ISSN: 0973-7510
E-ISSN: 2581-690X
Due to their ability to wipe out pathogens, botanical medicines have been historically used to effectively combat severe ailments throughout time immemorial. Furthermore, owing to the limitations of current medical approaches, investigators have begun looking into generating fresh formulations that have enhanced antioxidant and antimicrobial capabilities. These activities of combination generated from the fruit and seed of medicinal plants were collected in this investigation from different geographical areas for determining antioxidant and antimicrobial activity. One of the medicinal plants, i.e., Zanthoxylum armatum, belongs to the family Rutaceae and has medicinal values as mentioned in the literature. The objective of our study is to analyse the antimicrobial and antioxidant potential of formulations (fruits and seeds with different proportions) from district Bageshwar (location Shama Dura) against specific pathogens. In order to achieve the objective, fruits and seeds of Zanthoxylum armatum were examined through Fourier-transform infrared spectroscopy (FTIR) and estimated in vitro for their synergistic antibacterial potential against three pathogenic microorganisms: Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus using disc-diffusion assays. In addition, antioxidant activity was also performed using 2,2-diphenylpicrylhydrazyl (DPPH) and estimating its phenolic and total flavonoid content. The results of these studies showed that the maximum zone of inhibition was observed in formulation (40:60; 9–11 mm), whereas the least was observed in formulation (20:80; 3–4 mm) against different bacterial strains. In addition, the formulated samples of fruit and seed combinations may have shown higher antioxidant activity (86.37%), total phenolic (416.2 mg GAE/g) and total flavonoid (166.4 mg rutin/g) content. In contrast, FTIR was used to detect the presence of functional groups in the formulated fruit and seed of Zanthoxylum armatum. According to the aforesaid finding, the formulation shows strong antibacterial and antioxidant action without compromising cell viability.
Formulation, Antibacterial Potential, Pathogenic Microorganisms, Antioxidant Activity
Throughout the evolution of humanity, medicinal plants have assisted in an assortment of biological tasks. Evaluation of the biological impacts of traditional pharmaceuticals is an important aspect of ethnopharmacology, a holistic and interdisciplinary methodology for finding new drugs. The evaluation approach, which has been influenced by the widespread consumption of old-fashioned treatments, empirically investigates plants as an indicator of chemically active compounds and is influenced by ethnomedical data. The majority of them have the advantage of possessing a wide range of compounds with structural variability, rendering secondary metabolic products an appropriate choice for use in the medical, pharmaceutical, cosmetic, and agro-food industries.1,2
In India, the Indian subcontinent offers a wide variety of plants and animals because of its geography, seasonal variations, and altitude. In Ayurveda, there are currently approximately 3000 plant species with medicinal properties. The fundamental principle of Indian Ayurveda is the application of plant compounds to relieve a wide range of ailments.3,4 The World Health Organisation (WHO) identifies a medicinal plant as any kind of plant possessing therapeutic (curative) aspects or that has an encouraging pharmacological (related to pharmaceuticals) effect on the animal body. Almost all of the naturally occurring species that might have been found here deliver a broad spectrum of therapeutic benefits. Currently, a medication combo approach instead of one administration might be employed to treat ailments with greater effectiveness, as many illnesses have complicated aetiologies.3-5 In Western countries, combinatorial or chronic ailments (e.g., cancer, hypertension, etc.) are frequently cured with successful combination therapy. Since the dawn of time, we have relied heavily on botanicals as the primary source of treatments and remedies.5,6 Investigators are particularly interested in phytochemicals and compounds manufactured by plants as natural alternatives to synthetic or chemical-based compounds. One of the medicinal plants, i.e., Zanthoxylum armatum, belongs to the family Rutaceae and is well known as Timur. This plant may have been used as a medicinal medicine since ancient times for the cure of various diseases, such as toothache and other problems of the teeth, gum bleeding, asthma, fever, and dyspepsia.7,8 It was also claimed that Zanthoxylum armatum also has a number of potentials, like antimicrobial, antioxidant, anti-inflammatory, antinociceptive, antiproliferative, antifungal, hepatoprotective, pesticidal, and anthelmintic.7-10 Due to the high number of medicinal benefits and the spike in global demand, all the species of Zanthoxylum armatum are put in the endangered category by the IUCN. Drug development investigations are essential to prevent the occurrence of numerous medical conditions for which there are no efficient treatments.
Fruit and seeds include a vast variety of bioactive compounds with prospective applications in the culinary and pharmacological sectors, fulfilling the growing need for natural ingredients that are often chosen since they have fewer side effects than synthetic ones. Researchers have long been interested in the functional characteristics, as well as the proximate and mineral contents, of several fruit seeds, including tomato, apple, etc. Fruit by-products are an excellent source of biologically active components, which include proteins (bioactive peptides), carotenoids (lycopene), phytochemicals (flavonoids), and vitamins (tocopherol).6-10 These ingredients are also highly beneficial for one’s wellness, which makes fruit by-products an attractive option for the formulation of several kinds of food products that have extensive functioning and nutraceutical potential. As a result, the current study is to investigate the fruit and seed of Zanthoxylum armatum from Bageshwar district, which is located in Kumaun, Uttarakhand, for its antioxidant and antibacterial properties.
Collection and extraction
Collection of plant material from Kumaun region—Shama Dhura, Bageshwar—provided the Zanthoxylum armatum (fruits and seeds) that were collected in November 2022.
Extraction
A maceration procedure was used for making the formulation that was used in the present research, which contained both primary and secondary metabolites. Using 70% alcohol, samples were wiped down to eradicate dust, and their surfaces were allowed to dry for up to six days underneath the shade. After weighing every fruit and seed sample to the nearest 30 g, methanol was chosen as the solvent for dissolving them. These samples were then kept on a revolving platform for six to seven days. In order to allow the solvents to evaporate, they were then heated to 60°C in a water bath. Samples were subsequently filtered two times with syringe filters that were made using Whatman No. 1 filter paper, and the samples were subsequently preserved at 4°C.
Preparation of formulation
The fruits and seeds were individually separated out, lyophilized, and ground into a powder. 2 g of the lyophilized powdered samples were taken out separately for the formulation preparation by using 100 mL of an ethanol/water (80/20) combination. This was done by first agitating the solution for two hours at room temperature and then sonicating it for fifteen minutes. Following a 15-minute centrifugation at 4000 rpm for each sample, the pellet was extracted once more. After collecting the supernatants, the solvents were compelled to evaporate at 40°C in a rotating evaporator under vacuum. Ultimately, 1% DMSO was used to dissolve the dry leftovers, creating new formulations using PBS.
The formulation of fruits and seeds with varying amounts was created by allowing the fruit and seed powder in PBS and controlling the pH levels. The fruit and seed formulation of Zanthoxylum armatum was mixed in different ratios (20/80, 40/60, 60/40, and 80/20), and its pH was 6.8-7.2 (Table). Four identical parts of the formulations were kept at ambient temperature (25°C) and refrigerated at 2°C, respectively. After that, the samples’ total antioxidant, phenolic, and flavonoid content—as well as their antimicrobial content—were assessed while they were being kept.
| No. | Formulations of fruit and seed | Total volume |
|---|---|---|
| 1 | Control (PBS) | 1 ml |
| 2 | Control (Ethanol/water) | |
| 3 | Fruit formulation (F) | |
| 4 | Seed formulation (S) | |
| 5 | 200 F :800 S | |
| 6 | 400 F :600 S | |
| 7 | 600 F :400 S | |
| 8 | 800 F :200 S | |
| 9 | Standard |
FTIR analysis
Perhaps among the best and most productive methods to assess various types of chemical bonds (functional groups) within molecules is FTIR. For this FTIR investigation, desiccated powders from solvent-based extractions of plant material were chosen. The fruit and seed powder was mixed with PBS and prepared in different proportions. The formulation was kept at room temperature for 2–3 days and then centrifuged at 10,000 rpm for 15 minutes. The supernatant was collected and stored at -20°C until analysis. The sample of each plant specimen was loaded into an FTIR spectroscope (Shimadzu, IR Affinity 1, Japan), with a scan range of 400 to 4000 cm-1 and a resolution of 4 cm-1.11
Total phenolic contents
The Folin-Ciocalteu technique was used to evaluate the formulation’s total phenolic content. Since the conversion of phosphotungstic acid to phosphotungstic blue is the only step in the process, the absorbance values of aromatic phenolic groups are directly connected with them. In this experiment, four test tubes containing 250 µl of Folin-Ciocalteu’s reagent and 750 µl of a 20% sodium carbonate solution each were used, and 50 µl of each formulation from the appropriate district was added individually. The remaining 5 ml of the total volume was made up with distilled water. A UV/visible light spectrophotometer were used to assess absorbance at 765 nm after two hours in comparison to a control that included all reaction chemicals other than the sample aqueous extract. Estimates of the total amount of polyphenols were made and expressed in the form of gallic acid equivalent (mg GAE/100 g).12,13
C = c × V / m
where,
C = Total phenolic contents (mg/100g fruit and seed formulation, in GAE)
c = Concentration of gallic acid (mg/mL)
V = Volume of formulation (mL)
M = Weight of formulation (fruit and seed) (g)
Flavonoids
A spectrophotometer was used to assess the formulation’s total flavonoid content. Quercetin was employed in this study as a benchmark to assess the total flavonoids present in the formulations of the various districts. In this instance, 1 ml of the formulation was added to a 10 ml volumetric flask, which had previously been filled with distilled water to a level of 5 mL. Next, 0.3 ml of sodium nitrite solution containing 5% (w/v) sodium nitrite was added. 0.6 ml of 10% (w/v) aluminium chloride was added after 5 minutes. Following the mixing of 2 ml of 1 M sodium hydroxide for 10 minutes, 2.1 mL of distilled water was added. On a UV/visible light spectrophotometer, absorbance was instantly recorded at 510 nm. The information was presented using quercetin equivalents (QE) in mg per 100 g of extract.12,13
Antioxidant activity
Radical Scavenging Activity— Zanthoxylum armatum DPPH Inhibition has been evaluated following the Brand-Williams et al. conventional method, consisting of formulations of the fruit or seed (in triplets) in the centrifuge tube.14 200 microliters of distilled water have been collected in the absence of the sample. Then, the empty container and the formulations (fruits and seed) of Zanthoxylum armatum were added together (using different concentrations) with 1 mL of DPPH solution (8 mg/100 mL of ethanol). After agitating every five minutes, this configuration was maintained at ambient temperature for 30 minutes. The tubes were then centrifuged for 10 minutes at 4000 rpm. The absorbance at 517 nm was then measured against the PBS using a UV-vis spectrophotometer after 0.5 mL of the supernatant had been transferred into brand-new tubes containing 1 mL of formulation. Each crude sample was analyzed in triplicate. The percentage of inhibition was calculated against a blank
I%=(Ablank – Asample )/(Asample) ×100
Where, Ablank is the absorbance of the control reaction (containing all reagents except the test compound) and Asample is the absorbance of the test compound.
Culturing of microbial strains
Three microbiological strains—Escherichia coli MTCC 68, Pseudomonas aeruginosa MTCC 2582, and Staphylococcus aureus MTCC 737—were tested against the prepared fruit and seed formulation. Muller-Hinton Agar (MHA) medium was used to support the microbial growth. The required amount of the medium was prepared, autoclaved, cooled to 40°C, and mounted onto sterilised Petri plates, where it was allowed to set. The right number of test organism colonies were grown in the correct plates before being inoculated, and they were kept there for 18 to 24 hours at 37°C in a chamber incubator. All of these experiments were carried out aseptically in a biosafety room. The necessary colonies were selected from the overnight cultures and inoculated in Muller-Hinton broth (MHB) to prepare the cell suspension. These samples were incubated at 37ºC for 18-24 hours and then the absorbance of the cell suspension was taken at 600 nm using UV-Vis spectroscopy.12-14 The standard absorbance reported for the bacterial suspension at 600 nm is between 0.08 and 1. At varied doses of formulation (final volume 50 µl) having 8 µl (10:40), 16 µl (20:30), 24 µl (30:20), and 32 µl (40:10) from a stock solution of formulation (1 mg/ml; final volume in each well, i.e., 50 µl), the antibacterial activity of formulations was assessed using the disc diffusion method. Microbial suspension (108 CFU/mL) was gently applied to the Mueller-Hinton agar (MHA). Following that, 6mm-diameter discs were coated with formulations of different concentrations of fruit and seed, sterilised for 15 minutes at 121°C. As positive controls, ampicillin (2 mg) was utilized. The discs were tagged and incubated for 24 hours at 37°C after being dried and set in proximity to the bases of the plates containing the organisms. The findings of the studies were reported as the width (mm) of the inhibitory zones in duplicate.14
FTIR analysis
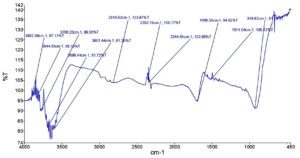
The FTIR spectrum of different proportions of fruit and seed formulation (60:40) is shown in Figure 1. It contains information on the peak values and likely functional groups (identified by FTIR analysis) found in different proportions of fruit and seed of Zanthoxylum armatum.
No. |
Absorption (cm-1) |
Appearance |
Group |
Compound Expected |
|---|---|---|---|---|
1 |
3786.23 |
medium sharp |
O-H stretching |
alcohol |
2 |
3696.44 |
Strong broad |
O-H stretching |
alcohol |
3 |
3601.44 |
Strong broad |
O-H stretching |
alcohol |
4 |
2810.52 |
medium |
C-H stretching |
aldehyde |
5 |
2344.15 |
strong |
O=C=O stretching |
carbon dioxide |
6 |
1696.56 |
strong |
C=O stretching |
conjugated acid |
7 |
1514.04 |
strong |
N-O stretching |
nitro compound |
Figure 1. FTIR analysis of Zanthoxylum armatum (fruit and seed, 40:60 ) formulation from Bageshwar district
Total phenolic and flavonoid contents
The extraction yield of different proportions of fruit and seed may have varied the phenolic content in a descending order of proportions (40:60 > 60:40 > 80:20 > 20: 80) (Table). So the formulation (40:60) resulted in the highest amount of total phenolic and total flavonoid content as compared to other proposed formulations. Gallic acid (total phenolics) and rutin (total flavonoids) were used as standards for these studies.
Table:
Determination its total phenolic and flavonoid contents in formulation (fruit and seed with different proportions in Zanthoxylum armatum
No. |
Formulations of fruit and seed |
Total phenolic mg GAE/g formulation |
Total flavonoids mg rutin/g formulation |
|---|---|---|---|
1 |
Fruit formulation (F) |
357.4 ± 11.26** |
192.2 ± 6.24 |
2 |
Seed formulation (S) |
301.2 ± 9.34 |
76.68 ± 5.12 |
3 |
200 F :800 S |
266.6 ± 9.78 |
96.24 ± 5.66 |
4 |
400 F :600 S |
416.2 ± 10.78** |
166.4 ± 3.44** |
5 |
600 F :400 S |
357.4 ± 11.26* |
154.2 ± 5.44* |
6 |
800 F :200 S |
328.7 ± 12.42 |
112.4 ± 4.78 |
7 |
Standard |
486.2 ± 14.22*** |
228.4 ± 5.34*** |
Each value represents a mean ± SE (n = 3). Readings were obtained from the calibration curve of gallic acid (25 mg/L) using the equation y = mx + c, where m is the slope, c is the intercept, and y is the optical density. Statistical analysis, i.e., *P<0.05, **P<0.01, and ***P<0.001 (one-way ANOVA test, Bonferroni multiple comparison test)
Antioxidant activity
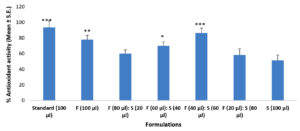
As shown in Figure 2, it may indicate that the formulation of fruit and seed (60:40) has a significant amount of free radical scavenging activity as compared to other concentrations of fruit and seed. In comparison to the control, ascorbic acid was used as a standard and showed significant enhancement.
Figure 2. Antioxidant activities of formulation (fruit and seed) from Zanthoxylum armatum at various concentrations. Each value represents a mean ± SE (n = 3), *P<0.05, **P<0.01 and ***P<0.001 (One-way ANOVA test)
Antimicrobial activity
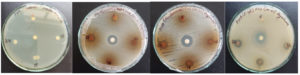
The effect of formulations using different concentrations of fruit and seed of Zanthoxylum armatum is shown in Figure 3. These studies may suggest that fruit and seed formulations (40:60) showed inhibition in bacterial (Staphylococcus aureus, and E. coli) strains as compared to control. Methanol was used as a negative control for these studies and showed a less toxic effect against these bacterial strains.
Plant constituents in the form of metabolites have been explored all over the world to create novel antibacterial chemicals, antioxidants, and food preservation agents. They are thought to be potential sources of natural bioactive molecules.15 The antibacterial and antioxidant properties of plant metabolites in the form of formulations have been the subject of several studies. In preference to manmade antimicrobial and antioxidant additives for food, numerous naturally found bioactive compounds within plants represent a good alternative. The vast majority of those compounds are obtained from plants, and investigations on their antioxidant and antibacterial abilities have been presented regularly in the previous ten years.16 Generally, plant-derived metabolites are used either in the form of formulations or combinations of different metabolites from plant components that are extracted from the same plant. In the formulation, the phytochemical constituents of a single formulation are insufficient or difficult to achieve desirable effects. In some cases, formulations from different parts of the plant show a synergistic or additive interaction that enhances the therapeutic effect. In short, the phytochemicals from different plant components act on multiple targets to enhance their pharmacological activity.14-17
According to the literature, Ayurvedic practitioners extensively apply polyherbal mixtures for managing acute and persistent wounds. These formulations are frequently produced through the incorporation of ingredients from plants in proportional amounts, and they are subsequently used effectively in the formulation (solid or liquid) for the purpose of treating infectious disorders. The most commonly cited instances of the aforementioned formulation in actuality are those recalled in Triphala and Trikadu. By integrating fruit and seeds in different proportions and evaluating their antibacterial and antioxidant properties, the current research is additionally utilised and associated with the fabrication of fruit and seed formulations. To optimise the effectiveness of this mixture, which possesses antioxidant and antibacterial qualities, it is necessary to balance each plant component, notably the fruit and seed. Since the chemical composition of the fruit and seeds from Zanthoxylum armatum are somewhat distinct, which have been identified through FTIR (the disparity on the assumption of functional groups), varying proportions of the fruit and seeds exhibited different interactions on antioxidant and antibacterial properties. The multiple fruit and seed proportions are employed to identify the ideal formulations that generate the ideal conditions for the antioxidant and antibacterial capabilities to occur.18,19
The antioxidant value of the seed and fruit mixture has been identified in this work and was assessed through an in vitro procedure, i.e., a free radical scavenging assay (DPPH). One of the quickest procedures entails combining different proportions of fruit and seed formulations with DPPH and quantifying their absorbance values after a specific period of time. This technique has been used to decrease the oxidation rate of naturally occurring substances that encounter molten oxygen in the air. The free radical scavenging activity of the phenolic compounds was of extreme importance from both an industrial and biological perspective in the present research.18-20 The antioxidants of essence are thus preferred over synthetic antioxidants. The assortment of approaches explored in determining the medicinal value of antioxidants has gone up as well. However, a new study has brought up concerns about the appropriateness of these synthetic compounds because of their unknown consequences, notably their enzyme inhibition characteristics. Experts have invested an immense amount of effort into creating a new formulation of antioxidant chemical compounds with fewer hazardous adverse reactions due to the negative consequences of these synthetic compounds. In the present setting, plant-based antioxidants, which exhibit greater biodegradability, fewer side effects, and more secure modes of action, are increasingly replacing synthetic antioxidants. However, in addition to the natural source, the isolation and extraction procedures used also have an impact on the antioxidant quality and capacity of natural antioxidants and extracts. It was found that the formulation (seed and fruit) scavenged free radicals in the procedures in a concentrated way. The different proportions of seed and fruit combinations contain the highest amount of DPPH, according to the DPPH scavenging activities, which were measured in terms of percentage inhibition. The outcomes were contrasted with ascorbic acid, which is typically used. Better scavenging or antioxidant capability is indicated by a larger inhibition percentage. In addition, total phenolic and flavonoid content was also estimated, showing a positive correlation between the free radical scavenging assay and phenolic compounds. The results of these studies showed that the formulation of seed and fruit had a higher phenolic and flavonoid content as compared to the control. These phenolic compounds showed their medicinal importance by having health-promoting activities, and they are commonly reported in fruits, vegetables, plants, etc.21, 22
It is essential to successfully treat viral illnesses carried on by resistant pathogens through antibacterial agents. Antibiotics are a class of medicines with antibacterial characteristics that can be used to treat, manage, and prevent bacterial infections. Antibiotics are substances, either synthetic or natural, that operate to stop bacterial growth. Various bacteria have varied reactions to antibiotics. Different bacteria react differently to various antibiotics. In this study, we tested the formulation of seed and fruit and evaluated their antimicrobial activity. For estimation, we used methanolic extracts of selected plants (fruit and seed in different proportions) that were evaluated in the form of formulations for antimicrobial activity using the agar-well diffusion method. According to the findings, the formulation has exhibited growth inhibition against gram-positive and gram-negative bacteria. In short, these studies showed that the formulation is highly active against the gram-positive and gram-negative bacteria.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
The study was approved by the IBSC Committee, Department of Microbiology and Department of Biotechnology, Graphic Era Deemed to be University, Dehradun, India, with project number GEU/IBSC/project3-2021.
- Modorresi A, Darah I, Shaida Fariza S. Antioxidant Activity and Total Phenolic Content of Some Medicinal Plants in Urticaceae Family. JABS. 2009;3(2):27-31.
- Guder A, Korkmaz H. Evaluation of in-vitro Antioxidant Properties of Hydroalcoholic Solution Extracts Urtica dioica L., Malva neglecta Wallr. and Their Mixture. Iran J Pharm Res. 2012;11(3):913-923.
- Zengin G, Cakmak YS, Guler GO, Aktumsek A. Antioxidant properties of methanolic extract and fatty acid composition of Centaurea urvillei DC. subsp. hayekiana Wagenitz. Rec Nat Prod. 2011;5:123-132.
- Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006;97(4):654-660.
Crossref - La Vecchia C, Altieri A, Tavani A. Vegetables, fruit, antioxidants and cancer:a review of Italian studies. Eur J Nutr. 2001;40:261-267.
Crossref - Jang HD, Chang KS, Huang Y, et al. Principal phenolic phytochemicals and antioxidant activities of three Chinese medicinal plants. Food Chem. 2007;103(3):749-756.
Crossref - Kala CP, Farooquee NA, Dhar U. Traditional uses and conservation of timur (Zanthoxylum armatum DC.) through social institutions in Uttaranchal Himalaya, India. Conserv Soc. 2005;3(1):224.
- Mushtaq MN, Ghimire S, Akhtar MS, Adhikari A, Auger C, Schini-Kerth VB. Tambulin is a major active compound of a methanolic extract of fruits of Zanthoxylum armatum DC causing endothelium-independent relaxations in porcine coronary artery rings via the cyclic AMP and cyclic GMP relaxing pathways. Phytomedicine. 2019;53:163-170.
Crossref - Negi J, Bisht V, Bh A, Singh P, Sundriyal R. Chemical constituents and biological activities of the genus Zanthoxylum:a review. Afr J Pure Appl Chem. 2011;5(12):412-416.
- Phuyal N, Jha PK, Raturi PP, Rajbhandary S. Zanthoxylum armatum DC.:current knowledge, gaps and opportunities in Nepal. J Ethnopharmacol. 2019;229:326-341.
Crossref - Boskey A, Camacho PN. FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials. 2007;28(15):2465-2478.
Crossref - Chun OK, Kim DO, Lee CY. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J Agric Food Chem. 2003;51(27):8067-8072.
Crossref - Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64(4):555-559.
Crossref - Leong LP, Shui G. An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 2002;76(1):69-75.
Crossref - Gupta M, Thakur S, Sharma A, Gupta S. Qualitative and quantitative analysis of phytochemicals and pharmacological value of some dye yielding medicinal plants. Oriental J Chem. 2013;29(2):475-481.
Crossref - Gupta A, Srivastava S, Singh A, Arya P, Bajpai AB, Kumar V. Total phenolic, flavonoid content and antioxidant potential of Phaseolus vulgaris. J Med Pharm Alli Sci. 2023;12(3):5780-5784.
Crossref - Gupta A, Sutariya S, Shah S, Palekar SS, Somani H, Kumar Vijay. Immuno pharmacological studies of Gymnosporia Montana (roth) benth. J Med Pharm Alli Sci. 2022;11(3):4970-4977
Crossref - Kelm M, Nair M, Strasburg G, DeWitt D. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine. 2000;7(1):7-13.
Crossref - Spencer JP, Abd El Mohsen MM, Minihane AM, Mathers JC. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr. 2008; 99:12–22.
Crossref - Kahkonen MP, Hopia AI, Vuorela HJ, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47(10):3954-3962.
Crossref - Wojdylo A, Oszmianski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105(3):940-949.
Crossref - Gracia SMT, Heinonen M, Frankel EN. Antioxidant activity of anthocyanin in LDL and lecithin liposome systems. J Agric Food Chem. 1997;45(9):3362-3367.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.