for antagonistic plants for control of Phytophthora spp. in soil. Korean J Mycol. 1989;17:39-47.
- Ndala RI, Mbega ER, Ndakidemi PA. Different plant extracts against Phytophthora infestans (Mont.) de Bary in tomato in vitro. American J Plant Sci. 2019;10(4):698-708.
Crossref - Shebannavar MN, Devappa V, Ramachandra RK, Anjaneya Reddy B, Sangeetha CG. Evaluation of different Botanicals for the Management of Late Blight (Phytophthora infestans) of Potato in Karnataka. Biological Forum. 2022;14(4):1182-1187.
- Islam, S, Azad MAK, Islam MR, Sultana MS, Khatun JA, Islam MH. Efficacy of some botanical extracts on the control of late blight disease in experimental potato field. Adv Biosci Biotechnol. 2021;12(2):426-435.
Crossref - Majeed A, Ahmad H, Chaudhry Z, Jan G, Alam J, Muhammad Z. Assessment of leaf extracts of three medicinal plants against late blight of potato in Kaghan Valley, Pakistan. J Agric Tech. 2011;7(4):1155-1161.
- Rohner E, Carabet A, Buchenauer H. Effectiveness of plant extracts of Paeonia suffruticosa and Hedera helix against diseases caused by Phytophthora infestans in tomato and Pseudoperonospora cubensis in cucumber. J Dis Protect. 2004;111(1):83-95.
Crossref - Yusuf Y, Izzet K, Ayhan G, et al. In vitro antifungal activities of 26 plant extracts on mycelial growth of Phytophthora infestans (Mont.) deBary. Afr J Biotechnol. 2011;10(14):2625-2629.
Crossref - Mehmood B, Azad A, Rahim N, et al. Management of late blight of potato caused by Phytophthora infestans through botanical aqueous extracts. Int J Phytopathol. 2022;11(1):35-41.
Crossref - El-Baky NA, Amara AAAF. Recent approaches towards control of fungal diseases in plants: An updated review. J Fungi (Basel). 2021;7(11):900.
Crossref - Haider E, Khan MA, Atiq M, Shahbaz M, Yaseen S. Phytoextracts as management tool against fungal diseases of vegetables. Int J Biocsi. 2020;16(3):303-314.
Crossref - Lydia GM, Gichimu BM, Muturi PW, Ezekiel KN. Essential oils as biocontrol agents of early and late blight diseases of tomato under greenhouse conditions. Int J. Agrono. 2021;2021(1):1-10.
Crossref - Ben Naim Y, Cohen Y. Replacing mancozeb with alternative fungicides for the control of late blight in potato. J Fungi. 2023;9(11):1046.
Crossref - Gudero G, Hussien T, Dejene M, Biazin B. Integrated management of tomato late blight [Phytophthora infestans (Mont.) De Bary] through host plant resistance and reduced frequency of fungicide in Arbaminch Areas, Southern Ethiopia. J Biol Agric Healthcare. 2018;8(9):1-10.
- Meya AI, Mamiro DP, Kusolwa PM, et al. Management of tomato late blight disease using reduced fungicide spray regimes in Morogoro, Tanzania. Int J Basic Appl Res. 2014;13(2):8-17.
- Artemii AI, Egor OU, Tatiana SG. Phytophthora infestans: An overview of methods and attempts to combat late blight. J Fungi. 2021;7(12):1071.
Crossref - Binyam T. Late blight of potato (Phytophthora infestans) biology, economic importance and its management approaches. J Biol Agric Healthcare. 2014;4(25):215-225.
- Nauman KM, Shoukat SAR, Aziz N, et al. Integrated pest management strategies for controlling potato late blight and enhancing crop and yield. J Agric Food. 2024; 5(1):1–15.
Crossref - Lal M, Yadav S, Singh BP. Efficacy of new fungicides against late blight of potato in subtropical plains of India. J Pure Appl Microbiol. 2017;11(1):599-603.
Crossref - Cooke RJ, Schepers HTAM, Hermansen A, et al. Epidemiology and integrated control of potato late blight in Europe. Potato Res. 2011; 54(2):183–222.
- Krause RA, Massie LB, Hyre RA. BLITECAST, a computerized forecast of potato late blight. Plant Dis Rep. 1975;59:95-98.
- Small IM, Joseph L, Fry WE. Development and implementation of the BlightPro decision support system for potato and tomato late blight management. Comput Electron Agric. 2015;115:57-65.
Crossref - Abuley IK, Nielsen BJ. Integrating cultivar resistance into the TOMCAST model to control early blight of potato, caused by Alternaria solani. Crop Protection. 2019; 117:69-76
- Fry WE, Apple AE, Bruhn JA. Evaluation of potato late blight forecast modified to incorporate host resistance and fungicides weathering. Phytopathology. 1983;73(7):1054–1059.
- Singh VK, Shailbala, Pundhir VS. Forecasting models: an effective tool for potato late blight management. In: Singh VK, Singh Y, Singh A, editors. Eco-friendly innovative approaches in plant disease management. International Book Publishers and Distributors, Dehradun; 2012. p.101-112.
- Meno L, Abuley IK, Escuredo O, Seijo MC. Factors influencing the airborne sporangia concentration of Phytophthora infestans and its relationship with potato disease severity. Scientia Horticulturae. 2023;307:111520.
Crossref - Gudero GM, Hussien T, Dejene MW, Biazin B. Integrated management of tomato late blight [Phytophthora infestans (Mont.) de Bary] through host plant resistance and reduced frequency of fungicide in Arbaminch areas, Southern Ethiopia. J Biol Agric Healthcare. 2018; 8(9):94-109.
- Fry WE, Goodwin SB. Resurgence of the late blight of potato and tomato. Plant Dis. 1997;81(12):1349-1361.
Crossref - Shailbala, Kumar A. Eco-friendly management of late blight of potato- A review. J Appl Nat Sci. 2017;9(2):821-835.
Crossref - Smith LP. Potato blight forecasting by 90 per cent humidity criteria. Plant Pathol. 2007;5(3):83-87.
Crossref - Meno L, Escuredo O, Seijo MC. Opportunity of the NEGFRY Decision Support System for the sustainable control of potato late blight in A Limia (NW of Spain). Agriculture. 2024; 14: 652.
Crossref - Fry WE, Apple AE, Bruhn JA. Evaluation of Potato Late Blight Forecasts Modified to Incorporate Host Resistance and Fungicide Weathering. Phytopathol. 1983;73(7):1054-1059.
Crossref - Schepers HTAM. Prophy: a computerized expert system for control of late blight in potatoes. In: Proceedings of the XIII International Plant Protection Congress; 1995; Netherlands. p. 948.
- van der Waals JE, Denner FDN, van Rij N, Korsten L. Evaluation of PLANT-Plus, a decision support system for control of early blight on potatoes in South Africa. Crop Prot. 2003; 22(6): 821-828.
Crossref - Colturato AB, Chavier LFC. Decision support system for late blight in tomato and potato. Acta Horticulturae. 2019;1233:19–26.
Crossref - Meno L, Escuredo O, Rodríguez-Flores MS, Seijo MC. Modification of the TOMCAST Model with Aerobiological Data for Management of Potato Early Blight. Agronomy. 2020; 10(12):1872.
Crossref - Zwankhuizen MJ, Govers F, Zadoks JC. Development of potato late blight epidemics: disease foci, disease gradients, and infection sources. Phytopathology. 1998; 88(8):754-763.
Crossref - Pérez W, Arias R, Taipe A, et al. A simple, hand-held decision support tool to help resource-poor farmers improve potato late blight management. Crop Protection. 2020;134:105186.
Crossref - Singh VK, Shailbala, Pundhir VS. Forecasting models for potato late blight management – A review. Agricultural Reviews. 2013;34(2):87-96.
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.