ISSN: 0973-7510
E-ISSN: 2581-690X
The objective of this research is to evaluate alternative source for collagen. The common source of collagen is from bovine and pig. There is need to find an alternative source of collagen due to its demand. Broiler Chicken feet waste is one of the alternative sources because of its abundant collagen protein content. On an average each chicken shop generates minimum of 30-40 kg waste every day in Hyderabad. Collagen is a fibrillar protein composing different forms of the connective tissue: bone, cartilage, tendon and skin. Collagen is the major component of the extracellular matrix, and more than 27 genetic isoforms have been identified. Collagens type I, II and III are the most abundant and well investigated for biomedical applications. Chicken feet, rather than being transformed into meal for animal feed, a large quantity of chicken feet could be used to produce collagen, which is valued for its unique functional properties. The purpose of this research project was to extract and characterize collagen from chicken feet. Biochemical composition of chicken feet collagen such as moisture, protein, fat and ash content was 5.85, 29.11, 35.43 and 28.60%, respectively. Acid- solubilized collagen (ASC) was isolated from the chicken feet. Molecular weight of ASC was analysed and confirmed by SDS- PAGE. Secondary structure of ASC was confirmed by FT-IR spectrum. Chicken feet thus appear to be a good alternative source of high-quality collagen.
Collagen, Chicken feet, SDS –PAGE, FTIR. Acid Solubilized Collagen.
India is the fourth largest producer of poultry meat in the world, producing approximately 3.8 million tonne of poultry meat a year. The market is estimated to be worth about Rs 90,000 crore. (Index Mundi, 2015). Per capita chicken consumption in India has been on the rise, with eating habits changing predominantly in the metro cities. Poultry meat production increased from 0.069 million tons in 1961 to 3.725 million tons in 2014. Poultry production accounts for about 0.66 percent of India’s GDP and 7.72 percent GDP from the livestock sector (Prabakaran, 2014; Rajendran et al., 2014).Organic solid waste by-products such as bones, skins, feathers and feet’s are generated from Broiler chicken vendors. The most Common methods for disposing these wastes are burning, incineration, compositing as fertilizer and animal feed. The disposal of these presents significant environmental, biological, and financial problems for the poultry industry. It contains useful proteins such as keratin and collagen and thus broiler chicken feet were utilized as resources for collagen production. Chicken feet are an integral part in Trinidadian, South African, Peruvian Philippines and Jamaican cuisine. Chicken feet are used as popular salad and appetizer too and mostly sold as street food by deep frying and seasoning with vinegar, fresh green pepper, mustard and crushed garlic. Therefore, the objective of this study was to isolate and characterize collagen from chicken feet using different acids. The characterization of collagen was carried out in terms of relative molecular weight distribution, amino acid composition, FTIR spectra and thermal characteristics.
Collagen is a fibrous structural protein present in the extracellular matrix and connective tissue of animals (Ramshaw et al., 2009). Collagen is formed mostly by the fibroblast of connective tissue and also by variety of other epithelial cells (Kadler et al., 2007). Collagen molecules are 280 nm long, with molar mass of 360,000 Da; they are stabilized by hydrogen bonds and intermolecular bonds (Silva and Penna, 2012), which are composed of three helical polypeptide chains, each with about 1000 amino acids, which are called an ± chain. They are arranged in the form of a triple helix with two identical chains (±1) and the third which differs to some extent in its chemical composition (±2). The triple helix molecules have terminal globular domains and are called procollagen. These globular regions are cleaved in varying degrees to give a polymerized structure (tropocollagen), which is the basic unit of collagen. The tropocollagen molecules are stabilized by hydrophobic and electrostatic interactions (Damoradan et al., 2010). Microscopically collagen is found as elongated fibrils (Szpak & Paul, 2011). Nearly 28 types of collagen have been identified so far which is composed of 46 distinct polypeptide chains according to their structure. All of them have a characteristic triple helix but the length of the helix and the size and nature of non-helical portion varies from one to another type (Miller, 1984). Among these the five most common ones are: Collagen I, II, III, IV, V. The Type I collagen is present primarily in connective tissue, in tissues such as skin, tendons and bones. It consists of three polypeptide chains, two of which are identical, which are called chain ±1 (I) and ±2 (I), and which are composed of different amino acids. Type II collagen occurs in cartilage tissue and it is believed that the ±1 (II) subunit is similar to the ±1 (I) subunit. Type III collagen is strongly dependent on age: very young skin can contain up to 50%, but with the passage of time that percentage can be reduced to 5-10%. Other types of collagen are only present in very small quantities, mainly in specific organs such as the basement membranes, cornea, heart muscle, lungs and intestinal mucosa (Schrieber and Gareis, 2007; Karim and Bhat, 2009).
Collagen provides protection to skin by inhibiting the absorption of toxins and pathogens (Fratzl, 2008). It has role in biological functions of a cell (cell survival, proliferation and differentiation), helps in healing of damaged bones or blood vessels and maintains structural integrity (Buehler, 2006).
The main sources for collagen extraction are byproducts from the slaughter of pork and beef (Silva and Penna, 2012), used in cosmetics, health supplement and other non-bio medical industries(Higgins, 2010). Collagen from Porcine -pig rind is one source of collagen for processing products like sausage casings and films. In the medical world too, porcine collagen sheet material proves to be very useful as an implant for reconstructive surgery. Collagen from Fish is similar to mammal and avian type I collagen, fish type I collagen contains three polypeptide chains, each consisting of about 1,000 amino acid residues while weighing approximately 100kDa (Saito et al., 2001). Thus fish processing wastes could be promising cost effective collagen sources through recycling of those wastes. The isolated collagen may well find application in biomedical and pharmaceutical fields as a potential material for construction of tissue engineering scaffold, wound dressing system and drug delivery device (Pati et al., 2010).
Collagen is used in pharmaceutical industries as microparticles, injectable dispersions, shields in ophthalmology sponges, drug delivery system. Its application in the pharmaceutical as well as biomedical field is due to its characteristics such as weak antigenicity, cell attachment ability, biodegradability and biocompatibility (Leitinger and Hohenester, 2007).Collagen type I is considered to be the gold standard for this field due to its high biocompatibility. It is used as the basic matrix for cell culture system. Biomaterials based on collagen are widely used in tissue engineering such as injectable matrices, scaffolds intended for bone regeneration etc. These biomaterials are produced mainly from fibril- forming collagen which includes type I, II, III, V, XI (Oliveira et al., 2009; Parenteau-Bareil et al., 2010).
Collagen based dressing in the form of sponge for wounds or burns in the form of collagen films and powders, surgical suture. In urogenital disorders, corneal defects, study of neural migration, dental purpose, bone grafting, arthritis and obesity (Sanz-Herrera et al., 2011). Collagen has various applications in the departments such as Cardiology (heart valve) Dermatology (for skin replacement, augmentation of soft tissue, skin tissue engineering, artificial skin dermis) Surgery (as hemostatic agent, wound repair and dressing, nerve repair, blood vessel prostheses) Orthopaedic (tendon, bone and ligament repair, cartilage reconstruction) Ophthalmology (corneal grafts, contact lenses) Urology (dialysis membrane hemodialysis, sphincter repair) Vascular (vascular graft, vessel replacement) (Rose & Oreffo, 2002).
Studies on the extraction of collagen from poultry slaughter waste of emu skin (Dromaius novaehollandiae) (Nagai et al., 2015), and chicken feet (Hashim et al., 2014), chicken sternal cartilage (Cao and Xu,2008), chicken skin (Munasinghe et al., 2015) and chicken tarsus (Almeida et al.,2012) etc. has been researched.
Extraction of Collagen Process
Collagen can be obtained by chemical hydrolysis and enzymatic hydrolysis (Zavareze et al., 2009). All the preparative procedures were performed at 20°C. The methods for extraction of collagen from broiler chicken feet waste consisted of the following steps: sample collection & preparation, removing non collagenous tissue, solubilizing collagen, centrifuging and precipitating collagen, concentration measurement and characterizations of collagen.
Sample collection and preparation
Broiler Chicken feet was used as the raw material. This is thrown as a waste in every butcher shops in the local market of Hyderabad Telangana state. It is collected and brought to the laboratory in ice bag. Chicken feet was cleaned under running tap water to remove blood strains and dirt using sterilized double distilled water at least three times. Then it was cut into small pieces (0.5x 0.5 cm) using sterile knife and kept on ice prior to collagen extraction.
Pre-treatment:- Removing non collagenous tissue
In order to remove non collagenous substance and to make the tissue very loose, the chicken feet waste was treated with 0.1 N of NaOH for 24 hours. Then, the solution was placed for gentle stirred. The solution was changed every 8 hours, and then washed with distilled water. (Kaewdang et al 2014).
Extraction of collagen procedure
Acid solubilized collagen (ASC)
Acid hydrolysis is performed either with organic acids such as acetic acid, citric acid and lactic acid, or inorganic acids such as hydrochloric acid (HCL). However, organic acids are more efficient than inorganic acids (Wang et al., 2008). Organic acids are capable of solubilizing non-crosslinked collagens and also of breaking some of the inter-strand cross-links in collagen, which leads to a higher solubility of collagen during the extraction process (Liu et al., 2015). Therefore, acidic solutions, especially acetic acid, are commonly used to extract collagen. To extract acid-solubilized collagen, the pretreated collagen sample was soaked in 0.5 M acetic acid with a sample per solution ratio of 1: 20 (W/V) for 48 h at 25°C. This acetic acid suspension was filtered by using cheese cloth to remove the bone residue. In order to precipitate the acid solubilized collagen, it was salted out by adding NaCl to 0.9 M and centrifuged at 30,000 × g for 60 m. The pellet was collected and then dissolved in 0.5 M acetic acid, then dialyzed against 0.1M acetic acid and deionized water in a dialysis membrane and air dried. The dialysate was referred acid- solubilised collagen (ASC). (Nagai et al 2015)
Biochemical Analysis
Moisture content
Three grams of sample was weighted(W1) and placed to the dish and spread uniformly. The sample was placed in an oven for 3h at 105ºC. Then transferred the sample to the desiccator to cool and reweighed the sample (W2). (AOAC 2000). The formula for calculating the moisture % is (W1 – W2) / W1 x 100
Fats content
Five grams of sample was wrapped in a filter paper and placed in the thimble and transfer into soxhlet apparatus. petroleum ether was used as a solvent and heat rate of 150 drop/min was maintained for 14 hr. Solvent recovery was done by Rotary evaporator. Then it was placed in oven at 80 -90ºC until the solvent is completely evaporated. The fat content in the sample was determined by the ratio of weight of fat to the weight of sample multiplied by 100. (AOAC 2000)
Crude protein
The crude protein content in chicken feet was determined according to micro Kjedahl method mentioned in (AOAC, 2000)
Ash content
Weighed about 5 gm of sample into the crucible and placed in furnace at 550ºC overnight. The ash content in the sample was determined by the ratio of weight of ash to the weight of sample multiplied by 100. (AOAC 2000)
Yield of Collagen
The yield of the collagen isolated was reported on dry weight basis according to the method of (B. Jamilah K., 2011) as stated:% Yield (dry weight) of collagen = [Dry weight of collagen/ (Wet weight of sample – moisture content of sample)x 100
pH and Swelling percentage
The pH of crude collagen was measured using pH meter at the end different soaking time intervals 12 hr,24 hr,36 hr,48hr (Ockerman 1984).The pH of these solutions were neutralized to 7 using 0.1 N NaOH/0.1 N HCL. Then the solution was centrifuged for 15 minutes by using a high speed centrifuge. The supernatant was discarded and the precipitate was air dried to obtain a dry collagen (Liu et al., 2001). The swelling percentage of dry collagen was calculated by following the formula: Swelling Percentage = [weight of total solid after soaking/weight of bone before soaking] x 100%
Characterization of collagen
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Preparation of sample
One gram of sample was dissolved in 10ml of 5% (w/v) SDS solution and then heated at 75º C for one hour. The Supernatant was collected and mixed with buffer containing 0.5 M tris-HCl, pH 6.8 containing 4% (w/v) SDS, 20% (v/v) glycerol, and 10% (v/v) ²ME) at the ratio of 1:1 (v/v) and boiled for three min.
Samples were analyzed for determination of Molecular weight by SDS PAGE following the procedure of (U.K. Laemmli 1970) with an electrophoresis unit. The samples were analyzed using 12% separating gel and 5% stacking gel. Both the Gels contained 30% acrylamide –bisacrylamide. Ten micrograms of extracted sample was loaded into the wells. Electrophoresis was done at a constant voltage of 100 Volts and 10 mA, until the collagen subunits had passed the resolving gel. The gel was stained with 0.1% (w/v) Coomassie blue R-250 in 15% (v/v) methanol and 5% (v/v) acetic acid for 30 min and destained with 10% (v/v) acetic acid and 35% (v/v) methanol. Rabbit collagen was used as reference ladder for determining the molecular weight of the two chains ±1and ±2.
Fourier Transforms Infrared Spectroscopy (FTIR)
Infrared (IR) spectroscopy is an analytical technique that detects the vibration characteristics of chemical functional groups in a sample. A chemical functional group tends to absorb IR radiation in a specific wave number (cm-1) range, as bonds and groups of bonds tend to vibrate at characteristic frequencies. The term Fourier Transform-IR (FT-IR) refers to the fact that a Fourier transform algorithm is required to turn the raw data into a spectrum. The absorption of a beam of IR light passing through a sample is examined at all wave numbers at once, and peaks at specific wave number ranges are revealed.
The structural conformation of extracted chicken feet collagen, the functional group possessed by the collagen has been investigated by this Fourier transform infrared (FTIR) spectra using the Bio-rad FTIR (model FT – 155) spectrophotometer. The FTIR spectra were obtained from discs that contained 1 mg of collagen powder in approximately 10 mg potassium bromide (KBr). To form a disc, all required equipment was cleaned with acetone. A mixture of a sample and KBr was then ground and well blended, then placed in a palletizer to form a miniature thin disc. The disc was then inserted in the infrared spectrophotometer. Spectra from 4000 to 500 cm -1 were obtained at a data acquisition rate of 2 cm-1 per point
Biochemical Analysis
Table 1 summaries the results of various biochemical parameters like Moisture content, fat, content, protein content, Ash content, yield of collagen. pH & swelling percentages of chicken feet collagen. Experimental results reveal that acetic acid is an ideal solvent and efficient for extracting collagen. Statistical results showed that it is significant (P < 0.06). Yield of collagen using Acetic acid on dry weight basis was 8.24 ± 0.87%. Our result was higher than collagen yield from silky fowl feet (7.31 ± 0.20%, dry basis) (F.Y. Cheng, 2009). This suggested that acid solubilization can be a good alternative method for isolation of chicken feet collagen. Table 2 summarizes the effect of soaking chicken feet in acetic acid in different intervals of time on pH, swelling percentage. Swelling of collagen in acetic acid-extracted material is inversely proportional to the concentration of acid (Liu et al., 2001). Recent studies on extracting chicken feet collagen reported that a higher swelling capacity seems to contribute to a higher collagen extraction yield (Cheng et al., 2009).
Table (1):
Biochemical Analysis of Chicken feet.
Test |
Composition (%) |
|---|---|
Moisture |
58.02 ± 0.40 |
Fat |
3.90 ± 1.10 |
Protein |
18.10 ± 0.20 |
Ash |
7.94 ± 1.62 |
Yield of Collagen |
8.24 ± 0.87 |
Table (2):
The effect of soaking chicken feet in acetic acid in different intervals of time on pH, swelling percentage.
Items |
12 hr |
24 hr |
36 hr |
48 hr |
|---|---|---|---|---|
pH |
3.41 |
3.49 |
3.64 |
3.58 |
Swelling (%) |
212.73 |
238.40 |
227.60 |
232.50 |
Characterization of collagen
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Two distinct ± chains corresponding to ±1and ±2 were detected. These bands were clearly seen indicating presence of type I collagen. The extracted chicken feet collagen was type I collagen that consisted of two distinct ± chains (±1: 150kDa and ±2 : 125 kDa) and a single cross-linked ² chain at 245 kDa (Skierka and Sadowska, 2007; Woo et al., 2008; Cheng et al., 2009).
Fourier Transforms Infrared Spectroscopy(FTIR)
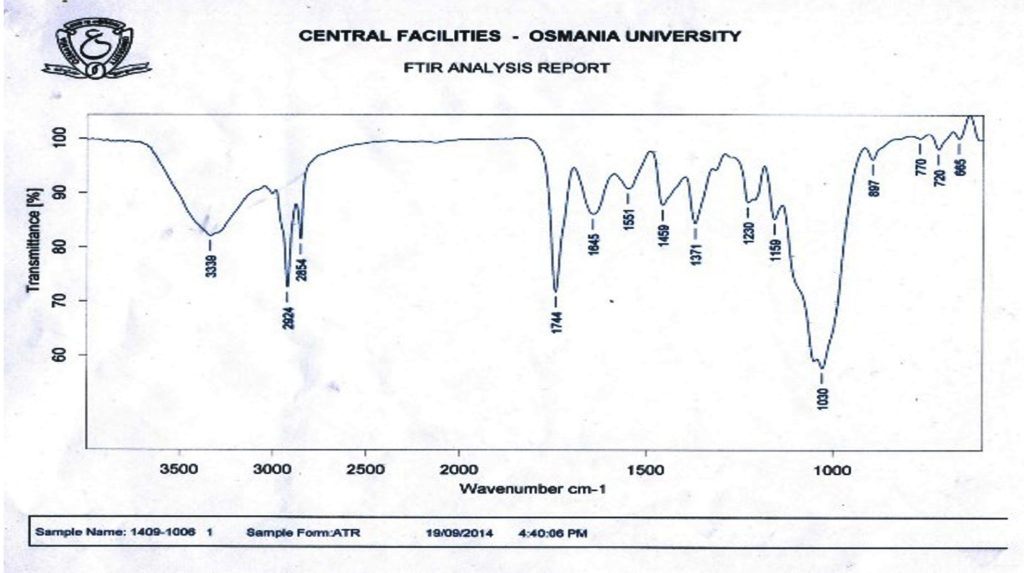
Fourier transform infrared (FTIR) spectroscopy has been used to study changes in the secondary structure of collagen. FTIR spectra of chicken feet collagen exhibited the characteristic peaks of absorption peaks as shown in Fig. 1.0.
The absorption peak of amide chicken collagen isolated using acid was found at 3339 cm.-1 When N-H group is involved with H-bond in peptide chain, the position starts to shift to lower frequencies. Amide peaks of chicken collagens were found at 2924 cm,-1 representing asymmetrical stretch of CH2. The absorption peaks of chicken feet collagens were found at 1744 cm.-1 This observation confirmed that the formation of hydrogen bond between N-H stretch (X position) and C- or O- (Gly) of the fourth residue is responsible for introducing triple helix. The higher hydrogen bonding in triple helical structure would result in higher structure order of collagen. Differences of band absorptions were possibly attributed to the different molecular structure between collagens. These results implied the collagen still preserved native conformation during purification processes. The peak (1230–1371cm-1) is associated with N-H deformation and C-N stretching vibrations. This considerably corresponded to pyrrolidine ring vibration of proline and hydroxyproline as described by (Muyonga et al. 2004). From the FT-IR results, absorption of ASC shows the spectral data which are indicative of collagen secondary structure.
In this study collagen type 1 was extracted from chicken feet waste and high yield was observed in ASC. From the FTIR spectrum analysis the secondary structure of collagen was predicted. Molecular weight characterization of collagen from broiler chicken waste was determined by SDS-PAGE analysis. The sharp bands showed up two distinct ± chains corresponding to ±1 and ±2, then small amounts of ²– and ³– chains were detected.
Chicken skin, a byproduct derived from chicken meat processing, is highly underutilized, constituting huge cost for waste disposal and danger to the environment (Feddern et al., 2010 ). Chicken feet are mainly used to produce animal feed and low-end meat products. However, an area of research that is yet to be explored is the development of chicken feet based products with functional and health promoting values. Extraction and characterization of collagen from chicken feet could effectively increase its economic value-added and hence increase its comprehensive utilization.
- A.O.A.C., “Official Methods of Analysis”, Association of OfficialAnalytical Chemist. Arlington, VA., USA, 1999.
- Almeida, P.F., Vanalle, R.M. and Santana, J.C.C. Produção de Gelatina: Uma perspectiva competitiva para a cadeia produtiva de frango de corte. Produto and Produção, 2012; 13(2): 22-39
- B. Jamilah, K. W. Tan, M. R. Umi Hartina, and A. Azizah, “Gelatins from Three Cultured Freshwater Fish Skins Obtained by Liming Process”, Food Hydrocolloids, 25, pp. 1256–1260, 2011.
- Buehler, MJ. Nature designs tough collagen: Explaining the nanostructure of collagen fibrils. PNAS. 2006; 103(33): 12285-12290.
- Cao, H. and Xu, S.Y. Purification and characterization of type II collagen from chicken sternal cartilage. Food Chemistry, 2008; 108(2): 439-445.
- Damodaran S., Parkin, K. and Fennema, O.R. Química de alimentos de fennema. p. 726-730. 4ª ed. São Paulo: Artmed 2010.
- F. Y. Cheng, F. W. Hsu, H. S. Chang, L. C. Lin, and R. Sakata, “Effect of Different Acids on the Extraction of Pepsin-Solubilised Collagen Containing Melanin from Silky Fowl Feet”, Food Chem., 113: 563–567, 2009.
- Feddern, V., Kupski, L., Cipolatti, E.P., Giacobbo, G., Mendes,G.L.,Badiale- Furlong, E., et al. Physico – chemical composition, fractionated glycerides and fatty acid proûle of chicken skin fat. European Journal of Lipid Science and Technology, 2010; 112(11),1277e1284.
- Fratzl P. 2008. Collagen: Structure and Mechanics. New York: Springer. p 1-496.
- Hashim, P., Ridzwan, M.M. and Bakar, J. Isolation and characterization of collagen from chicken feet. International Journal of Biological, Veterinary, Agricultural and Food Engineering, 2014; 8(3): 242-246
- Higgins JH. 2010. Bovine collagen – leading medical and industrial applications. URL: http://ezinearticles.com/?Bovine-Collagen—Leading-Medical-and Industrial Applications & id=3520525 [accessed September 2010]
- Index Mundi. 2015. India Broiler Meat (Poultry) Production by Year. Available at http://www.indexmundi.com/agriculture/?country=in&commodity=broilermeat&graph=production-growth-rate. Accessed Jan. 26, 2015.
- J. Muyonga, C. G. Cole, and K. Duodu, “Extraction and Physico & Chemical Characterisation of Nile Perch (Lates niloticus) Skin and Bone Gelatin”, Food Hydrocolloids, 2004; 18, pp. 581–592.
- Kadler KE, Baldock C, Bella J, Boot-Handford RP. Collagens at a glance. Journal of cell science, 2007; 120: 1955-1958.
- Kaewdang, O., Benjakul, S., Kaewmanee, T. and Kishimura, H. Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chemistry, 2014; 155: 264-270+
- Karim, A.A. and Bhat, R. 2009. Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocolloids 23(3): 563– 576.
- Leitinger, B Hohenester E. Mammalian collagen receptors. Matrix Biol. 2007; 26:146-155.
- Liu D.C, Lin Y.K and Chen M.T. Optimum condition of extracting collagen from chicken feet and its characteristics. Journal of animal science, 2001;14.
- Lullo DD, Gloria A, Shawn SM, Jarmo K, Ala-Kokko, Leena, Antonio S, James D. Mapping the Ligand-binding Sites and Disease associated Mutations on the Most Abundant Protein in the Human, Type I Collagen. J. Biol. Chem. 2002; 277 (6): 4223-4231.
- Miller, EJ. Biomedical and industrial application of collagen; In: Extracellular Matrix Biochemistry, K. A. Piez and A. H. Reddi, eds. Elsevier, New York, 1984; pp. 41-81.
- Munasinghe, K.A., Schwarz, J.G. and Whittiker, M. 2015. Utilization of Chicken By-Products to Form Collagen Films. Journal of Food Processing 2015: 1-6.
- Nagai, T. 2015. Characterization of collagen from emu (Dromaius novaehollandiae) skins. Journal of Food Science and Technology, 1984; 52(4): 2344-2351.
- Okerman, w.H. Quality of post-mortem muscle tissue. The Ohio State University, Ohio, 1984; USA p. 51
- Oliveira S, Ringshia R, Legeros R, ClarkE, Terracio L, Teixeira C, Yost M. An improved collagen scaffold for skeletal regeneration. Journal of Biomedical Materials, 2009; 94 (2): 371–379.
- Parenteau-Bareil, R., Gauvin, R., Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863-1887.
- Pati F, Dhara S, Adhikari B. 2010. Fish collagen: a potential material for biomedical application. Sch of Med Sci & Technol (Indian Inst. of Technol, Kharagpur, India).
- Prabakaran, R. 2014, Indian Poultry Industry – Current Status, Practical Challenges and Opportunities. Pages 1-14 in Proceedings of the 31st Annual Conference and National Symposium of Indian Poultry Science Association (IPSACON 2014), Dec. 18-20, Namakkal, India.
- Rajendran, K., K. Mani, P. Shamsudeen and T. Vasanthakumar. 2014. Broiler Industry – Understanding the Integration and Role of Private Industries. Pages 103-105 in Proceedings of the 31st Annual Conference and National Symposium of Indian Poultry Science Association (IPSACON 2014), Dec. 18-20, Namakkal, India.
- Ramshaw JA, Peng Y, Glattauer V, Werkmeister JA. Collagens as biomaterials. J. Mater. Sci. Mater. Med; 2009; 20 (1): S3–S8.
- Rose FR, Oreffo RO: Bone tissue engineering: hope vs hype. Biochem Biophys Res Commun 2002, 292:1-7.
- Saito M, Takenouchi Y, Kunisaki N, Kimura S. Complete primary structure of rainbow trout type I collagen consisting of á1(I) á3(I) heterotrimers. Eur J Biochem, 2001; 268: 2817-2827.
- Sanz-Herrera JA, Reina-Romo, E. Cell-Biomaterial Mechanical Interaction in the Framework of Tissue Engineering: Insights, Computational Modeling and Perspectives. Int. J. Mol. Sci., 2011; 12: 8217-8244.
- Schrieber R. and Gareis H. 2007. Gelatine handbook: theory and industry practice. Hardcover. 348p.
- Silva, T.F. and Penna, A.L.B. Colágeno: Características químicas e propriedades funcionais. Revista do Instituto Adolfo Lutz, 2012; 71(3): 530-539.
- Skierka, E. and Sadowska, M. The influence of different acids and pepsin on the extractability of collagen from the skin of Baltic cod (Gadus morhua). Food Chemistry, 2007; 105(3): 1302-1306.
- Szpak and Paul. Fish bone chemistry and ultrastructure: implications for taphonomy and stable isotope analysis. Journal of Archaeological Science, 2011; 38 (12): 3358–3372.
- U. K. Laemmli. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature, 1970; 227; 680 – 685.
- Wang, L., Yang, B., Du, X., Yang, Y. and Liu, J. Optimization of conditions for extraction of acidsoluble collagen from grass carp (Ctenopharyngodon idella) by response surface methodology. Innovative Food Science and Emerging Technologies, 2008; 9(4): 604- 607.
- Woo, J.W., Yu, S.J., Cho, S.M., Lee, Y.B. and Kim, S.B. Extraction optimization and properties of collagen from yellowfin tuna (Thunnus albacares) dorsal skin. Food Hydrocolloids, 2008; 22(5): 879-887.
- Y. K. Lin, and D. C. Liu, “Effects of Pepsin Digestion at Different Temperatures and Times on Properties of Telopeptide-Poor Collagen from Bird Feet”, Food Chem., 2006; 94: pp. 621–625.
- Zavareze, E.R., Silva, C.M., Mellado, M.S. and PrenticeHernández, C. Funcionalidade de hidrolisados proteicos de cabrinha (Prionotus punctatus) obtidos a partir de diferentes proteases microbianas. Química. Nova, 2009; 32(7): 1739-1743.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.