ISSN: 0973-7510
E-ISSN: 2581-690X
Food loss and wastage occur in large quantities globally every year and it occurs in the entire supply chain right from the production to the processing stage. The loss of food is due to various factors like adopting traditional cultivation practices, low investment in the food sector, and more loss from poor pests and disease management of agricultural crops. The most important and major cause is due to microbial spoilage; fungi are most harmful to the consumers and also to the agriculture sector. Synthetic chemical strategies can prevent fungal growth and may reduce wastage but still causes accumulation of chemical substances in the environment and food chain in a long run. For these reasons, the use of bio-control technologies can be a great solution to agriculture and food sector as well. In view of this, the present study has been conducted using an efficient Azotobacter species, which belongs to the PGPR group. In this study, antifungal compounds produced by Azotobacter have been extracted by following solvent extraction protocols and identified using GCMS methods. The antifungal compounds were tested against the major fungal pathogens viz., Aspergillus, Fusarium, and Penicillium species. The metabolites produced by Azotobacter species were efficient in controlling the growth of the fungal species. These compounds can be used as a potential bio-preservative in the food sector instead of synthetic chemicals. Thus, these compounds can further be analyzed and tested on the food sample, having a great scope in the future to replace the chemical preservatives.
Food Loss, Bio-protectants, Antifungal, Azotobacter, Secondary Metabolites
Globally, food insecurity and sustainable development to reduce hunger is a major challenge due to the ever-increasing population.1 To achieve food security in a sustainable way, we need an improved food security system associated with economic, climatic, and economic shocks to the food system.2,3 Addressing the under-nutrition by considering the total food production can help in effectively optimizing food systems. The amount of food available for consumption even after exempting loss conversion must meet global needs, thus the nutrient required for the entire population must be considered.4 The quantity of food loss and wastage varies between various countries depending on income, urbanization, and economic growth.5
About 25% of the global post-harvest loss is due to microbial spoilage.6,7,8,1,9 Microbial spoilage of fresh fruits and vegetables has been a problem globally as it causes human health risks. The source of contamination is water, soil, human handling, pests, storage, and transportation equipment.8 Microbial spoilage causes deterioration of the food and thus changes the property of the food right from the appearance, flavor, and odor of the product and thus makes it unfit for human consumption.
Among all the microorganisms, fungi are the most common and major spoilage-causing microbe as they can tolerate harsh climatic conditions and is capable of producing spores.10 Food spoilage occurs mainly due to four main groups of fungal species such as Zygomycetes, Penicillium, Aspergillus, and Fusarium.9 These fungal species produce mycotoxins which are dangerous to human health. To avoid food spoilage and mycotoxin residues in foods, different fungicides are being added to agricultural produce at various levels, which start from production to consumer.11
Persistent Organic Pollutants (POPs) or synthetic chemicals (herbicides, fungicides, and insecticides) used by food sectors mainly cause toxicity, and bioaccumulation in living beings and in the environment as well for a very long time.12 These chemicals are used as they are cheap and more effective in protecting the crop. According to the UNEP (1993), many documents have reported various health issues, which include cancers, hematological morbidity, heart dysfunction, immune system deficiencies, and inborn deformities that can be attributed to the use of toxic chemicals.
A better way to reduce any harmful effect of toxic chemicals on humans and the environment is by using the bio-control method as it can be used at both pre and post-harvest stages.10 Streptomyces natalensis produces Natamycin (E235), which is a commonly used antifungal food bio-preservative in the food industry.10 Similarly, many bacterial species produce different antifungal metabolites, which can be an effective method in preventing fungal contamination and accumulation of mycotoxin residues in food. Among the bacterial species, Azotobacter is one of the most versatile PGPR bacteria having multiple benefits.13,14 Azotobacter species produce phytohormones like auxins, cytokinins, gibberellic acid, indole acetic acid, and substances such as thionin, riboflavin, nicotin, giberalin, etc., that stimulate root development and plant growth, help in protection from phytopathogen, and improves nutrient uptake.15
In addition, Azotobacter produces an antifungal compound that protects the plant from various soil-borne diseases caused by various fungi such as Alterneria, Aspergillus, Fusarium, Curvularia, etc. Azotobacter produces several types of antifungal compounds such as azotobactin, azotochelin, aminochelin, HCN, testin, viscosinamide, zwittermicin A, etc. 2,4-DAPG is one of the efficient antibiotics that work well against various pathogens and possess antifungal, antihelminthic, and antibacterial properties.14,16 Azotobacter has wide potential as an antibiotic, which can be made use in agriculture, and various food industrial applications as a bio-control agent as an alternative to chemical substances.14
Azotobacter cultures
A total of 30 different previously isolated Azotobacter strains were used for the present study. The growth parameters have been checked for these isolates before conducting the experiments. Based on the viability and growth rate, active Azotobacter strains have been selected for further studies.17-19
Sub culturing of Azotobacter strain
Azotobacter strains were sub cultured on Waksman 77 media (Mannitol, Calcium Carbonate, Dipotassium hydrogen phosphate, Magnesium Sulphate, Sodium Chloride, Manganous Sulphate, Ferric Chloride, and Agar).13,16-18 The media was poured onto sterilized Petri plates and the bacteria was streaked onto the plates under sterile conditions. The plates were then kept for 5 days in incubator to observe the growth rate of different strains of Azotobacter species.
Fungal isolates
Aspergillus, Penicillium, and Fusarium cultures were used for the present study. The representative isolates were obtained from the University of Mysore and the identity of the isolates will be reconfirmed based on cultural and morphological characters by comparing standard strains.
Sub culturing of fungal species
The 3 fungal species; Aspergillus flavus, Fusarium verticillium, and Penicillium expansum were sub cultured on PDA Petri plates and was incubated at room temperature for 3 days to observe the complete growth.
Bio efficacy of Azotobacter species against fungal species
Modified Waksman 77 agar medium was designed and prepared to facilitate growth of both bacteria and fungi on same medium by adding the composition of both Waksman broth and potato dextrose agar.17-19 Bio-efficacy of different Azotobacter species against Aspergillus, Penicillium and Fusarium isolates were studied following dual culture method. The inoculated plates were incubated at 28±2°C for 3 to 4 days and after incubation period, the zone of inhibition will be measured from the edge of the bacterial colony up to the edge of fungal mycelia.20 The efficient Azotobacter species were used for the extraction of antifungal antibiotics; purification and characterization were done as per the standard protocols.21
Preparation of solvent mixture
Solvent extraction mixture has been prepared by using equal volumes (1:1:1:1) of diethyl ether, ethyl acetate, hexane, and n-butanol as per the standard protocols.21
Extraction and purification of antifungal compounds
The efficient Azotobacter strains were inoculated in 200mL each of Waksman broth and incubated at 32±2°C for 5 days for the maximum growth of the bacteria. After incubation, the cultured broth was centrifuged at 10,000 rpm for 25 mins. The Cell-Free Supernatant (CFS) was collected and the residue was discarded. In a separating funnel, an equal volume of CFS and solvent mixture has been taken and mixed continuously for 10mins. After 10 mins of mixing, the reaction mixture was kept undisturbed for 30 mins to separate the solvent layer and compound mixture. The solvent eluted out and evaporated to 50% of the total solvent extract.21,22
The extract was concentrated by using a rotary evaporator at 55oC vacuum. The extract with antifungal adherence was carried out for further purification through Thin Layer Chromatography (TLC). The concentrated extract was separated on silica gel sheets by TLC. Solvent system Toluene, ethyl acetate with concentration (7:3) for different Azotobacter species (6:4) was standardized and detected under UV at 254nm. The extract was dried and dissolved in ethyl acetate for further confirmation of antifungal activity. Active fractions of the extract were further scrapped and treated with acetone thrice and were reconfirmed through bio-autography. The active fractions were subjected to GC-MS (Thermo Scientific USA) as per the protocol.23
Detection of metabolites
The analysis of the active fraction was performed by a Shimadzu QP-2010 Gas Chromatograph coupled to the Shimadzu GCMSQP–2010 Mass Spectrometer with a SGEBPX-5 column (30m length, 0.25µm film thickness). Helium was used as a carrier gas at a constant flow rate of 0.8 mL/min. The active fraction was dissolved in methanol and 1.0µL of the sample was injected using AOC5000 auto injector with a split ratio 100:1. The initial temperature was set to 50°C, and then increased at a rate of 3°C/min to 280°C and held isothermally for 5min. For MS detection, the ion source temperature was set to 200°C, and an electron ionization mode with ionizing energy of 70eV and a scan mass range of 100–1200amu was employed. The compounds were identified by comparing their relative retention times and fragmentation patterns of mass spectra with those reported in the literature as well as at the National Institute of Standards and Technology (NIST17.lib) data library.
Viability of the Azotobacter strains
Among 30 Azotobacter isolates, the seven most active Azotobacter strains viz., A. vinelandii RCR-4 (KF470806), A. salinestris GVT-1 (KF470807), A. tropicalis KOP-11 (KF470799), A. chroococcum SND-4 (KF470801), A. armeniacus GVT-11 (KF470809), Azotobacter sp. DVD-7 (KF470804) and A. nigricans YG-7 (JX262165) have been used for bio-efficacy studies. Previously cultural, morphological, biochemical, and molecular studies confirmed the identity of the isolate as Azotobacter species.17-19
Bio efficacy of Azotobacter species against fungal species
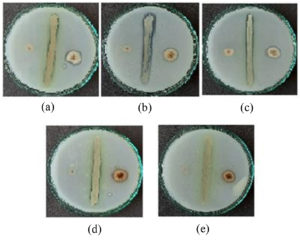
The bio-efficacy assay of Azotobacter species was tested against three different food-borne fungal species using a dual culture method. For the dual culture method, a modified Waksmann and PDA were used to grow both cultures. After the incubation period (28±2°C for 3 to 4 days), the results were recorded by observing the extent of inhibition of fungal mycelia by Azotobacter strains.20 Zone of inhibition was measured from the edge of the bacterial colony up to the edge of fungal mycelia. Among 7 Azotobacter strains, 5 showed satisfactory results against the 3 selected fungal strains after a period of 3 days after inoculation (Figure 1). The efficacy of Azotobacter strains against the three fungal species has been discussed in Table 1; A. salinestris showed maximum inhibition against all three fungal species, followed by A. vinelandii, A. chroococcum, A. tropicalis, and A. nigricans.
Table (1):
Efficacy of different Azotobacter strains against fungal species
Azotobacter species |
Aspergillus flavus |
Fusarium verticillium |
Penicillium expansum |
|---|---|---|---|
A. vinelandii |
+ |
+++ |
++ |
A. chroococcum |
+++ |
++ |
+ |
A. tropicalis |
++ |
++ |
++ |
A. salinestris |
++ |
+++ |
+++ |
A. armeniacus |
– |
++ |
– |
A. species |
– |
++ |
– |
A. nigricans |
++ |
+++ |
+ |
Note: (+) 2-4mm, less inhibition; (++) 6-8mm, moderate inhibition; (+++) 8-12mm, good inhibition
Figure 1. Screening of the selected Azotobacter strain against the three different fungal strains (a) GVT-1; (b) RCR-4; (c) KOP-11; (d) SND-4; (e) GVT-11
Extraction and profiling of antifungal compounds
GC-MS analysis
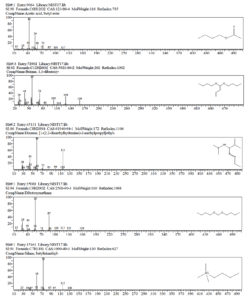
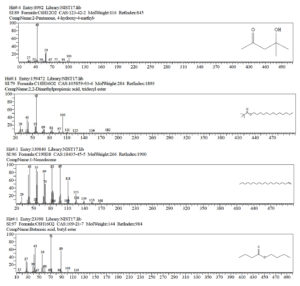
Solvent extraction was carried out to obtain the compound and the purified compound is subjected to GC-MS analyses to identify the antifungal compound produced by Azotobacter species. A total of 39 compounds were identified using this technique (Figure 2; Table 2). Out of the 39 compounds, a few compounds detected through the analyses were part of the extraction method (1-Butanol). There are 9 major and 30 minor compounds that are detected; the important one includes, Acetic acid, butyl ester; Butane, 1,1-dibutoxy-; Butanoic acid, butyl ester; Diazene, [1-(2,2-dimethylhydrazino)-2-methylpropyl] ethyl-; dibutoxymethane; silane, butyltrimethyl-; 1-Nonadecene; 2,2-Dimethylpropionic acid, tridecyl ester and 2-Pentanone, 4-hydroxy-4-methyl (Figure 3). Similarly, Streptomyces sp. Sp1 showed antimicrobial properties against fungal and bacterial isolates. The compounds such as propanoic acid, 2-methyl-, butyl ester, butane, 1,1-dibutoxy-, tetradecane, hexadecane and heneicosane from Streptomyces sp. Sp1 have been detected and identified using GC-MS analyses. Among them, heneicosane and butane, 1,1-dibutoxy- have been identified to possess the highest antimicrobial activity.24 Similarly, A. salinestris GVT-1 isolate showed maximum inhibition of Aspergillus flavus growth after the 4-day incubation.25,26
Table (2):
Compounds identified using GC-MS technique
No. |
Compound |
Retention time |
Peak area % |
|---|---|---|---|
1. |
1-Butanol |
2.020 |
1.01 |
2. |
Formic acid, butyl ester |
2.300 |
0.17 |
3. |
1-Chloro-2-methyl-2-propanol |
2.404 |
0.04 |
4. |
1-Butanol, 2-methyl- |
2.494 |
0.07 |
5. |
Butane, 1-isothiocyanato- |
2.619 |
0.07 |
6. |
Butane, 1,1′-[(1-methylethylidene) bis(oxy)] bis- |
2.947 |
0.44 |
7. |
Acetic acid, butyl ester |
3.510 |
4.92 |
8. |
2-Pentanone, 4-hydroxy-4-methyl- |
4.011 |
4.14 |
9. |
4-Heptanone |
4.682 |
0.10 |
10. |
o-Xylene |
5.138 |
0.50 |
11. |
Silane, butyltrimethyl- |
5.323 |
2.90 |
12. |
Silane, triethyl- |
6.549 |
0.13 |
13. |
Propanoic acid, 2-methyl-, butyl ester |
6.700 |
0.77 |
14. |
Butanoic acid, 2-methylpropyl ester |
6.779 |
0.82 |
15. |
Butanoic acid, butyl ester |
7.895 |
9.78 |
16. |
1-Hexanol, 2-ethyl- |
8.836 |
0.46 |
17. |
Dibutoxymethane |
9.697 |
1.38 |
18. |
Butane, 1,1′-[(1 methylethylidene) bis(oxy)] bis- |
10.123 |
0.11 |
19. |
Butane, 1,1′-[ethylidenebis(oxy)]bis- |
10.715 |
0.36 |
20. |
Diazene, [1-(2,2-dimethylhydrazino)-2-methylpropyl]ethyl- |
11.507 |
55.79 |
21. |
Di-tert-Butyl ether |
12.955 |
0.34 |
22. |
Naphthalene |
13.290 |
0.13 |
23. |
1-Dodecanol |
13.599 |
0.11 |
24. |
1,1-Diisobutoxy-isobutane |
14.070 |
0.32 |
25. |
Butane, 1,1-dibutoxy- |
15.324 |
7.96 |
26. |
Propanoic acid, 2,2-dimethyl-, propyl ester |
16.897 |
0.13 |
27. |
1-Tetradecene |
19.086 |
0.43 |
28. |
Tetradecane |
19.305 |
0.13 |
29. |
2,4-Di-tert-butylphenol |
21.918 |
0.13 |
30. |
2,2-Dimethylpropionic acid, tridecyl ester |
22.200 |
1.42 |
31. |
1-Heptadecene |
24.408 |
0.98 |
32. |
Hexadecane |
24.671 |
0.20 |
33. |
1-Nonadecene |
30.636 |
1.30 |
34. |
Octadecane |
30.778 |
0.19 |
35. |
9-Heptadecanone |
31.976 |
0.38 |
36. |
Nonadecene |
33.669 |
0.95 |
37. |
Heneicosane |
33.749 |
0.10 |
38. |
1-Heptacosanol |
35.857 |
0.59 |
39. |
Octacosanol |
37.749 |
0.24 |
In order to satisfy the consumer demand for less processed and preservative-free foods, bio-preservation has received growing interest for improving food quality and safety.26 Biological control of Fusarium spp. using sustainable, safe, and eco-friendly antagonistic bacteria is gaining importance in recent years.27 Some of the microorganisms that possess antifungal activity against food pathogens are; Lactic acid bacteria, Propionibacterium, Bacillus, and Azotobacter.28,29,12,13 Azotobacter is a prokaryote that fixes atmospheric nitrogen into ammonia, which can be easily assimilated in plants. Azotobacter is a gram-negative, catalase and oxidase positive, non-spore forming bacteria.12,13 Many Azotobacter strains have been isolated such as Azotobacter vinelandii, A. beijerinckii, A. insignis, A. macrocytogenes, A. paspali, A. salinestris, A. armeniacus, A. brasilense, A. tropicalis, and A. nigricans.30-32 Among them, A. chroococcum and A. vinelandii are found almost in all the rhizosphere soils.18,31 In the present study, different Azotobacter strains have been tested against fungal species, in order to confirm their bio control efficacy. The antifungal compounds responsible for the control of fungal contamination is then extracted and identified using GC – MS technique. Butanoic acid, butyl ester; 1- Nanodecene; diazene, [1-(2,2-dimethylhydrazino)-2-methylpropyl]ethyl-; hexadecane; dibutoxymethane are some of the compounds produced by the Azotobacter strains used in this study. The compounds (hexadecane and octadecane) showed antifungal properties by inhibiting the mycelial growth in Verticillium dahlia, which causes vascular wilt disease in strawberries.33 Similarly, in the present study, tetradecene and nonadecene extracted from A. vinelandii were effective in inhibiting the growth of Fusarium verticillium which is a common source of contamination in cereals.31
Another study reported that, naphthalene, 1-Tetradecene, 2,4-Di-tert-butylphenol, hexadecane, octadecane, and 1- Nonadecene were found among the 37 compounds which could inhibit spore germination of Penicillium chrysogenum, Aspergillus niger, and Alternaria alternata.33,34 In the current study Octadecene, Hexadecane, and Naphthalene have also been produced by A. chroococcum and A. tropicalis. These compounds have been effective in controlling the growth of Aspergillus flavus in peanuts and Penicillium expansum commonly found in apples. In another study, 2,4-Di-tert-butylphenol compound isolated from Pseudomonas monteilii PsF84 from the tannery waste exhibited antifungal activity against F. oxysporum, which is a wilt causing soil-borne fungus.35 Similarly it was documented that, B. amyloliquefaciens produced formic acid, butyl ester, and acetic acid, butyl ester was produced by B. thuringiensis and identified using GC-MS analysis.36 Formic acid, butyl ester, and acetic acid have also been reported to be produced by A. vinelandii in the current study. Diacetyl and benzaldehyde produced by B. velezensis were effective in controlling the growth of B. cinerea infection in grapes.37,38 Another study also showed that 3 methyl 1 butanol and 2- phenylethyl methyl ether produced by R. aquatilis were effective in controlling C. gloeosporioides which causes infection in the leaves and fruits of many plants.39,40 Therefore, nonadecene, octadecene, hexadecane, tetradecene, acetic acid, formic acid, and butyl ester are some of the compounds among the 39 antifungal compounds that have been identified in the current study, and which have also been proven to possess antifungal effects from previous studies.
The results of this study show that Azotobacter possesses certain antifungal compounds which have been extracted and purified. From the dual culture method, Azotobacter strains were found to be effective in controlling the growth of Aspergillus, Penicillium, and Fusarium species. Azotobacter strains were able to produce different antimicrobial compounds and these metabolites can be used in the food industry as an alternative to synthetic chemicals. The compounds that are produced by Azotobacter compounds that have minimum antifungal properties should be studied extensively. Proper research should be done on these compounds to recognize the bio-efficacy of each compound and accordingly utilize them in the food industries as a better alternative to synthesized chemicals. The metabolites produced by Azotobacter will be a novel strategy for the agriculture sector.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Food Technology, Ramaiah University of Applied Sciences, Bangalore, India, for their immense co-operation and support in completing the project successfully.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SML and CG conceptualized the study. AMB performed profiling and characterization of the compounds. SML protocol development for solvent extraction. VP conducted the research experiments. CG and KNH wrote the manuscript. AT, ADB and CG reviewed the manuscript. AT, ADB and KNH edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This study was carried out under RUAS seed grant (Ref.No.RUAS/DSR/Seed Money/2022/1006).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Odeyemi OA, Alegbeleye OO, Strateva M, Stratev D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr Rev Food Sci Food Saf. 2020;19(2):311-331.
Crossref - Alexander P, Brown C, Arneth A, Finnigan J, Moran D, Rounsevell MDA. Losses, inefficiencies and waste in the global food system. Agric Syst. 2017;153:190-200.
Crossref - Mehrabi Z, Ramankutty N. Synchronized failure of global crop production. Nat Ecol Evol. 2019;3(5):780-786.
Crossref - Wood SA, Smith MR, Fanzo J, Remans R, DeFries RS. Trade and the equitability of global food nutrient distribution. Nat Sustain. 2018;1(1):34-37.
Crosssref - Melissa Mary George, Nisha K, Lekhana SM and Chenaappa Gurikar. Patulin: A potentially harmful food contaminant. International Journal of Chemical Studies. 2022; 10(3): 11-18
- Garg N, Abdel-Aziz SM, Aeron A, eds. Microbes in Food and Health. Springer International Publishing; 2016:1-362.
Crossref - Gram L, Ravn L, Rasch M, Bruhn JB, Christensen AB, Givskov M. Food spoilage-interactions between food spoilage bacteria. Int J Food Microbiol. 2002;78(1-2):79-97.
Crossref - Kaczmarek M, Avery SV, Singleton I. Microbes associated with fresh produce: Sources, types and methods to reduce spoilage and contamination. Adv Appl Microbiol. 2019;107:29-82.
Crossref - Petruzzi L, Corbo MR, Sinigaglia M, Bevilacqua A. Microbial spoilage of foods. The Microbiological Quality of Food. Elsevier. 2017:1-21.
Crossref - Leyva Salas M, Mounier J, Valence F, Coton M, Thierry A, Coton E. Antifungal microbial agents for food biopreservation-A review. Microorganisms. 2017;5(3):37.
Crossref - Chennappa G, Shivaprasad DP, LuisSabillonc B, NanjeGowda NA, Kaliramesh S. Impact of mycotoxins and their metabolites associated with food grains. Grain & Oil Science and Technology. 2022;6(1):1-9
Crossref - De Souza RM, Seibert D, Quesada HB, de Jesus Bassetti F, Fagundes-Klen MR, Bergamasco R. Occurrence, impacts and general aspects of pesticides in surface water: A review. Process Saf Environ Prot. 2020;135:22-37.
Crossref - Chennappa G, Naik MK, Amaresh YS, Nagaraja H, Sreenivasa MY. Azotobacter: A Potential Biofertilizer and Bioinoculants for Sustainable Agriculture. In: Microorganisms for Sustainability, 2017;6.
- Patil SV, Mohite BV, Patil CD, Koli SH, Borase HP, Patil VS. Azotobacter. Beneficial Microbes in Agro-Ecology. Elsevier. 2020:397-426.
Crossref - Ojha RB, Jnawali AD, Marahatta S. Role of Azotobacter in Soil Fertility and Sustainability. Adv Plant Agric Res. 2015;2(6):250-253.
- Chennappa G, Sreenivasa MY, Nagaraja H. Azotobacter salinestris: A Novel Pesticide-Degrading and Prominent Biocontrol PGPR Bacteria. Microorganisms for Sustainability. Springer. 2018:23-43.
Crossref - Chennappa G, Naik MK, Adkar-Purushothama CR, Amaresh YS, Sreenivasa MY. PGP, abiotic stress tolerant and antifungal activity of Azotobacter sp. isolated from paddy soils. Indian Journal of Experimental Biology. 2016; 322-331
- Chennappa G, Adkar-Purushothama CR, Suraj U, Tamilvendan K, Sreenivasa MY. Pesticide tolerant Azotobacter isolates from paddy growing areas of northern Karnataka, India. World J Microbiol Biotechnol. 2014;30(1):1-7.
Crossref - Chennappa G, Adkar-Purushothama CR, Naik M, Suraj U, Sreenivasa MY. Impact of Pesticides on PGPR Activity of Azotobacter sp. Isolated from Pesticide Flooded Paddy Soils. Greener J Agric Sci. 2014;4(4):117-129
Crossref - Nagaraja H, Chennappa G, Rakesh S, Naik MK, Amaresh YS, Sreenivasa MY. Antifungal activity of Azotobacter nigricans against trichothecene-producing Fusarium species associated with cereals. Food Sci Biotechnol. 2016;25(4):1197-1204.
Crossref - Bonartsev AP, Bonartseva GA, Myshkina VL, et al. Biosynthesis of poly (3-hydroxybutyrateco-3-hydroxy-4-methylvalerate) by Strain Azotobacter chroococcum 7B. AceaNaturae. 2016; 8(3):77-87.
Crossref - Bhosale HJ, Kadam TA, Bobade AR. Identification and production of Azotobacter vinelandii and its antifungal activity against Fusarium oxysporum. J Environ Biol. 2013;34(2):177-182.
- Sangmanee P, Hongpattarakere T. Inhibitory of multiple antifungal components produced by Lactobacillus plantarum K35 on growth, aflatoxin production and ultrastructure alterations of Aspergillus flavus and Aspergillus parasiticus. Food Control. 2014;40:224-233.
Crossref - Kawuri R, Darmayasa IBG. Bioactive Compound from Extract Filtrat Streptomyces sp.Sp1. as Biocontrol of Vibriosis on Larvae of Macrobrachium rosenbergii shrimps. Hayati. 2019;26(1):15.
Crossref - Oliveira PM, Zannini E, Arendt EK. Cereal fungal infection, mycotoxins, and lactic acid bacteria mediated bioprotection: from crop farming to cereal products. Food Microbiol. 2014;37:78-95.
Crossref - Nagaraja H, Chennappa G, Deepa N, et al. Antifungal potential of Azotobacter salinestris Strain Azt 31 against phytopathogenic Fusarium spp. associated with cereals. J Fungi. 2022;8(5):473-493.
Crossref - Mousa EM, Mahdy ME, Galal NM. Role of mycorrhizae and some biocontrol agents to control root-knot nematodes on tomato. Menoufia Journal of Plant Protection. 2021;6(4):11-12.
Crossref - Stackebrandt E, Cummins CS, Johnson JL. Family Propionibacteriaceae: The Genus Propionibacterium. The Prokaryotes. Springer. 2006:400-418.
Crossref - Falardeau J, Wise C, Novitsky L, Avis TJ. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J Chem Ecol. 2013;39(7):869-878.
Crossref - Page WJ, Shivprasad S. Azotobacter salinestris sp. nov., a Sodium-Dependent, Microaerophilic, and Aeroadaptive Nitrogen-Fixing Bacterium. Int J Syst Bacteriol. 1991;41(3):369-376.
Crossref - Aquilanti L, Favilli F, Clementi F. Comparison of different strategies for isolation and preliminary identification of Azotobacter from soil samples. Soil Biol Biochem. 2004;36(9):1475-1483.
Crossref - Gurikar C, Sreenivasa MY, Nanje Gowda NA, Lokesh AC. Azotobacter – A potential symbiotic rhizosphere engineer. Rhizosphere Engineering. Elsevier. 2022;1:97-112.
Crossref - Li X, Wang X, Shi X, et al. Antifungal Effect of Volatile Organic Compounds from Bacillus velezensis CT32 against Verticillium dahliae and Fusarium oxysporum. Processes (Basel). 2020;8(12):1674.
Crossref - Talie MD, Wani AH, Malik WS, Bhat MY. A new species of Rhizopogon from Kashmir valley, India. Kavaka Trans Mycol Soc India. 2020;55:128-133.
Crossref - Dharni S, Sanchita, Maurya A, et al. Purification, characterization, and in vitro activity of 2,4-Di-tert-butylphenol from Pseudomonas monteilii PsF84: conformational and molecular docking studies. J Agric Food Chem. 2014;62(26):6138-6146.
Crossref - Ajilogba CF, Babalola OO. GC-MS analysis of volatile organic compounds from Bambara groundnut rhizobacteria and their antibacterial properties. World J Microbiol Biotechnol. 2019;35(6):83.
Crossref - Calvo H, Mendiara I, Arias E, Gracia AP, Blanco D, Venturini ME. Antifungal activity of the volatile organic compounds produced by Bacillus velezensis strains against postharvest fungal pathogens. Postharvest Biol Technol. 2020;166(111208):111208.
Crossref - Chennappa Gurikar, Nanje Gowda N.A, David H.E and Nethravati, B.P. Role of Bacillus species in soil fertility with reference to rhizosphere engineering, Rhizosphere Engineering. 2022; 1:65-76.
Crossref - Kong WL, Rui L, Ni H, Wu XQ. Antifungal Effects of Volatile Organic Compounds Produced by Rahnella aquatilis JZ-GX1 Against Colletotrichum gloeosporioides in Liriodendron chinense × tulipifera. Front Microbiol. 2020;11:1114.
Crossref - Deepa N, Chennappa G, Deepthi BV, Naik MK, Amaresh YS, Sreenivasa MY. Antifungal potential of Azotobacter species and its metabolites against Fusarium verticillioides and biodegradation of fumonisin. Journal of Applied Microbiology. 2022; 1-15.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.