Silver nanoparticles (AgNPs) are known for their exceptional physicochemical characteristics, including remarkable stability and conductivity, catalytic activity, and antibacterial capabilities. The emerging approach of plant-mediated synthesis of AgNPs is eco-friendly, non-hazardous, sustainable in biomedical applications, and highly cost-effective. Its non-toxicity and rapidity instill confidence in its potential, making it a secure choice. Plant-derived AgNPs combine nanotechnology features with the therapeutic potential of plant bioactive compounds, offering significant potential for medicinal applications. With their ease of availability and unique phytochemical composition, Ficus plants outperform other plant species in synthesizing AgNPs, adding more confidence to this efficient and economically secure synthesis process. This article underscores the benefits and advances of the Ficus plant in AgNP synthesis and highlights its promising antimicrobial, anticancer, antibacterial, and cytotoxic activities. The potential of the Ficus plant in AgNP synthesis is genuinely intriguing and inspiring, opening up new possibilities in nanotechnology. However, this process has challenges and limitations, such as precise control of the synthesis conditions, inconsistent synthesis efficiency, potential variability in the complex phytochemical compositions, scalability issues, and safety concerns. This article also discusses the key challenges of the Ficus-based AgNP synthesis. It suggests mitigation strategies, underscoring the urgent need for further research and motivating the researchers to engage in this vital topic.
Plant-mediated Synthesis, AgNPs, Ficus Plant, Antibacterial Activities, Antimicrobial Agents, Cancer Therapy
Nanoparticles (NPs) hold immense potential in various diverse applications, with nanoscale dimensions ranging from 1-100 nm. They are categorized into inorganic and biological NPs. Inorganic NPs include ferrous and magnetic NPs, whereas organic NPs include carbon NPs. The growing interest in gold and silver (noble metals) is akin to that for fullerenes, quantum dots, and carbon nanotubes, owing to their superior characteristics and valuable flexibility.1-5 Silver NPs (AgNPs) stand out from other nanometal particles due to their exceptional physicochemical characteristics, including remarkable stability and conductivity, catalytic activity, and antibacterial capabilities. Their potential applications are diverse and inspire hope and optimism for the future, making them a subject of growing interest and research.
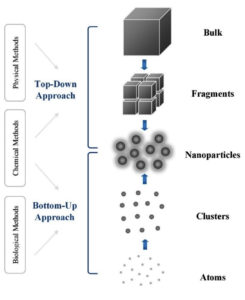
With their distinctive electrical and physical properties and magnetic capabilities, AgNPs are fascinating candidates with many applications. AgNP synthesis is a beautiful example of nature’s contribution to science. It involves numerous biomolecules from plants that function as reducing and stabilizing agents, producing high-quality and stable AgNPs.4 AgNPs can be formulated using top-down and bottom-up approaches. The top-down approach uses various techniques, each with unique complexity and intricacy, to break the bulk material into nanoscale particles. It offers high pattern shape and size controllability but typically incurs high costs. The bottom-up method, a precise and reliable approach, initiates nanoparticle synthesis through molecules or atoms using biological and chemical techniques. This method’s precision and reliability reassure us of its effectiveness despite needing more control over particle shape and size precision.
Physical techniques are expensive with little yield; traditional chemical methods are unsafe and toxic.4,5 In contrast, biological processes are cost-effective and safe, providing a promising alternative approach to AgNP synthesis. Several techniques have been used to manufacture AgNPs in an environmentally friendly manner. These techniques, which take advantage of various organisms, including microorganisms and arthropods, are risk-free, effective, and economical. Research has utilized various biological organisms, including bacteria, fungi, viruses, yeast, arthropods, and plants, showcasing the versatility of biological processes in AgNP synthesis. Plant synthetic approaches are beneficial and eco-friendlier than other biological entities.
Microbial synthesis, being slow, allows plants to emerge as the ideal bioresource for AgNP synthesis. Their phytochemicals and metabolite composition equip them to act as bio-reductants, actively reducing metal ions. Plants’ bioactive compounds and functional groups, such as terpenoids, flavones, aldehydes, amides, and ketones, further enhance their suitability.6,7 Notably, neem, aloe vera, and green tea extracts have been successfully used in AgNP synthesis. Plant extracts offer a simple and cost-effective method for producing AgNPs from diverse sources, facilitating rapid green synthesis. Moreover, they stabilize NPs and act as reducing agents, ensuring nucleation stability and AgNP growth.8 The green-synthesized AgNPs are renowned for their superior yields, cost-effectiveness, and environmental friendliness, surpassing chemically manufactured AgNPs.
The choice of plant material significantly influences the properties of the synthesized AgNPs, including their size, shape, and stability, affecting their biological activities. Ongoing research is exploring a variety of plant species to optimize the synthesis process and enhance the functional properties of AgNPs. Based on their phytochemical compositions, plants play a significant role in advancing the AgNP synthesis process. Ficus plants, with their unique combination of abundant bioactive compounds, sustainability, and versatility, stand out as particularly advantageous for producing and stabilizing AgNPs. The Ficus plant, a medicinal plant with diverse properties, is not merely a passive ingredient but an active player in the synthesis of AgNPs. Ficus plants actively contribute to the synthesis of AgNPs, and the AgNPs they mediate have been shown to have significant applications in various scientific fields, including antibacterial and antimicrobial activities, antidiabetic, cytotoxic, antioxidant, and antihemolysis.6,9,10 This article highlights the recent developments in Ficus-derived AgNPs, particularly their promising antimicrobial, anticancer, antibacterial, and cytotoxic activities. It keeps the readers informed about the latest advancements and enlightens them about the active and crucial role of the Ficus plant in this process. The main contributions of this paper are summarized as follows:
- Highlight the unique benefits and advances of the Ficus plant in AgNP synthesis, underscoring its superiority over other plant-based approaches.
- Discuss the impact of the synthesis parameters on the Ficus-based AgNP synthesis to produce AgNPs with desirable morphologies and sizes, thus enhancing their properties.
- Explore the appropriate characterization techniques for measuring and understanding the AgNP properties.
- Highlight the significant contributions and advances in AgNP synthesis using Ficus plants, emphasizing their potential in biomedical and environmental applications. This discussion is intended to inspire and instill hope about the future of nanotechnology.
- Discuss the key challenges of the Ficus-based AgNP synthesis and suggest mitigation strategies, emphasizing the urgent need for further research on this vital topic.
The review is organized as follows: Introducing the AgNP synthesis process, exploring the benefits and advances of the Ficus plant in AgNP synthesis, and discussing optimizing the synthesis parameters to create AgNPs with high yields and desirable morphologies and sizes. Next is the appropriate characterization technique for measuring and understanding the AgNP properties. The potential contributions of the Ficus-based AgNPs in biomedical and environmental applications are demonstrated next. Key challenges and limitations of Ficus-based synthesis and mitigation strategies are also discussed. Finally, a conclusion of the paper is drawn, and future work is considered.
Synthesis of AgNPs
Chemical and physical approaches, while widely used, come with significant drawbacks. They involve environmentally hazardous reactants, resulting in unstable yields, complex purification processes, and low conversion efficiency. Moreover, they are costly and consume high levels of energy.11 These drawbacks and potential environmental and biological risks due to toxic and dangerous chemicals underscore the urgent need for alternative methods. The biological approach, for instance, provides a sustainable, environmentally friendly, and economically viable method for synthesizing AgNPs.12-14 Figure 1 shows the two primary strategies for synthesizing AgNPs. The top-down method uses physical and chemical techniques to reduce the bulk material size to nanoscale proportions. In contrast, the bottom-up method creates NPs from smaller components, such as chemical and biological processes.15,16 A comparison of the AgNP synthesis methods is given in Table 1; details are discussed below.
Table (1):
Methods for AgNP synthesis
| Method | |||
|---|---|---|---|
| Chemical | Physical | Biological | |
| Examples | Reduction, photochemical electrochemical, sonochemical and microwave | Laser ablation, evaporation condensation, pyrolysis, gas and vapor phase, and arc discharge | Fungi, bacteria, algae, plant extract |
| Advantages | Reproducible, reliable, and quick. | Quick, with no harmful chemicals used. | Ecologically sustainable, economically advantageous, and very stable. |
| Approach | Bottom-up approach | Top-down approach | Bottom-up approach |
| Toxic | Toxic | Toxic | Non-toxic |
| Challenges | Hazardous, costly | Insufficient production, excessive energy use, and elevated expenses | Possible hazard of contamination by harmful microorganisms during the purifying process |
| References | 17 | 18 | 19 |
Physical methods
Physical techniques, including evaporation, condensation, laser ablation, irradiation, and lithography, can help produce AgNps. While they induce drawbacks, such as substantial power consumption and low yield potential, they also offer significant advantages. They do not involve solvent contamination and hazardous substances like reducing agents or stabilizers, offering rapid synthesis reaction rates and the exceptional purity of the AgNps. This high purity provides reassurance about the quality of the product, suggesting a promising future for AgNps production.
Chemical methods
Chemical synthesis remains the predominant approach for creating AgNPs. Under certain circumstances, Ag+ ions derived from a silver salt precursor undergo electron transfer and are converted into elemental silver.20 Several chemical methods, including reduction, polyol, and radiolysis, have been developed to produce AgNPs. Organic and inorganic chemicals are the most effective and uncomplicated candidates for generating AgNPs without aggregation, resulting in high yield at minimum preparation cost.21 Compared to physical procedures, the primary benefit of chemical procedures is their high throughput. Some disadvantages of chemical approaches include their high cost and the harmful and poisonous nature of the chemicals and compounds utilized in producing AgNPs, such as borohydride, which is a potent reducing agent and may cause skin and eye irritation, 2-mercaptoethanol, which is toxic if inhaled or swallowed, citrate, which can cause respiratory irritation, and thio-glycerol, which is harmful if swallowed or inhaled. Producing AgNPs of a precise size is a significant challenge, requiring extra measures to avoid particle aggregation and ensure uniformity.22 Multiple perilous and toxic by-products are generated during the synthesis process. In most instances, chemical synthesis methods cause certain chemically harmful compounds, limiting their use in medicinal applications.
Biological methods
Physically and chemically producing AgNPs is costly, time-consuming, and environmentally harmful. Therefore, creating an ecological and economically sustainable technique without toxic chemicals is crucial. Biological techniques, which include fungi, bacteria, yeasts, and plant sources, are safe alternatives. Their safety in diverse health management applications and controlling numerous biological processes is reassuring.23,24 This approach is gaining popularity for the medical applications of AgNPs. The biological manufacture of AgNPs involves three critical components: the synthesis solvent medium, the eco-friendly reducing agent, and the nontoxic stabilizing agent.25,26 Within the biological framework, organisms, including fungi, bacteria, and plants, involve bioactive substances that may serve as a bio-reducing agent capable of converting Ag+ ions into AgNPs.27 Microorganisms, including fungi, yeasts, and bacteria, can help create AgNPs.5 Plants, with their variety, availability, and non-toxicity, are the unsung heroes in AgNP synthesis, among other biological synthesis techniques. Plant-derived AgNPs exhibited comparable and better bioactivities to AgNPs, including antimicrobial, anti-angiogenesis, anti-cancer, and anti-diabetic properties. Plant-derived AgNPs’ biocompatibility makes them suitable as therapeutic agents in healthcare.27,28 The versatility of AgNPs in various applications, from healthcare to environmental remediation, is a fascinating aspect that we will explore in detail in this study. The AgNP biosynthesis using microorganisms or plants is discussed below in detail.
AgNP biosynthesis using microorganisms
The creation of AgNPs by microorganisms, a fascinating process, may occur either via an external or intracellular process.5 AgNPs accumulate inside the cells in the intracellular mode, and these NPs containing living cells may be extracted with specific processing. On the other hand, the extracellular process refers to the external release of microorganisms, which are then separated and used for synthesis. Microorganisms that exhibit resistance to silver ions demonstrate high effectiveness in producing AgNPs. The Pseudomonas stutzeri AG259 strain is a prevalent bacterial species often present in silver pits. It was the first strain to create microbial NPs.29 Enzymes like nitrate reductase are abundant and readily available in microorganisms, facilitating their production into AgNPs. Silver ions concurrently absorb the transferred ions as nitrate (NO3) is catalyzed into nitrite (NO2) by the nitrate reductase enzyme, which reduces silver ions to AgNPs.5 A few recent investigations have shown the potent antibacterial qualities of AgNPs, offering a promising solution against bacterial infections. According to several antimicrobial tests, bacteria and fungi are more efficient in reducing and stabilizing agents than various microbes, highlighting the potential of AgNPs in antibacterial treatments. Table 2 lists the antibacterial characteristics of biosynthesized AgNPs using fungi and bacteria.30
Table (2):
Synthesis and antimicrobial properties of AgNPs mediated from bacteria and fungi
| Biology Entity | Method Used | Tested Microorganisms | Shape and Sizes (nm) | Ref. |
|---|---|---|---|---|
| Bacteria | ||||
| Streptomyces strains | Intracellular | S. aureus, S. typhi, P. aeruginosa, K. pneumoniae, E. coli, P. vulgaris | Spherical;
1.17-13.3 nm |
32 |
| Streptomyces hirsutus strain SNPGA-8 | Extracellular | P. aeruginosa, C. glabrata, E. faecalis, C. albicans, A. alternate, F. oxysporum, S. aureus, E. coli | Spherical;
18.99-32.09 nm |
33 |
| Cytobacillus firmus | Extracellular | S. aureus, P. aeruginosa, E. feacalis, C. albicans, E. coli | Spherical;
30-60 nm |
34 |
| Lactobacillus brevis | Extracellular | S. aureus, E. coli | Spherical;
45 nm |
35 |
| Fungi | ||||
| Penicillium oxalicum | Extracellular | A. flavus, P. albicans, A. niger, A. luchuensis | Spherical;
13-23 nm |
36 |
| Chlorococcum humicola | Extracellular | K. pneumoniae, S. typhi, E. coli, F. solani, F. moniliforme Penicillium sp. (MTCC6489) | Spherical;
12.83 nm |
37 |
| Cladosporium cladosporioides | Extracellular | B. subtilis, S. aureus, E. coli, S. epidermis, C. albicans | Spherical;
30-60 nm |
38 |
In addition, bacteria may continue to proliferate following the creation of AgNPs. However, their primary drawbacks, shown as nano factories, are the sluggish pace of synthesis and the restricted sizes and shape range that may be achieved compared to traditional approaches. Alternatively, AgNPs are produced using fungi and plant extracts. Fungi can synthesize metallic NPs due to their capacity for metal accumulation, tolerance, high binding capacity, and ability to uptake metals intracellularly. With their convenience and ease of use in a research facility, Fungi is a more suitable alternative to bacteria.31,32 Fungi can release abundant enzymes to diminish the AgNO3 solution, synthesizing AgNPs. These organisms have a high tolerance to heavy metals, serving as reducing and stabilizing agents. They provide a sense of security about their potential use in AgNP synthesis. Also, fungi can readily grow in vast quantities and generate NPs with precise size and form. Fungi outperform other microbes because they can generate significant amounts of enzymes and proteins, which are utilized for the rapid and environmentally friendly production of AgNPs.5 However, they have several drawbacks that need to be addressed. One potential solution is to identify the specific fungus to use through advanced genetic and biochemical techniques. This approach, which can streamline the selection process and improve the efficiency of AgNP synthesis, underscores the need for technological advancements in the field. Understanding its growth characteristics is crucial, but the need for sterile conditions is equally important, a factor that cannot be overstated. Accounting for the time required to develop fungal and complete synthesis is also crucial.31 Another potential solution is to develop novel methods for maintaining sterile conditions, such as automated systems and advanced sterilization techniques. Synthesizing AgNPs using microorganisms is more time-consuming than utilizing plant extracts due to the intricate procedures involved in acquiring the reducing and capping agents. This scenario is because bacteria are difficult to cultivate, maintain cultures, standardize inoculum sizes, and other issues, encouraging the researchers to use plant extracts such as bark, tissue, or the whole plant to synthesize AgNPs.
AgNP biosynthesis using plants
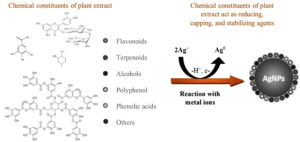
Synthesizing AgNPs using plants, shown in Figure 2, is a quick, cost-effective, environmentally benign, energy-efficient process without harmful chemicals.39 The potential for further research is a significant advantage of this method, as plants are readily available, safe-handled, and contain many active agents that help reduce Ag+ ions.40-42 Plants possess various active biomolecules, such as sugars, phenolic acids, terpenoids, alkaloids, polyphenols, and proteins. These biomolecules are essential for reducing and stabilizing silver ions in biological processes. Non-toxic phytochemicals, such as phenols and flavonoids, possess distinctive properties that reduce Ag+ ions and efficiently encapsulate AgNPs, thus avoiding clumping. Phenolic compounds include hydroxyl and carboxyl groups that can form bonds with metals. Hence, with their proven therapeutic significance, medicinal plants are not only safe and readily available but also intriguingly effective in producing AgNPs, sparking further interest and research in this area.
Extensive research has been conducted on producing AgNPs utilizing various plants, particularly Juniperus procera, Azadirachta indica, Olea europaea, and Ficus.40-43 Table 3 lists the antibacterial characteristics of biosynthesized AgNPs mediated by plants. Ficus plants, with their unique phytochemical profiles, offer some advantages in AgNP synthesis compared to other plants. These benefits, which are critical in reducing and stabilizing silver ions during nanoparticle synthesis, stand out as a fascinating area of study. Next, we will discuss the phytochemicals substances, synthesis, optimizations, characterizations, and applications of AgNPs synthesized using Ficus plants, inspiring further exploration and research in this intriguing field.
Table (3):
Antimicrobial characteristics of Plant-mediated AgNPs
Plants |
Parts |
Shape and size |
Microorganisms |
Ref. |
|---|---|---|---|---|
Melaleuca alternifolia |
Leaf |
Spherical; 11.56 nm |
S. aureus, methicillin-resistant Staphylococcus aureus, P. aeruginosa, S. epidermidis, S. pyogenes, K. pneumoniae, T. mentagrophytes, and C. albicans |
46 |
Moringa oleifera |
Flower |
Spherical; 8 nm |
K. pneumonia and S. aureus |
47 |
Asparagus racemosus |
Root |
Spherical; 10-17 nm |
E. coli, F. branchiophilum, Y. ruckeri, S. aureus, B. subtilis, A. hydrophila, E. tarda, K. pneumonia, and P. fluorescence |
48 |
Carya illinoinensis |
Leaf |
Spherical; 12-30 nm |
E. coli, P. aeruginosa, S. aureus, and L. monocytogenes |
49 |
Tecoma stans |
Flower |
Spherical; 50-60 nm |
E. coli and S. aureus |
50 |
Aegle marmelos |
Fruit |
Spherical; 10-200 nm |
B. cereus, P. aeruginosa, S. typhi, S. aureus, E. coli, S. dysenteriae and Y. pestis |
51 |
Carum copticum |
Seeds |
Spherical; 21.48 nm |
P. aeruginosa, S. marcescens, and C. violaceum |
52 |
Psidium guajava |
Leaf |
Spherical; 25 nm |
B. aryabhattai, B. megaterium, E. coli, Alcaligenes faecalis, S. cerevisiae, B. subtilis, A. creatinolyticus, A. niger, and R. oryzae |
53 |
Nauclea latifolia |
Fruit |
Irregular, 12 nm |
E. coli, C. albicans, Rhizopus sp., Staphylococcus sp. Klebsiella sp., A. niger, C. fruendii, and S. aureus |
54 |
Ficus ingens |
Leaf |
Spherical; 81.37 nm |
S. typhi, B. cereus, and E. coli |
55 |
Hibiscus cannabinus |
Seeds |
Spherical; 7-11 nm |
S. aureus, E. coli, and B. cereus |
56 |
Lysiloma acapulcensis |
Root |
Spherical; 1.2 to 62 nm |
E. coli, P. aeruginosa, S. aureus, and C. albicans |
57 |
Syzygium aromaticum |
Flower |
Polydisperse; 23 nm |
Staphylococcus spp. E. coli, A. niger, Penicillium spp. Pseudomonas spp., Bacillus spp., and A. flavus |
58 |
Murraya koenigii |
Leaf |
Spherical; 35-80 nm |
E. coli, P. aeruginosa, E. faecalis, and C. albicans |
59 |
Pimpinella anisum |
Seeds |
Irregular; 16-48 nm |
E. coli, C. albicans, S. aureus, and A. flavus |
60 |
Myristica fragrans |
Fruit |
Irregular; 31.31 nm |
E. coli, S. aureus, B. subtilis, and P. aeruginosa |
61 |
Ficus plants
Ficus, a fruit rich in vitamins, minerals, water, and fat, is a nutritional powerhouse. It is one of the richest plant sources of calcium and fiber, belonging to the Moraceae family’s Ficus genus, which comprises around 800 species.44 Mawa et al. found that Ficus plants are high in antioxidants and phenolic compounds.45 Ficus plants offer distinct advantages compared to other plant species. They are abundant in bioactive substances and phytochemicals, such as antioxidants, phenolic compounds, polyunsaturated fatty acids, phytosterols, and vitamins. These rich phytochemical substances, act as reducing and capping agents, facilitating rapid and efficient synthesis of AgNPs. The high concentration of phytochemicals in Ficus extracts accelerates the reduction of silver ions, resulting in rapid AgNP formation with a higher yield than other plant extracts.
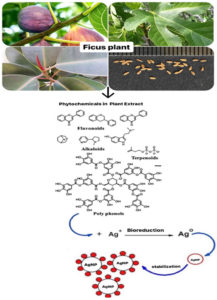
Ficus plants are widely distributed and adaptable to diverse climates, making them a sustainable and reliable source for AgNP synthesis. This is a significant advantage compared to other plants that may require more specific growth conditions or time-consuming cultivation processes. Ficus plant parts, such as bark, leaves, young shoots, fruits, seeds, and latex, are both nutritional and medicinal, used to produce AgNPs and for various pharmacological activities. The chemical diversity in Ficus extracts enables controlling the shape and size of the synthesizing AgNPs, offering diverse applications, including catalysis, medicine, and environmental remediation. Figure 3 comprehensively presents the traditional uses and pharmacological treatments of the Ficus-mediated AgNPs, providing valuable insights into their potential benefits and enhancing our knowledge in this area.62
Figure 3. Traditional uses and contemporary pharmacological activities of AgNPs biosynthesized from Ficus plants
Phytochemicals substances of Ficus plants
Conducting phytochemical screening on the active morphological samples is quite beneficial as it provides us with significant insights into the composition of the elements present in each plant sample. Ficus plants have diverse phytochemical substances, including phenols, amino acids, and flavones, which have antioxidant action.63 Plant phytochemicals reduce the Ag+ form to Ag3 and produce AgNPs without involving toxic compounds. Using plant-based sources of AgNPs offers practical benefits such as reassuringly simple processing, making it a promising avenue for future research. The potential of these self-assembled molecules to cap the metal NPs generated in their presence during metal ion reduction and provide some shape control is a source of inspiration for future studies. Figure 4 presents the active biomolecules found in Ficus plants. These molecules are responsible for reducing and stabilizing Ag+ ions, hence producing AgNPs. The Ficus sycomorus extract includes a noteworthy quantity of tannins and large amounts of flavonoids, saponins, alkaloids, and glycosides. Alkaloids are also present in the Ficus fruit extracts. These chemical classes are linked to Ficus sycomorus’s antibacterial properties and are known to be physiologically active.64 The extensive research on the potential utility of these bioactivities in inhibiting cancer cell activities has provided a wealth of knowledge.65,66 Flavonoids, which exhibit many biological impacts, such as antibacterial, analgesic, cytostatic, hypoglycemic, anti-inflammatory, and antioxidant qualities, showcase the impressive versatility of these compounds.67 Table 4 shows the Ficus plant’s phytochemical analysis, revealing the presence of bioactive agents that may contribute to the plant’s medicinal efficacy.
Table (4):
Phytochemical analysis of Ficus extracts
Phytochemicals |
Leaf |
Stem |
Bark |
Fruit |
|---|---|---|---|---|
Flavonoid |
+ |
+ |
+ |
+ |
Tannin |
+ |
+ |
+ |
+ |
Carbohydrate |
+ |
+ |
+ |
+ |
Protein |
+ |
– |
– |
+ |
Saponin |
+ |
+ |
+ |
+ |
Terpenoids |
+ |
+ |
+ |
+ |
Steroids |
+ |
+ |
+ |
+ |
Glycoside |
+ |
+ |
+ |
+ |
Phenol |
+ |
+ |
+ |
+ |
Key: + = Present and – =Absent
Optimization of the AgNP synthesis
The synthesis parameters, such as extract concentration, temperature, pH, and light, are crucial factors that substantially influence the properties of AgNPs.68 Understanding and optimizing these parameters can help produce AgNPs with desirable morphologies and sizes, thus enhancing their properties. Next, we will delve into the impact of the synthesis parameters (extract concentration, pH, and temperature) on the AgNPs biosynthesized using Ficus plants.
Extract concentration
The AgNP formation is significantly affected by the extract concentration used in the synthesis process. Plant extracts are critical for reducing Ag+ ions, and the efficiency of AgNP formation is greatly improved when the appropriate concentration is used.69 The surface plasmon resonance (SPR) strength increases as the extract concentration rises. This increase in SPR strength suggests a substantial rise in the yields of AgNPs. Optimizing the AgNP synthesis by varying Ficus extract concentration can be performed in the following experimental steps. The Ficus part is collected, dried, and ground into a fine powder. The Ficus extract is prepared using water, ethanol, or methanol solvents at different concentrations: 1%, 5%, 10%, and 20% (w/v). Then, Ficus extract is mixed with 1 mM AgNO3 solution in varying ratios. The Ficus extract concentration is systematically varied, like 1 mg/mL, 5 mg/mL, and 10 mg/mL, while the AgNO3 concentration is kept unchangeable. The mixture at different concentrations is separately incubated under controlled conditions while stabilizing the other synthesis, including temperature, pH, reaction time, and light intensity. The AgNP yield is quantified using centrifugation, drying, and weighing techniques. The AgNPs’ size, morphology, and stability are determined using relevant characterization techniques. A similar procedure is adopted for synthesizing AgNPs at different pH values and temperatures.
The AgNP synthesis rate generally rises as extract concentration increases, but beyond a specific concentration, it stabilizes or declines. Smaller and more uniform AgNPs are often produced at lower extract concentrations. Higher extract concentrations may lead to aggregation or reduced stability. The optimal extract concentration for AgNP synthesis depends on the plant material and reaction conditions. In Ficus-based synthesis, previous studies suggested that an extract concentration of around 10% (w/v) is effective for AgNP synthesis, balancing yield, particle size, and stability. 70,71 However, the optimal concentration can differ significantly depending on the particular Ficus species, the part of the plant utilized, and other reaction conditions. As a result, initial experiments are crucial to identify the most appropriate extract concentration for the specific conditions being used. Ramesh et al. biosynthesized AgNPs from different doses of Ficus hispida Linn. f. leaf extracts (1%, 3%, and 5%), and the prepared UV-Vis spectrum is shown in Figure 5.72
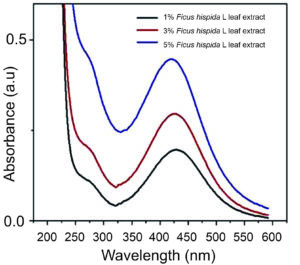 Figure 5. UV-Vis spectrum of AgNPs biosynthesized using various Ficus leaf extract concentrations.72
Figure 5. UV-Vis spectrum of AgNPs biosynthesized using various Ficus leaf extract concentrations.72
pH
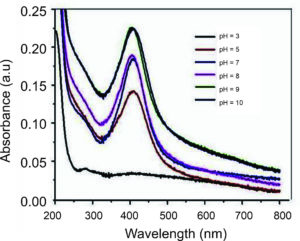
The profound impact of pH on biomolecules, changing their electrical charges and potentially diminishing their ability to coat, stabilize, and determine AgNP properties and reaction kinetics in the synthesis process, thereby influencing AgNP growth, is a significant finding.73,74 The research by Ramesh et al. is particularly enlightening, as it demonstrated that AgNPs derived from Ficus hispida Linn. f. leaf extracts, after incubation, exhibited high color intensity within a pH range of 6 to 9, as depicted in Figure 6.72 This finding was further supported by the collaborative efforts of other researchers, such as Sneha et al., who observed that an alkaline environment enhanced the Ficus leaf extract’s ability to reduce and stabilize the AgNP synthesis.75 Fresh Ficus leaves were cleaned, dried, and finely ground into powder. To prepare the Ficus extract, 10 g of the leaf powder was boiled in 100 mL of distilled water for 30 minutes and then filtered. The pH of the reaction mixture was adjusted to a range of 6-10 using NaOH or HCl. The pH is critical in the synthesis process, influencing the AgNPs’ properties and growth. Spectroscopic analysis of the reaction mixture of AgNPs prepared from Ficus racemosa revealed that the fruit at pH 9 yielded the best absorption. At the same time, the stem provided the best absorption at pH 8. The production of AgNPs with smaller surface areas at high pH values attributed to the functional groups’ bioavailability of the Ficus leaf extract is a significant implication of this research.
Temperature
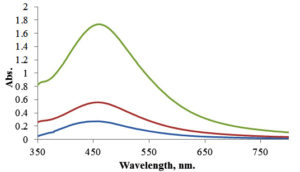
Temperature is a crucial determinant in AgNP production, with practical implications for our field. Typically, the reaction rate and nanoparticle formation increase as the temperature rises. As shown in Figure 7, Ramesh et al. extracted Ficus leaves to produce AgNPs at different temperatures, from 25 to 90 °C, and reported that the absorption peak increased when the reaction temperature increased. As the reaction temperature increases, Ag+ ions are rapidly reduced, resulting in successively homogeneous AgNPs with reduced particle sizes. Further increasing the reaction temperature beyond 90 °C decreased the absorption peak, suggesting 90 °C as the optimum reaction temperature for AgNP synthesis. These findings are consistent with other studies.76,77 The literature also proved that elevated reaction temperatures may expedite the synthesis rate, enhance the kinetic energy of molecules, rapidly deplete silver ions, and provide smaller particles with a uniform distribution, instilling confidence in the reliability of the process.78
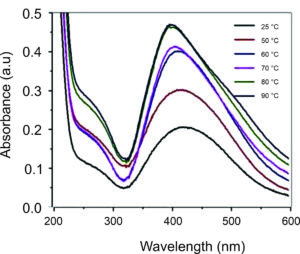 Figure 7. UV-Vis spectrum of AgNPs produced from Ficus leaf extract at various reaction temperatures.72
Figure 7. UV-Vis spectrum of AgNPs produced from Ficus leaf extract at various reaction temperatures.72
Furthermore, Sneha et al. biosynthesized the AgNPs from three parts of the Ficus racemosa plants: fruits, stems, and leaves.75 The maximum SPR peak intensity was obtained at temperatures between 80 and 100 °C, suggesting practical temperature ranges for AgNP production. The synthesis process was optimized by adjusting pH levels (ranging from 3 to 9), extract concentrations (from 0.5 to 8 mg/2 ml), reaction temperatures (50 °C to 100 °C), and AgNO3 concentrations (0.125 to 2 mM). The optimal conditions for AgNP synthesis were determined to be pH 9, extract concentration 8 mg, reaction temperature 80 °C, and AgNO3 concentration 2 mM for the fruits, pH 8, extract concentration 4 mg, reaction temperature 90 °C, and AgNO3 concentration 1.5 mM for the stems, and pH 8, extract concentration 8 mg, reaction temperature 100 °C, and AgNO3 concentration 2 mM for the leaves. The research highlighted the significant antibacterial effectiveness of the synthesized AgNPs against Gram-positive bacteria, including Staphylococcus equorum and Bacillus subtilis. These investigations emphasize the importance of optimizing synthesis parameters to customize the properties of AgNPs for targeted applications. By modifying variables such as pH, extract concentration, reaction temperature, and AgNO3 concentration, we can control the size, shape, and biological activity of the AgNPs, ultimately improving their performance across diverse fields and inspiring further research in this area.
Characterization of AgNPs
The necessity for analytical methodologies for nanoparticle analysis and characterization has arisen from the advent of nanotechnology, a field in which more contributions are crucial for significant advancement. Several characterization methods are accessible, including spectroscopic, separation, and microscopic techniques. Characterization of AgNPs is vital in determining the phase purity, shape, size, morphology, electronic transition plasmonic character, atomic environment, and surface charge.78
UV-Vis spectroscopy
UV-Vis spectroscopy, a precise and beneficial characterization technique, is instrumental in the preliminary characterization of produced nanomaterials. It allows for the examination of AgNPs for stability and structure. AgNPs, when subjected to UV-visible spectroscopy, exhibit an SPR band because of the free electron excitation. The absorption band in the visible region, a unique characteristic of AgNPs, is a crucial focus of research in this field.79,80 Vibration of the conduction electrons on the metal surface due to light excitation at specific wavelengths significantly intense the AgNP interaction. The produced AgNPs can be analyzed between 300 and 700 nm in size.81 Generally, broader peaks at longer wavelengths indicate increased AgNP size. Conversely, a narrower peak at a shorter wavelength suggests the development of smaller AgNPs. This work is crucial in our field, as demonstrated by Munoz-Carrillo et al., who found the best absorption of AgNPs produced from Opuntia Ficus–indica fruit at 435 nm wavelength.82 Also, Marzuk et al. biosynthesized the AgNPs from Ficus leaf extracts, highlighting the intriguing variation in synthesis methods.83 This variation underscores the need for further exploration and innovation in our field, and Figure 8 shows the maximum absorption obtained at 460 nm.
SEM Imaging
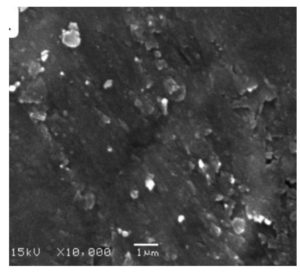
Most particles, including AgNPs, maintain a spherical form throughout their development, a considerable finding in the field. As previously reported, the AgNP size, a key characteristic, falls within the 10 to 50 nm range.84 The SEM technique is crucial in evaluating the AgNP morphological characteristics. Elkobrosy et al. created spherical AgNPs with different sizes utilizing Ficus, as shown in Figure 9.71 The AgNPs’ size and morphology variation are attributed to extract doses, reaction temperature, pH, and synthesis duration. These findings agreed with earlier reports.61,80 Organic compounds or reducing agents found in the extract may contribute to changing the AgNPs’ shape and size. These chemicals interact with AgNPs and cause their reduction. The produced AgNPs were uniformly spherical with a size range of 30-75 nm, and SEM images also revealed nano triangles and other morphologies.
On the other hand, spherical-shaped AgNPs made from vaca using Opuntia Ficus-indica were observed, with 64.28 ± 11.82 nm average particle size distribution.82 AgNPs synthesized using Ficus religiosa bark extract were spherical.86 This research is significant in nanotechnology and materials science, as it provides a good understanding of the characteristics and synthesis of AgNPs. These insights, combined with the contributions of our scientific community, can be seen in many applications, including drug delivery, catalysis, and sensing. The potential applications of this research are vast and inspiring, offering new possibilities and challenges for our scientific community and exciting potential for impact in various fields.
TEM analysis
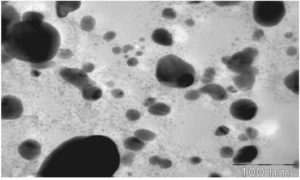
TEM, a reliable microscopy technique, is invaluable for determining the AgNPs’ shape and size. The resulting two-dimensional image, created by transmitting two electrons through the sample, is a testament to its reliability. This technique is beneficial for measuring the polymer wall of NPs.87 Figure 10 presents a TEM picture of AgNPs obtained from an extract of Ficus carica leaves.88 The AgNPs, with their spherical shape, clearly indicate the reliability of TEM analysis. Upon close examination, it is apparent that the AgNPs are encased by a subtle and delicate coating of other substances, which we hypothesize to be organic material derived from the Ficus carica leaf broth. The potential of TEM to uncover the nature of this organic coating is an intriguing aspect of this technique. The NPs obtained have diameters ranging from around 5 to 40 nm, with small particles becoming agglomerated. Furthermore, Elkobrosy et al. utilized TEM to analyze AgNPs produced from Ficus sycomorus leaves.71
The results, which are meticulously accurate, showed that most particles are spherical. Their diameters range between 17.1 and 57.5 nm, with at least 33 ± 1 nm average particle size. Salem et al. used TEM to identify AgNPs produced using Ficus sycomorus extracts, revealing that the predominant morphology of the particles is oval.64 A small number of AgNPs, either spherical or irregularly shaped, was also seen, with ≤20 nm size.
Zeta-Potential evaluation
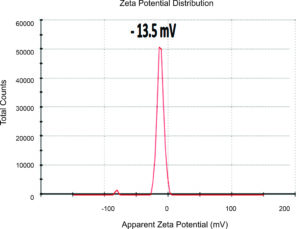
The AgNP surface potential, a novel aspect of this research, refers to the difference in electric potential between the medium in which the AgNPs are scattered and the outer surface of the dispersed NPs. This understanding can be crucial in various applications of AgNPs, inspiring optimism for their potential uses. The AgNPs’ surface potential can be measured using a zeta sizer.88,89 The solution pH may also affect the AgNP surface charge, which may reach a value of zero at a particular pH.2 Elkobrosy et al. determined the zeta potential of AgNPs produced from Ficus leaf extracts (Figure 11).71 The surfaces of the biosynthesized AgNPs had a net negative charge, with almost -13.5 mV zeta potential. Moreover, the zeta potential measurements of AgNPs made from Opuntia Ficus-indica fruits were -25:1 ± 0:03 mV at time 0 and -24:2 ± 0:06 mV after 120 hours.81 The produced AgNPs were generally stable over time with a negative charge. AgNPs with low zeta potential provide excellent stability in liquid preparation, which might be related to the significant electrostatic repulsion among the AgNPs.90 Also, AgNPs with <-30 mV zeta potential exhibit lower cytotoxicity than positively charged AgNPs since the latter easily interact with negatively charged cell membranes, as previously reported.88
DLS
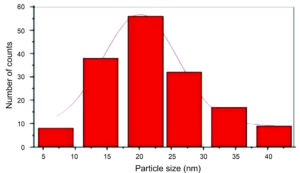
DLS, a technique that relies on light and particle interaction, is critical in understanding nanomaterials’ hazardous properties. Its precision in determining tiny particle size distributions, especially within the 2-500 nm range, is particularly noteworthy.91 By examining the changes in the intensity of scattered light over time, one may get data on the dimensions of the particle in a liquid solution.92 This understanding is vital when assessing the hazardous properties of a nanomaterial, especially in its liquid state.93 Hence, DLS assesses particle size distributions in aqueous or physiological solutions, providing researchers with a practical and applicable method.92 The size of AgNPs measured using DLS is often more significant than those measured using TEM due to Brownian motion’s complex influence. This method is instrumental in ascertaining the mean dimensions of AgNPs in liquid substances.94 The DLS report indicates the presence of various particle sizes biosynthesized using Ficus cordata leaf extract, with three distinct distribution curves observed. Figure 12 demonstrates the DLS of the AgNPs generated using Ficus leaf extracts, as reported by Ramesh et al.72 The most prevalent AgNP diameter observed was 150 nm, while the other AgNPs were more extensive due to agglomeration. This agglomeration may be attributed to the rapid reaction rate in forming AgNPs or adsorping a high molecular phytochemical concentration, increasing the dynamic diameter.
XRD
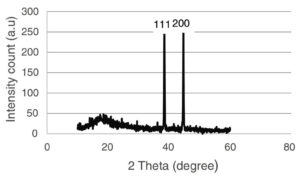
With its precision, XRD spectroscopy detects the AgNPs’ size and crystal structure.95 The characterization of NPs using XRD involves first identifying the phase of the sample material. The crystal type in the sample is then identified with the utmost accuracy, a testament to the reliability and trustworthiness of the search-match procedure in the areas where the peaks of high intensities are detected. This procedure, known for its reliability and trustworthiness, is a cornerstone in nanoparticle analysis. The study by Rahman & Prasanna has proven that the presence of three identifiable peaks at 33°, 46°, and 77° 2θ values correlates to the crystallographic planes 111, 200, and 311, respectively, for AgNPs that were generated by Ficus benghalensis leaf extract.81 These findings indicate a face-centered cubic structure for the AgNPs should be created. Another study revealed the cubic characteristics physiologically and found that XRD patterns of AgNPs manufactured by Ficus religiosa bark extract peak at 111 and 200 for the silver structure (Figure 13).85 These peaks represent the face-centered cube, demonstrating the precision of this research.
FTIR spectral analysis
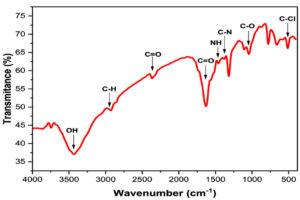
FTIR spectroscopy is vital for identifying the primary functional groups on the Ficus sycomorus surface. This surface plays a pivotal role in forming and stabilizing AgNPs, and it was effectively studied through this method. The absorption spectrum structure, a key element in our investigation, with its numerous peaks corresponding to high concentrations of specific chemical bond types, can provide a detailed map of the solution’s functional groups. These peaks demonstrate that the solution’s functional groups, including halogens, cycloalkanes, alcohols, carboxylic acids, amides, alkynes, alkanes, alkyl amines, and halogens, all serve as stabilizing agents.96,97 Alkobrosy et al. demonstrated that the AgNPs’ functional groups detected by FTIR analysis (Figure 14).71 These findings confirmed that AgNPs derived from Ficus plants have many functional groups that significantly contribute to reducing AgNO3 and facilitating AgNP synthesis. The control spectrum of AgNPs produced from the Ficus racemosa fruit, stem, and leaf extracts displayed peaks indicating the presence of several different biomolecules.80 This research opens new possibilities for applying AgNPs derived from Ficus plants, inspiring hope for their potential in various fields.
Applications of AgNPs biosynthesized from Ficus plant
Environmental applications
Because of their antimicrobial, antiviral, and antifungal properties, AgNPs have been proven promising in aquaculture. The Ficus-mediated AgNP synthesis method provides a sustainable and adaptable solution for tackling environmental issues such as water and air purification, soil remediation, and pollution monitoring while reducing ecological impact. Here, we discuss essential insights into the environmental applications of Ficus-derived AgNP synthesis and next explore its beneficial pharmaceutical activities.
Ficus-based AgNP synthesis demonstrates potent antimicrobial properties, making them highly effective in purifying water by eliminating harmful bacteria, viruses, and other microorganisms. When exposed to light, the biosynthesized AgNPs serve as catalysts in degrading organic pollutants, including dyes and pesticides. This unique property contributes significantly to water purification. Moreover, Ficus-based AgNP synthesis provides a viable alternative to traditional treatment methods for removing heavy metal contamination in wastewater.85 AgNPs, with their potential to break down complex organic pollutants in industrial wastewater, play a crucial role in lowering toxicity and minimizing environmental harm. They can help decrease microbial load in sewage sludge, enhancing the efficiency of wastewater treatment processes. However, using AgNPs in wastewater treatment may cause environmental and health threats. They can be released into the environment, potentially harming aquatic life. Therefore, the potential environmental and health impacts of using AgNPs in wastewater treatment require careful consideration and extensive investigation. The need for immediate action and the urgency of this investigation cannot be overstated. Key challenges of Ficus-derived AgNP synthesis and possible mitigation strategies are discussed next. Riaz et al. produced AgNPs from Ficus religiosa bark extracts and employed them to eliminate chromium from artificial wastewater.85 The treatment findings indicated a clearance effectiveness of over 74.8%.
Furthermore, AgNPs assist in degrading persistent organic pollutants, such as pesticides and herbicides, in contaminated soils, thereby improving soil health. They can inhibit harmful soil pathogens, fostering the growth of beneficial microorganisms and supporting plant development. Additionally, AgNPs derived from Ficus can be integrated into filters or coatings to break down volatile organic compounds (VOCs) and other air pollutants, enhancing air quality. They can be used in air filtration systems to capture and neutralize airborne pathogens, reducing the spread of infectious diseases. Ficus-based AgNPs can be incorporated into biosensors for real-time tracking of environmental factors, assisting in pollution control and management. Their potential in creating nanofertilizers and nanopesticides is significant, as it decreases the reliance on chemical inputs and offers hope for a more sustainable and less polluted environment.
Biomedical applications
Ficus plants offer beneficial pharmaceutical activities, such as antidiabetic, anti-inflammatory, nematocidal, antiphotoaging, antioxidant, antidiabetic, antiulcerogenic, and antibacterial agents. Table 5 reports some medical applications of AgNPs biosynthesized using different species and parts of Ficus plants.
Table (5):
Medical applications of AgNPs biosynthesized using different species and parts of Ficus plants
Ficus |
Part |
Application |
Mechanism |
Ref. |
|---|---|---|---|---|
Ficus benghalensis |
leaves |
Antibacterial activity |
Inhibitory effects against E. coli MTCC1302 |
98 |
Ficus sycomorus |
stem bark |
Antibacterial activity |
Inhibitory effects against the multi-drug resistant (MDR) Shigella species |
85 |
Ficus palmata |
leaves |
Antidiabetic activity |
Exhibited in vitro antidiabetic properties by effectively inhibiting α-amylases and α-glucosidases |
99 |
Ficus sycomorus |
leaves |
Nematocidal Activities |
Showed efficacy against both the root-knot nematode and many bacterial strains |
71 |
Ficus benghalensis |
roots |
antimicrobial activity |
Aerial roots exhibited potent antimicrobial activity against oral microbes against |
78 |
Ficus deltoidea |
leaves |
antimicrobial activity |
Had antimicrobial activity against Helicobacter pylori. |
100 |
Ficus deltoidea |
leaves |
Cytotoxicity Activity |
The presence of harmful elements in Ficus deltoidea leaves was assessed using atomic absorption spectroscopy, which indicated that the leaves do not contain any poisonous components. |
101 |
Antibacterial activity
The AgNP dosage influences their antibacterial activity, a captivating area of research. The enhanced antibacterial properties of AgNPs with increasing concentration, as demonstrated by their improved interaction with sulfur-containing proteins in bacteria, is a promising avenue for further exploration.72 The bactericidal activity of AgNPs, derived from Ficus benghalensis leaf extracts, was tested against E. coli MTCC 1302, illustrating significant antibacterial efficacy. The AgNPs exhibit substantial antibacterial activities against E. coli MTCC1302 due to their significant surface-to-volume ratio.98 The AgNPs derived from Ficus sycomorus stem bark extracts exhibit significant inhibitory effects against the multi-drug resistant (MDR) Shigella species, surpassing the crude extracts and silver nitrate solutions. This AgNP biosynthesis, presenting a promising treatment for shigellosis, instills hope and optimism about the potential of enhancing the efficacy of local herbs through nano-biotechnology models, sparking intrigue and excitement about the future of antibacterial research.
Antidiabetic activity
The antidiabetic effects of mineral AgNPs were assessed, and they have potential applications in treating diabetes. The AgNPs derived from Ficus palmata leaves exhibited in vitro antidiabetic properties by impressively inhibiting a-amylases and b-glucosidases. An analysis determined the amylase inhibition (AAIs) of AgNPs produced from Ficus leaf extract. The results showed that AgNPs derived using Ficus palmata exhibited the most significant inhibition. Notably, the level of inhibition caused by AgNPs rises proportionally with the concentration of produced AgNPs, suggesting potential for further research and development in this area.99
Nematocidal activity
The in vitro nematocidal properties of AgNPs, derived from Ficus sycomorus, have been assessed on the root-knot nematode Meloidogyne incognita. Elkobrosy et al. demonstrated that the biosynthesized AgNPs were efficacious against both the root-knot nematode and many bacterial strains, illustrating their potential control of plant-parasitic nematodes.71 Importantly, their simplicity of application, stability, cost, and environmental friendliness make them a reassuring choice for nematode control, ensuring ease of use for agricultural professionals.
Antimicrobial activity
The AgNPs derived from Ficus benghalensis aerial root extracts exhibited potent antimicrobial activity against oral microbes. Biosynthesized AgNPs may help treat many oral diseases and have extensive applications in dentistry.43
Cytotoxicity activity
Shafaei et al. rigorously applied atomic absorption spectroscopy to assess toxic elements in Ficus deltoidea leaves.101 Their findings, which confirmed the absence of these elements, were further validated by the evaluation of standardization parameters such as volatile, total ash, moisture, and acid insoluble ash, along with the microbial limit test (MLT) to determine microbial contamination.102 These findings are significant in the field of botany and toxicology.
Challenges, limitations, and mitigation strategies
Ficus-based AgNP synthesis is a promising, green, and versatile approach to AgNP production. It aligns with the global need for sustainable and eco-friendly technologies while providing AgNPs with significant medicinal and environmental applications. Despite its challenges, this method’s potential to deliver high-quality, consistent AgNPs is inspiring. The key challenges of Ficus-based AgNP synthesis and possible solutions are discussed below.
Variability in phytochemical composition
The variability in photochemical substances of Ficus extracts, depending on the species, growth conditions, season, and extraction method, can lead to inconsistent AgNP characteristics. This inconsistency can affect the functionality of the AgNPs in applications. Therefore, standardizing the plant source, growth conditions, and extraction process cannot be overstated. Optimizing the synthesis parameters (extract concentration, temperature, pH, AgNO3 concentration, and reaction time) is crucial to ensure consistency in the synthesized AgNPs.
Tailoring nanoparticle morphology and dimensions
Obtaining uniform size and shape of AgNPs is challenging due to plant-mediated synthesis’s complex and dynamic nature. The presence of polydisperse nanoparticles can limit their applicability in specific fields. However, adjusting reaction conditions such as pH, temperature, AgNO3 concentration, and extract volume is crucial in controlling nucleation and growth, thereby customizing the desired morphology and size of AgNPs. The use of capping agents or surfactants to stabilize the nanoparticles and prevent aggregation further underscores the integral role of the work. Post-synthesis purification methods like centrifugation or filtration can isolate AgNPs with the desired size and shape.
Low yield and scalability issues
Plant-based AgNP synthesis methods often produce lower yields than chemical or physical methods, posing a challenge for large-scale production. However, we can maximize yield by optimizing the extract concentration and reaction time. Investigating continuous flow synthesis techniques is essential for achieving scalable production. Moreover, bioreactors or automated systems can be a proper solution, streamlining and scaling up the synthesis process and instilling confidence in the feasibility of large-scale production.
Stability and aggregation
AgNPs biosynthesized using Ficus extracts hold great potential, but they may aggregate over time due to insufficient stabilization, reducing their efficiency. Stabilizing agents, such as polymers, proteins, or surfactants, can enhance colloidal stability. Maintaining controlled storage conditions for biosynthesized AgNPs helps prevent oxidation and aggregation. Additionally, adding biocompatible coatings on the surface of AgNPs can improve their stability, further enhancing the promise of this technology.
Toxicity and environmental concerns
While plant-based AgNP synthesis is environmentally friendly, the potential toxicity of AgNPs to humans and the environment continues to be a concern. Comprehensive toxicity evaluations, including cytotoxicity and ecotoxicity tests, are essential to ensure safe usage. Next, key observations on current safety issues and risk reduction measures associated with plant-based AgNP toxicity are discussed.
Safety concerns
Cytotoxicity and Genotoxicity
AgNPs’ nanoscale size and large surface area give them a heightened reactivity that can lead to severe cellular damage. This can result in DNA fragmentation and programmed cell death in plants, animals, and microorganisms, underscoring the serious potential for toxicity.
Environmental Accumulation
AgNPs, with their potential to accumulate in soil and water environments, pose a significant threat to ecosystems. Their presence has the potential to severely disrupt the delicate balance and impact of non-target organisms, such as beneficial microbes and aquatic species. Long-term exposure can result in bioaccumulation and biomagnification within the food chain, further exacerbating the situation.
Plant Toxicity
High concentrations of AgNPs can have a detrimental effect on plant health. They can disrupt the crucial process of photosynthesis and hinder nutrient absorption, inhibiting seed germination, root elongation, and overall plant growth.
Human Health Risks
Inhalation, ingestion, or skin contact with AgNPs may threaten human health, potentially causing inflammation, organ damage, and carcinogenic effects.
Mitigation strategies
Controlled Synthesis
Controlled synthesis has significant potential benefits in regulating the size, shape, and surface characteristics of AgNPs. By producing larger, more stable particles, we can minimize risks. Plant extracts contain natural stabilizing agents that can further enhance this process, preventing AgNP aggregation and improving biocompatibility.
Surface Functionalization
It is encouraging to note that coating AgNPs with biocompatible materials can significantly reduce their toxicity. Modifying AgNPs with particular ligands to target specific applications and minimize unintended side effects is crucial.
Dose Optimization
The significance of dose optimization in achieving the desired results while reducing toxicity cannot be overstated. Using the minimum effective concentration of AgNPs can ensure the safety of various applications. Dose-response studies play a crucial role in establishing safe limits for many applications.
Environmental Monitoring and Remediation
Establishing monitoring programs to measure AgNP concentrations in soil, water, and air is crucial to ensuring ecological safety. This proactive approach should reassure us that we are taking the necessary steps to protect our environment. Creating remediation strategies, such as the crucial role of plants and microbes in absorbing and neutralizing AgNPs in polluted environments, is essential, further enhancing our environmental safety measures.
Regulatory Frameworks and Guidelines
Equally important is establishing protocols for the safe disposal and recycling of AgNPs, reflecting our collective responsibility to use nanotechnology. By creating safety guidelines and exposure thresholds for AgNPs in agricultural and environmental contexts, we are providing a secure framework for the governance of AgNPs, empowering each of us to play a crucial role in this process.
While Ficus-mediated synthesis of AgNPs offers a sustainable and eco-friendly alternative to conventional methods, addressing the challenges of variability, scalability, stability, and toxicity is crucial for widespread adoption. We can overcome these limitations by refining synthesis parameters, understanding the underlying mechanisms more deeply, and utilizing advanced characterization techniques. This can inspire and motivate us to explore innovative applications of AgNPs in medicine, catalysis, and, most importantly, environmental remediation.
There is a growing concern about nanotechnology and green chemistry in producing AgNPs with unique and fascinating properties. These properties, such as their high surface area to volume ratio and unique optical, electrical, and catalytic properties, make them highly desirable for various applications. There is an increasing awareness of green chemistry and the employment of environmentally friendly methods to synthesize AgNPs, leading to a preference for expanding methodologies that are better for the environment. Natural resources such as bacteria, algae, fungi, and plants are widely utilized for AgNP synthesis. Using plants compared to other organisms can be advantageous in avoiding the leisurely path of microorganisms and maintaining the culture medium. Other advantages of using plants as reducing agents include their environmental benefits and health-friendly nature. The safety of AgNPs synthesized using plants is a key factor, providing a sense of reassurance to scientists. Also, plants have several unique compounds that help improve the synthesis rate.
The literature indicates that the AgNP properties are affected by several parameters. Understanding and addressing these aspects is crucial for AgNPs’ environmentally friendly production, and research in this fascinating field has the potential to make a meaningful and beneficial impact. For instance, in the synthesis process, increasing the biological substance concentration leads to higher contents of biomolecules, which is responsible for reducing Ag+ to Ag0, intensifying the solution color. Manipulating the pH values can influence the synthesis process and AgNPs’ physical properties. An alkaline environment leads to the formation of smaller NP and vice versa. In contrast, in an acidic environment, many biomolecules interact, leading to agglomeration and forming larger AgNPs. Also, higher temperatures are crucial in the nucleation process, a key factor in producing high-quality AgNPs.
This article explores the appropriate characterization techniques for measuring and understanding the AgNP properties. The SEM technique evaluates the morphological characteristics, and TEM examines the AgNPs’ size and morphology. XRD establishes the crystallinity degree, while UV-Vis spectroscopy helps ascertain sample formation via SPR. FTIR spectroscopy determines the primary functional groups and their potential role in forming and stabilizing AgNPs. DLS evaluates the AgNPs’ size distributions in aqueous or physiological solutions. AgNPs have various pharmaceutical, medicinal, and biological applications. However, it is crucial to understand and measure the potential risks, particularly the AgNP toxicity, and to conduct an intensive investigation. This emphasis on thorough research should make the audience feel the gravity of their work, understanding that their efforts are crucial in concluding the safety of AgNPs.
Ficus offers beneficial pharmaceutical activities, including antidiabetic, antinociceptive, antiinflammation, antioxidant, antimelanogenic, and antibacterial agents. Also, Ficus-derived AgNPs have diverse green activities, making them useful in various advanced treatment techniques that may help combat current and future pandemics. The urgency of future research cannot be overstated. It should examine the effects of AgNPs on biological systems and study living creatures’ responses to short-term and long-term exposure to AgNPs to reduce their impact on society and the environment. Furthermore, research into the internal and environmental variables influencing AgNP toxicity is crucial. While internal variables are tied to AgNPs’ characteristics, external aspects are controllable by human intervention. Consequently, to prevent the damage that AgNP toxicity does to people and the environment, it will be required to increase future studies on the variables that influence AgNPs. Research into the processes of AgNP toxicity is intended to eventually eradicate whatever harm AgNPs may bring to humans or the environment.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
NSA conceptualized and supervised the study. WFAM performed the literature review, data analysis and wrote the manuscript. NSA reviewed, edited and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Bawazeer S, Rauf A, Naz H, et al. Synthesis, Characterization and Bioactivity Profiling of Gold Nanoparticles of Trachyspermum ammi Crude Extract. J Pure Appl Microbiol. 2021;15(2):667-676.
Crossref - Botelho CM, Fernandes MM, Souza JM, et al. New textile for personal protective Equipment-Plasma chitosan/silver nanoparticles nylon fabric. Fibers. 2021;9(1):3.
Crossref - Krishna G, Maringanti SC. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J Exp Nanosci. 2016;11(9):1-8.
Crossref - Rahuman HBH, Dhandapani R, Narayanan S, et al. Medicinal plants mediated the green synthesis of silver nanoparticles and their biomedical applications. IET Nanobiotechnol. 2022;16(4):115-144.
Crossref - Mustapha T, Misni N, Ithnin NR, Daskum AM, Unyah NZ. A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. Int J Environ Res Public Health. 2022;19(2):674.
Crossref - Akhtar Y, Rashid A, Atif M, et al. Green Synthesis of Silver Nanoparticles Using Doxycycline: Evaluation of Antimicrobial Potential and Organ-specific Toxicity. J Pure Appl Microbiol. 2024;18(4):2496-2506.
Crossref - Thatyana M, Dube NP, Kemboi D, Manicum AE, Mokgalaka-Fleischmann NS, Tembu JV. Advances in phytonanotechnology: A plant-mediated green synthesis of metal nanoparticles using Phyllanthus plant extracts and their antimicrobial and anticancer applications. Nanomaterials. 2023;13(19):2616.
Crossref - Abdi V, Sourinejad I, Yousefzadi M, Ghasemi Z. Mangrove-mediated synthesis of silver nanoparticles using native Avicennia marina plant extract from southern iran. Chem Eng Commun. 2018;205(8):1069-1076.
Crossref - Khleifat K, Qaralleh H, Al-Limoun M, et al. Antibacterial Activity of Silver Nanoparticles Synthesized by Aspergillus flavus and its Synergistic Effect with Antibiotics. J Pure Appl Microbiol. 2022;16(3):1722-1735.
Crossref - Kefale GY, Wodag AF. Ficus sycomorus as a sustainable source of leather dyeing material: An ecofriendly approach. J Eng. 2023;2023(1):776239.
Crossref - Yaqoob AA, Umar K, Ibrahim MNM. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications-a review. Appl Nanosci. 2020;10(5):1369-1378.
Crossref - Al-Hayanni HSA, Alnuaimi MT, AL-Lami RA, Zaboon SM. Antibacterial Effect of Silver Nanoparticles Prepared from Sophora flavescens Root Aqueous Extracts against Multidrug-resistance Pseudomonas aeruginosa and Staphylococcus aureus. J Pure Appl Microbiol. 2022;16(4):2880-2890.
Crossref - Aboyewa JA, Sibuyi NRS, Meyer M, Oguntibeju OO. Green synthesis of metallic nanoparticles using some selected medicinal plants from southern africa and their biological applications. Plants. 2021;10(9):1929.
Crossref - Liaqat N, Jahan N, Khalil-ur-Rahman, Anwar T, Qureshi H. Green synthesized silver nanoparticles: Optimization, characterization, antimicrobial activity, and cytotoxicity study by hemolysis assay. Front Chem. 2022;10:952006.
Crossref - Jamkhande PG, Ghule NW, Bamer AH, Kalaskar MG. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J Drug Deliv Sci Technol. 2019;53:101174.
Crossref - Lee SH, Bong- Hyun J. Silver nanoparticles: Synthesis and application for nanomedicine. Int J Mol Sci. 2019;20(4):865.
Crossref - Menazea AA. Femtosecond laser ablation-assisted synthesis of silver nanoparticles in organic and inorganic liquids medium and their antibacterial efficiency. Radiat Phys Chem. 2020;168:108616.
Crossref - Bafana A, Kumar SV, Temizel-Sekeryan S, Dahoumane SA, Haselbach L, Jeffryes CS. Evaluating microwave-synthesized silver nanoparticles from silver nitrate with life cycle assessment techniques. Sci Total Environ. 2018;636:936-943.
Crossref - Danagoudar A, Pratap GK, Shantaram M, Ghosh K, Kanade SR, Joshi CG. Characterization, cytotoxic and antioxidant potential of silver nanoparticles biosynthesised using endophytic fungus (Penicillium citrinum CGJ-C1). Mater Today Commun. 2020;25:101385.
Crossref - Li Z, Dong H, Wu Z, et al. Novel p-n type porous Ag2O/Bi5O7I heterojunction for Uv-Vis-NIR activated high efficient photocatalytic degradation of bisphenol A: Photoelectric properties and degradation mechanism. Appl Surf Sci. 2020;529:147162.
Crossref - Marinescu L, Ficai D, Oprea O, et al. Optimized synthesis approaches of metal nanoparticles with antimicrobial applications. J Nanomater. 2020;2020(4):6651207.
Crossref - Ovais M, Khalil AT, Ayaz M, Ahmad I, Nethi SK, Mukherjee S. Biosynthesis of metal nanoparticles via microbial enzymes: A mechanistic approach. Int J Mol Sci. 2018;19(12):4100.
Crossref - Almatroudi, A. Silver nanoparticles: Synthesis, characterisation and biomedical applications. Open Life Sci. 2020;15(1):819-839.
Crossref - Vishwanath R, Negi B. Conventional and green methods of synthesis of silver nanoparticles and their antimicrobial properties. Current Research in Green and Sustainable Chemistry. 2021;4:100205.
Crossref - Xu L, Wang YY, Huang J, Chen CY, Wang ZX, Xie H. Silver nanoparticles: Synthesis, medical applications and biosafety. Theranostics. 2020;10(20):8996.
Crossref - Zuhrotun A, Oktaviani DJ, Hasanah AN. Biosynthesis of gold and silver nanoparticles using phytochemical compounds. Molecules. 2023;28(7):3240.
Crossref - Simon S, Sibuyi NRS, Fadaka AO, et al. Biomedical applications of plant extract-synthesized silver nanoparticles. Biomedicines. 2022;10(11):2792.
Crossref - Prabhu S, Poulose EK. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett. 2012;2:32.
Crossref - Naganthran A, Verasoundarapandian G, Khalid FE, et al. A. Synthesis, characterization and biomedical application of silver nanoparticles. Materials. 2022;15(2):427.
Crossref - Sebesta M, Urik M, Bujdo M, et al. Fungus Aspergillus niger processes exogenous zinc nanoparticles into a biogenic oxalate mineral. J Fungi, 2020;6(4):210.
Crossref - Guilger-Casagrande M, de Lima R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front Bioeng Biotechnol. 2019;22:7:287.
Crossref - Ameen F, AlYahya S, Govarthanan M, et al. Soil bacteria Cupriavidus sp. mediates the extracellular synthesis of antibacterial silver nanoparticles. Journal of Molecular Structure. 2020;1202:127233.
Crossref - Gan L, Zhang S, Zhang Y, He S, Tian Y. Biosynthesis, characterization and antimicrobial activity of silver nanoparticles by a halotolerant Bacillus endophyticus SCU-L. Prep Biochem Biotechnol. 2018;48(7):582-588.
Crossref - Nayak PS, Arakha M, Kumar A, Asthana S, Mallick BC, Jha S. An approach towards continuous production of silver nanoparticles using Bacillus thuringiensis. RSC Adv. 2016;6(10):8232-8242.
Crossref - Shaker MA, Shaaban MI. Synthesis of silver nanoparticles with antimicrobial and anti-adherence activities against multidrug-resistant isolates from Acinetobacter baumannii. J Taibah Univ Med Sci. 2017;12(4):291-297.
Crossref - Neethu S, Midhun SJ, Radhakrishnan EK, Jyothis M. Green synthesized silver nanoparticles by marine endophytic fungus Penicillium polonicum and its antibacterial efficacy against biofilm forming, multidrug-resistant Acinetobacter baumanii. Microbial Pathogenesis. 2018;116:263-272.
Crossref - Ma L, Su W, Liu JX, et al. Optimization for extracellular biosynthesis of silver nanoparticles by Penicillium aculeatum Su1 and their antimicrobial activity and cytotoxic effect compared with silver ions. Mater Sci Eng C Mater Biol Appl. 2017;77:963-971.
Crossref - Win TT, Khan S, Fu P. Fungus-(Alternaria sp.) mediated silver nanoparticles synthesis, characterization, and screening of antifungal activity against some phytopathogens. J Nanotechnol. 2020;2020(1):1-9.
Crossref - Hano C, Abbasi BH. Plant-based green synthesis of nanoparticles: Production, characterization and applications. Biomolecules. 2021;12(1):31.
Crossref - Alsubhi NS, Alharbi NS, Felimban AI. Optimized Green Synthesis and Anticancer Potential of Silver Nanoparticles Using Juniperus procera Extract Against Lung Cancer Cells. J Biomed Nanotechnol. 2022;18(9):2249-2263.
Crossref - Alharbi NS, Alsubhi NS. Green synthesis and anticancer activity of silver nanoparticles prepared using fruit extract of Azadirachta indica. J Radiat Res Appl Sci. 2022;15(3):335-345.
Crossref - Alharbi NS, Felimban AI. Cytotoxicity of silver nanoparticles green-synthesized using Olea europaea fruit extract on MCF7 and T47D cancer cell lines. J King Saud Univ Sci. 2023;35(10):102972.
Crossref - Singh P, Dhankhar J, Kapoor RK, et al. Ficus benghalensis – A comprehensive review on pharmacological research, nanotechnological applications, and patents. J Appl Pharm Sci. 2023;13(10):059-082
Crossref - Nawaz H, Waheed R, Nawaz M. Phytochemical composition, antioxidant potential, and medicinal significance of Ficus. Modern Fruit Industry. 2020;1:20.
Crossref - Mawa S, Husain K, Jantan I. Ficus carica L. (moraceae): Phytochemistry, traditional uses and biological activities. Evid Based Complement Alternat Med. 2013.
Crossref - Ramadan MA, Shawkey AE, Rabeh MA, Abdellatif AO. Promising antimicrobial activities of oil and silver nanoparticles obtained from Melaleuca alternifolia leaves against selected skin-infecting pathogens. J Herb Med. 2020;20:100289.
Crossref - Bindhu MR, Umadevi M, Esmail GA, Al-Dhabi NA, Arasu MV. Green synthesis and characterization of silver nanoparticles from Moringa oleifera flower and assessment of antimicrobial and sensing properties. J Photochem Photobiol B. 2020;205:111836.
Crossref - Kumar PPNV, Kalyani RL, Veerla SC, Kollu P, Shameem U, Pammi SVN. Biogenic synthesis of stable silver nanoparticles via Asparagus racemosus root extract and their antibacterial efficacy towards human and fish bacterial pathogens. Mater Res Express. 2019;6(10):104008.
Crossref - Dalir SJB, Djahaniani H, Nabati F, Hekmati M. Characterization and the evaluation of antimicrobial activities of silver nanoparticles biosynthesized from Carya illinoinensis leaf extract. Heliyon. 2020;6(3):e03624.
Crossref - Hariram M, Ganesan V, Muthuramkumar S, Vivekanandhan S. Functionalization of kaolin clay with silver nanoparticles by Murraya koenigii fruit extract-mediated bioreduction process for antimicrobial applications. J Aust Ceram Soc. 2021;57:505-513.
Crossref - Devi M, Devi S, Sharma V, Rana N, Bhatia RK, Bhatt AK. Green synthesis of silver nanoparticles using methanolic fruit extract of aegle marmelos and their antimicrobial potential against human bacterial pathogens. J Tradit Complement Med. 2020;10(2):158-165.
Crossref - Qais FA, Shafiq A, Ahmad I, Husain FM, Khan RA, Hassan I. Green synthesis of silver nanoparticles using Carum copticum: Assessment of its quorum sensing and biofilm inhibitory potential against gram negative bacterial pathogens. Microbial Pathogenesis. 2020;144:104172.
Crossref - Wang L, Wu Y, Xie J, Wu S, Wu Z. Characterization, antioxidant and antimicrobial activities of green synthesized silver nanoparticles from Psidium guajava L. leaf aqueous extracts. Mater Sci Eng C Mater Biol Appl. 2018;86:1-8.
Crossref - Odeniyi MA, Okumah VC, Adebayo-Tayo BC, Odeniyi OA. Green synthesis and cream formulations of silver nanoparticles of Nauclea latifolia (african peach) fruit extracts and evaluation of antimicrobial and antioxidant activities. Sustain Chem Pharmacy. 2020;15:100197.
Crossref - Kavaz D, Umar H, Shehu S. Synthesis, characterization, antimicrobial and antimetastatic activity of silver nanoparticles synthesized from Ficus ingens leaf. Artif Cells Nanomed Biotechnol. 2018;46(3):S1193-S1203.
Crossref - Adnan M, Azad MOK, Madhusudhan A, et al. Simple and cleaner system of silver nanoparticle synthesis using kenaf seed and revealing its anticancer and antimicrobial potential. Nanotechnology. 2020;31(26):265101.
Crossref - Garibo D, Borbon-Nunez HA, de Leon JND, et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci Rep. 2020;10(1):12805.
Crossref - Ajitha B, Reddy YAK, Lee Y, Kim MJ, Ahn CW. Biomimetic synthesis of silver nanoparticles using Syzygium aromaticum (clove) extract: Catalytic and antimicrobial effects. Appl Organomet Chem. 2019;33(5):e4867.
Crossref - Chahande RK, Mehere BA, Pantawane PK, Chouke PB, Murai SR. Bio-inspired synthesis of silver nanoparticles assisted Murraya koneigii leaf extract and its antimicrobial activity. Materials Today: Proceedings. 2020;29(3):923-928.
Crossref - Zayed MF, Mahfoze RA, El-Kousy SM, Al-Ashkar EA. In-vitro antioxidant and antimicrobial activities of metal nanoparticles biosynthesized using optimized Pimpinella anisum extract. Colloids and Surfaces A: Physicochemical and Engineering Aspect. 2020;585:124167.
Crossref - Alsammarraie FK, Wang W, Zhou P, Mustapha A, Lin M. Green synthesis of silver nanoparticles using turmeric extracts and investigation of their antibacterial activities. Colloids Surf B: Biointerfaces. 2018;171:398-405.
Crossref - Salehi B, Mishra AP, Nigam M, et al. Ficus plants: State of the art from a phytochemical, pharmacological, and toxicological perspective. Phytother Res. 2021;35(3):1187-1217.
Crossref - Murugesu S, Selamat J, Perumal V. Phytochemistry, pharmacological properties, and recent applications of Ficus benghalensis and Ficus religiosa. Plants. 2021;10(12):2749.
Crossref - Salem WM, Sayed WF, Hardy M, Hassan NM. Antibacterial activity of Calotropis procera and Ficus sycomorus extracts on some pathogenic microorganisms. Afr J Biotechnol. 2014;13(32):3271-3280.
Crossref - Jyothisree G, Umadevi S. Phytochemical screening of ficus religiosa root bark. Int J Pharm Sci Invent. 2021;10(8):13-16.
Crossref - Thawabteh A, Juma S, Bader M, et al. The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens.Toxins. 2019;11(11): 656.
Crossref - Ullah A, Munir S, Badshah SL, et al. Important flavonoids and their role as a therapeutic agent. Molecules. 2020;25(22):5243.
Crossref - Hasan M, Ullah I, Zulfiqar H, et al. Biological entities as chemical reactors for synthesis of nanomaterials: Progress, challenges and future perspective. Mater Today Chem. 2018;8:13-28.
Crossref - Lade BD, Shanware AS. Phytonanofabrication: Methodology and factors affecting biosynthesis of nanoparticles. Smart nanosystems for biomedicine, optoelectronics and catalysis. IntechOpen. 2020:6.
Crossref - Sabira O, Drisya N, Ajaykumar AP, et al. From Ficus recemosa Leaf Galls to Therapeutic Silver Nanoparticles: Antibacterial and Anticancer Applications. Pharmaceutics. 2024;16(8):1025.
Crossref - Elkobrosy D, Al-Askar AA, El-Gendi H, et al. Nematocidal and Bactericidal Activities of Green Synthesized Silver Nanoparticles Mediated by Ficus sycomorus Leaf Extract. Life. 2023;13(5):1083.
Crossref - Ramesh AV, Devi DR, Battu G, Basavaiah K. A facile plant mediated synthesis of silver nanoparticles using an aqueous leaf extract of Ficus hispida linn. f. for catalytic, antioxidant and antibacterial applications. S Afr J Chem Eng. 2018;26:25-34.
Crossref - Azarbani F, Shiravand S. Green synthesis of silver nanoparticles by Ferulago macrocarpa flowers extract and their antibacterial, antifungal and toxic effects. Green Chem Lett Rev. 2020;13(1):41-49.
Crossref - Godoy-Gallardo M, Eckhard U, Delgado LM, et al. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact Mater. 2021,6(12):4470-4490.
Crossref - Sneha DK, Shinde SP, Chavan TS, Phatake YB, Mane SG, Dhawan SS. Green synthesis of silver nanoparticles by using stem, leaves and fruits extracts of umber (Ficus racemosa). Int J Pharm Investig. 2020;10(3):312-319.
Crossref - Park J, Joo J, Kwon SG, Jang Y, Hyeon T. Synthesis of monodisperse spherical nanocrystals. Angew Chem Int Ed Eng. 2007;46(25):4630-4660.
Crossref - Stavinskaya O, Laguta I, Fesenko T, Krumova M. Effect of temperature on green synthesis of silver nanoparticles using vitex agnus-castus extract. Chemistry Journal of Moldova. 2019;14(2):117-121.
Crossref - Liu H, Zhang H, Wang J, Wei J. Effect of temperature on the size of biosynthesized silver nanoparticle: Deep insight into microscopic kinetics analysis. Arab J Chem. 2020;13(1):1011-1019.
Crossref - Neelu C. Silver nanoparticles: Synthesis, characterization and applications. Silver Nanoparticles: Fabrication, Characterization and Applications; IntechOpen: London, UK. 2018.
Crossref - Siddiqui T, Zia MK, Muaz M, Ahsan H, Khan FH. Synthesis and characterization of silver nanoparticles (AgNPs) using chemico-physical methods. Indonesian Journal of Chemical Analysis. 2023;6(2):124-132.
Crossref - Rahman AI, Prasanna A. Characterization of silver nanoparticles biosynthesized using Ficus religiosa plant leaf extract. Int Res J Eng Tech. 2018;5(12):272.
- Munoz-Carrillo MG, Garciduenas-Pina C, Valerio-Garcia RC, Carrazco-Rosales JL, Morales-Dominguez JF. Green synthesis of silver nanoparticles from the Opuntia Ficus-indica fruit and its activity against treated wastewater microorganisms. J Nanomater. 2020;2020(1):6908290.
Crossref - Marzuk AFT, Adnan H, Hussein NN, Oda AM, Kamil SA, Salah M. Green Synthesis of Silver Nanoparticle by Ficus Cordata Leaf Extract and Study Its Antibacterial Activity. Orient J Chem. 2019;35(3).
Crossref - Mishra AK, Tiwari KN, Saini R, et al. Green synthesis of silver nanoparticles from leaf extract of Nyctanthes arbor-tristis L. and assessment of its antioxidant, antimicrobial response. J Inorg Organomet Polym Mater. 2020;30:2266-2278.
Crossref - Saheed Y, Umar AF, Iliyasu MY. Potential of silver nano particles synthesized from Ficus sycomorus Linn against multidrug-resistant Shigella species isolated from clinical specimens. Am J Life Sci. 2020;8(4):82-90.
Crossref - Riaz A, Nosheen S, Mughal TA. Fabrication of biogenic silver nanoparticles from Ficus religiosa bark extract and their application for chromium removal. Microsc Res Tech. 2022;85(11):3618-3622.
Crossref - Vega-Baudrit J, Gamboa SM, Rojas ER, Martinez VV. Synthesis and characterization of silver nanoparticles and their application as antibacterial agents. Int J Biosen Bioelectron. 2019;5(5):166-173.
Crossref - Aldebasi YH, Aly SM, Khateef R, Khadri H. Noble silver nanoparticles (AgNPs) synthesis and characterization of fig Ficus carica (fig) leaf extract and its antimicrobial effect against clinical isolates from corneal ulcer. Afr J Biotechnol. 2014;13(45):4275-4281.
Crossref - Wypij M, Jedrzejewski T, Trzcinska-Wencel J, Ostrowski M, Rai M, Golinska P. Green synthesized silver nanoparticles: Antibacterial and anticancer activities, biocompatibility, and analyses of surface-attached proteins. Front Microbiol. 2021;12:632505.
Crossref - Giri AK, Jena B, Biswal B, Pradhan AK, Arakha M, Acharya S, Acharya L. Green synthesis and characterization of silver nanoparticles using Eugenia roxburghii DC. extract and activity against biofilm-producing bacteria. Sci Rep. 2022;12(1):8383.
Crossref - Khalir WKAWM, Shameli, K, Jazayeri SD, Othman NA, Jusoh NWC, Hassan NM. Biosynthesized silver nanoparticles by aqueous stem extract of Entada spiralis and screening of their biomedical activity. Front Chem. 2020;8:620.
Crossref - Tomaszewska E, Soliwoda K, Kadziola K, et al. Detection limits of dls and Uv Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J Nanomater. 2013;2013(1):313081.
Crossref - Caputo F, Arnould A, Bacia M, et al. Measuring particle size distribution by asymmetric flow field flow fractionation: A powerful method for the preclinical characterization of lipid-based nanoparticles. Mol Pharm. 2019;16(2):756-767.
Crossref - Pleus R. Nanotechnologies-guidance on physicochemical characterization of engineered nanoscale materials for toxicologic assessment. ISO: Geneva, Switzerland. 2012. https://www.iso.org/standard/52334.html
- Zhang X, Liu Z, Shen W, Gurunathan S. Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci. 2016;17(9):1534.
Crossref - Ahmad K, Asif HM, Afzal T, et al. Green synthesis and characterization of silver nanoparticles through the Piper cubeba ethanolic extract and their enzyme inhibitory activities. Front Chem, 2023;11:1065986.
Crossref - Al-Otibi F, Perveen K, Al-Saif NA, et al. Biosynthesis of silver nanoparticles using Malva parviflora and their antifungal activity. Saudi J Biol Sci. 2021;28(4):2229-2235.
Crossref - Sati SC, Kour G, Bartwal AS, Sati MD. Biosynthesis of Metal Nanoparticles from Leaves of Ficus palmata and Evaluation of Their Anti-inflammatory and Anti-diabetic Activities. Biochemistry. 2020;59(33):3019-3025.
Crossref - Saxena A, Tripathi RM, Zafar F, Singh P. Green synthesis of silver nanoparticles using aqueous solution of Ficus benghalensis leaf extract and characterization of their antibacterial activity. Materials Letters. 2012;67(1):91-94
Crossref - Uyub AM, Nwachukwu IN, Azlan AA, Fariza SS. In-vitro antibacterial activity and cytotoxicity of selected medicinal plant extracts from penang island malaysia on metronidazole-resistant-helicobacter pylori and some pathogenic bacteria. Ethnobot Res Appl. 2010;8:95-106.
- Shafaei A, Farsi E, Ahamed BMK, et al. Evaluation of toxicological and standardization parameters and phytochemical investigation of Ficus deltoidea leaves. Am J Biochem Mol Biol. 2011;1(3):237-243.
Crossref - Ramakrishna K, Savithramma, N. Antioxidant activity and cytotoxic evaluation of phytofabricated silver nanoparticles of fig (Ficus mollis vahl). Plant Science Today. 2023;10(3):197-202.
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.