ISSN: 0973-7510
E-ISSN: 2581-690X
Respiratory tract infections (RTIs) have been critically associated with health care problems globally. Subsequently, increased antibiotic resistance rates have limited treatment options that are further exaggerated due to lack of newer novel drugs and therapies. Current study highlights, antibiotic resistance profiling along with extended-spectrum beta-lactamase (ESBL) producers of RTI pathogens from Bengaluru. During June 2020-May 2021, 1016 clinical samples collected, prevalence rate of 22.4% was exhibited, with highest in male (74.5%). Following age group, 30-35 years displayed highest (24.1%) though, lowest was in 45-50 years (1.3%). The standard microbiological characterization revealed Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli, Acinetobacter baumannii as predominant bacterial pathogens associated with RTIs. While, Antibiotic susceptibility test (AST) exhibited highest resistance rates for different antibiotics in the following pathogens, as K. pneumoniae for ampicillin (74.8%), P. aeruginosa for doripenem (66.6%), A baumannii to piperacillin/tazobactam (76.9%), E. coli for penicillin and β-lactamase inhibitors ranging between 56-92%, E. cloacae to ticarcillin/clavulanic acid besides cefuroxime (100%). However, prevalence of Gram-positive strains were lowest and exhibited highest resistance to penicillin, and fluoroquinolone (83.3%). ESBL producers were predominantly K. pneumoniae, followed by E. coli, and E. cloacae with 21.9%, 6.5% and 1.3%, respectively. Notably, all the Gram-negative strains showed 100% sensitivity towards colistin with remarkable sensitivity was observed in oxazolidinone, glycopeptides by S. aureus and Coagulase-neagtive Staphylococcus aureus (CoNS). The study emphasizes increased antimicrobial resistance antimicrobial and ESBL resistance, suggesting AST as a systematic approach for apprising treatment guidelines in current scenario. The present study denotes polypeptide colistin as choice of drugs for treating RTI pathogens, however its not recommended in all cases.
Antibiotic Resistance, Respiratory Tract Infections, Extended-spectrum beta-lactamase, Colistin
The rise of novel antibiotic-resistant nosocomial pathogens associated with respiratory tract infections (RTIs) is becoming a threat to human development, food security, and global health. Hence, antibiotics must be used appropriately to save them for the future as there is a decline in the discovery of novel antibiotics.1 The collective infections restricted to lower RTIs such as pneumonia, bronchitis, and upper respiratory tract infections namely tracheitis pharyngitis, sinusitis, and rhinitis are referred to as RTIs.2 The diseases caused by RTI are quite common however, with being as life-threatening it contributes to millions of deaths across the globe. Moreover, being 3rd leading cause of morbidity and mortality, RTI accounts for 4.25 million deaths annually in children and adults.3,4
Major bacterial pathogens causing RTI include K. pneumoniae, A. baumannii, P. aeruginosa, E. coil, and S. aureus. A substantial increase in antimicrobial resistance (AMR) among these pathogens has made antibiotic therapy ineffective.5 Some other pathogens causing RTI recently emerged as novel pathogens including coronavirus, influenza virus along with fungal pathogens such as Aspergillus spp., Pneumocystis spp., and bacterial pathogens like Bacillus anthracis, Staphylococcus aureus, Streptococcus pneumoniae and P. aeruginosa.6 The emergence of AMR in respiratory tract pathogens has led to life-threatening situations claiming approximately 2,603,913 deaths worldwide by 2019.7 It has been estimated that, by 2050 around 10 million people may die annually due to AMR, with an account of about 3,90,000 in Europe, 47,30,000 in Asia, and 41,40,000 in Africa.8 Eventually, multidrug- resistance (MDR) being detected with the drastic rise in respiratory pathogens was found to escalate management and treatment options in AMR. Hence, an immediate measure to control the spread of AMR in medically important respiratory pathogens is needed on an emergency basis.9
β-lactam antibiotics has been a key factor for treating bacterial infections globally with 65% of usage.10 The classified groups include penicillin, cephalosporins, monobactam, cephamycin, carbapenems and β-lactamase inhibitors that block cell wall synthesis by preventing penicillin-binding protein (PBP), leading to cell death.11 The mechanism for production of β-lactamases in Gram-positive and Gram-negative that acts as the most predominant source of resistance towards β-lactam antibiotics. The lactamases enzymes inactivate β-lactam antibiotics by binding covalently to their carbonyl group and hydrolyze the ring enabling them resistance.12 Additionally, these β-lactamases can be inhibited by b-lactamases inhibitors like clavulanic acid, tazobactam and sulbactam.13 ESBL producers have increased in frequent times and has become a worldwide threat that may also induce the resistance in non β-lactam drugs.14,15
The present investigation was carried out to understand the resistant pathogenic bacteria observed in patients admitted to the intensive care unit (ICU), in-patient (IP), and out-patients (OP) during the year 2020 COVID-19 pandemic. Following the signs and symptoms, samples were collected from RTI patients, and bacterial pathogens were isolated using specific microbiological techniques. Further, pure cultures of RTI bacterial pathogens thus obtained were identified by biochemical reactions, screened for antibiotic susceptibility test (AST) and Vitek® 2 system. Following with the results obtained from AST, the prevalence of antibiotic resistance was calculated and these isolates were additionally verified for their ESBL production.
Chemicals and microbiological Media: All the chemicals and reagents such as antibiotic discs, Glycerol, Peptone water, Nutrient broth, Mueller Hinton Agar (MHA), Blood agar, MacConkey agar, etc. were procured from Hi Media Laboratory Pvt. Ltd. (India).
Location and design of cross-sectional study
In the present investigation, prior to the sample collection, consent from the patients was taken and in case of critical patients informed consent was obtained from the patient’s relatives. The study was conducted from June 2020 to May 2021 following appropriate personnel protective equipment in the Department of Microbiology. In the present study, following the signs and symptoms of RTI, the patients from different sections such as the intensive care unit (ICU), in-patients (IP), and outpatients (OP) were selected and screened for antibiotic resistance pattern of bacterial isolates. The study included three different types of samples from the patients such as sputum, suction tip, and tracheal aspirate samples.
Clinical sample collection and isolation of RTI pathogens
The clinical respiratory tract samples were collected using sterile peptone water in an aseptic container along with the patient’s details such as gender, age, IP, OP, etc. Following which the collected samples were transferred to the laboratory and processed immediately using standard microbiological techniques, if failed to process immediately, the samples were stored at 4 °C in a refrigerator. The clinical samples were sub-cultured on sterile nutrient agar along with specific media such as blood Agar, chocolate agar and MacConkey agar. The inoculated plates were incubated at 37 °C for 24-48 hours in a pre-set bacteriological incubator. Subsequently, the bacterial isolates were observed for their cultural characteristics on specific media and morphological characteristics was analysed by Gram staining, motility, and biochemical characteristics.
Identification of RTI pathogens by biochemical tests
Following preliminary identification of bacterial isolates based on their colony morphology, these isolates were further sub-cultured and subjected to biochemical characterization. Various biochemical reactions such as mannitol fermentation, citrate utilization, urease production, growth on Triple Sugar Iron (TSI) agar, and coagulase tests were performed using standard protocols.16 The identified pure cultures of RTI causing pathogenic bacteria were preserved in glycerol stocks at -20 °C for further use.
Determination of antibiotic-susceptibility test (AST) of respiratory pathogens
The AST assay was performed as per Clinical and Laboratory Standards Institute guidelines.17 The method adopted is Kirby Bauer’s disc diffusion assay for analysing the antibiotic resistance in the pathogens. A 24 hour grown bacterial colony was inoculated in peptone water and inoculum size was adjusted to MacFarland’s constant. Following the inoculum of 106 CFU/ml was used to spread uniformly on sterile MHA plates and allowed to set for 5 minutes. The antibiotic discs with difference of 6 mm diameter apart were dispensed on plates using sterile forceps, and incubated at 37 °C for 24 hours. The zone of inhibition formed around the antibiotic discs was recorded for each antibiotic and the results were interpreted as per CLSI guidelines.18
The AST for the isolated RTI pathogens was also analysed utilising a Vitek® 2 (BioMerieux) for identification using susceptibility cards as (ID-GN for Gram-negative, ID-GP for Gram-positive), the AST-628 (for Staphylococci), AST-658 (for Enterococci and S. agalactiae), AST-ST03 (for Pneumococci), AST-280 (for Enterobacteriaceae), and AST-281 (for Gram-negative non- fermenters). Following which the antimicrobial results as resistant, sensitive and intermediate were recorded as per EUCAST guidelines.19 E. coli ATCC 25922 and Klebsiella pneumoniae ATCC 700603 was used as negative and positive control strain respectively for the effectiveness of the drugs.
The antibiotics used in the present study consisted of Ampicillin (10 mcg/disc), Ertapenem (10 mcg/disc), Imipenem (10 mcg/disc), Meropenem (10 mcg/disc), Doripenem (10 mcg/disc), Gentamicin (10 mcg/disc), Colistin (10 mcg/disc), Penicillin (10 units/disc), Amoxicillin/clavulanic acid (20/10 mcg/disc), Cefoperazone/Sulbactam (75/10 mcg/disc), Piperacillin/tazobactam (100/10 mcg/disc), Ticarcillin/clavulanic acid (75/10/disc), Cefuroxime (30 mcg/disc), Cefuroxime Axetil (30 mcg/disc), Ceftriaxone (30 mcg/disc), Ceftazidime (30 mcg/disc), Cefepime (30 mcg/disc), Amikacin (30 mcg/disc), Linezolid (30 mcg/disc), Teicoplanin (30 mcg/disc), Vancomycin (30 mcg/disc), Tetracycline (30 mcg/disc), Ciprofloxacin (5 mcg/disc), Levofloxacin (5 mcg/disc), Rifampicin
(5 mcg/disc), Benzylpenicillin (2 units/disc), Daptomycin (5 mcg/disc), Erythromycin (15 mcg/disc), Trimethoprim/Sulfamethoxazole (1.25/23.75 mcg/disc), Clindamycin (2 mcg/disc), and Oxacillin (5 mcg/disc).
Phenotypic Confirmation of Extended-Spectrum β-lactamases (ESbL) production
The antibiotic-resistant clinical isolates of Gram-negative bacteria such as K. pneumoniae, P. aeruginosa, A. baumannii, E. coli, and E. cloacae were further tested for ESBL production by Double Disc Synergy Test (DDST) using CLSI guidelines.20 A fresh bacterial inoculum equivalent to 0.5 McFarland standard was prepared from each 24-hour bacterial culture and inoculated on sterile MHA by spread plate technique and allowed to stand for 5 minutes. The antibiotic discs containing cefotaxime+clavulanic acid (30/10 mcg) along with cefotaxime (30 mcg) and ceftazidime+clavulanic acid (30/10 mcg) along with ceftazidime (30 mcg) were placed opposite to each other at an appropriate distance. The plates were incubated at 37 °C for 24 hours in an inverted position and the results were analysed. As recommended by CLSI guidelines, formation of ≥5 mm diameter of zone inhibition with combination versus cefotaxime and ceftazidime alone is indicated as positive for ESBL production.
Clinical samples collection and analysis of data
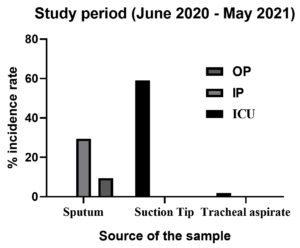
During the study period of 12 months interval, i.e. June 2020 – May 2021, a total of 1016 samples (n = 1016) were collected from patients attending with signs and symptoms for RTI. The detailed data of RTI samples collected from the patients belonging to different wards with their numbers and percentages are indicated below (Table 1 and Figure 1). The highest number of clinical samples of suction tips, i.e. 600 was collected from the ICU section contributing to 59.05% of the total clinical sample collected. In the total samples, sputum samples collected from IP and OP consisted 29.52% (300) and 9.44% (96) respectively. However, lowest of 1.96% consisted of the tracheal aspirate samples collected from the ICU section. Of the 1016 RTI samples collected, a total of 228 samples indicated the presence of respiratory tract pathogens corresponding to a prevalence rate of 22.4%.
Table (1):
Total number of clinical samples of RTI collected from different hospital wards
| RTI samples | Study period: June 2020 – May 2021 | |||||||
|---|---|---|---|---|---|---|---|---|
| ICU | % | IP | % | OP | % | |||
| Sputum | 00 | 00 | 300 | 29.52 | 96 | 9.44 | ||
| Suction Tip | 600 | 59.05 | 00 | 00 | 00 | 00 | ||
| Tracheal aspirate | 20 | 1.96 | 00 | 00 | 00 | 00 | ||
| Total isolates | 1016 | |||||||
The clinical samples collected from both the genders indicated highest prevalence in male RTI patients i.e., n = 170 as compared to female patients (n = 58). The study indicated a 74% incidence with an overall carriage rate of 16.6% RTI pathogens in male patients in comparison with 25% in females with a carriage rate of 5.7%. The detailed number of clinical samples and percentage of incidences of RTI pathogens isolated from clinical samples of both genders are indicated in Table 2 and Figure 2.
Table (2):
Incidence of RTI observed in clinical samples of patients concerning gender
Gender |
No. of samples (n = 1016) |
No. of positive isolates (n = 228) |
% incidence (n = 228) |
% Carriage rate (n = 1016) |
|---|---|---|---|---|
Male |
614 |
170 |
74.5 |
16.6 |
Female |
402 |
58 |
25.4 |
5.7 |
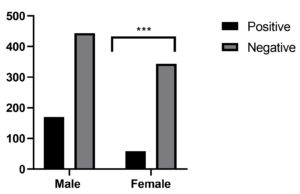 Figure 2. Strong association between the gender was observed for incidence rate of RTI in clinical samples of patients. ***p < 0.001, Male is distant pattern for male compared to female, Chi-square was using by GraphPad Prism
Figure 2. Strong association between the gender was observed for incidence rate of RTI in clinical samples of patients. ***p < 0.001, Male is distant pattern for male compared to female, Chi-square was using by GraphPad Prism
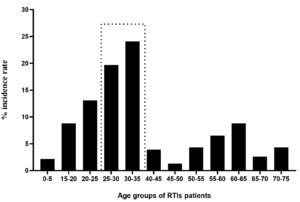
The study also highlighted the age-wise assessment for the prevalence of RTIs, the analysis indicating highest percentage of prevalence in the age groups of 30-35 years (24.1%) followed by 25-35 years (19.7%). The younger patients with an age group of 20-25 years and elderly group of 60-65 years indicated an incidence of RTIs with 8.77%. The results shows that incidence of RTIs was found to be with increasing trend from age 0-5 years to 35 years of age. Subsequently, the older patients with above 50 years of age except 60-65 years of age group were less prone to RTIs as compared to those below 50 years of age (Figure 3).
Figure 3. Incidence of RTI pathogens observed in clinical samples of patients concerning different age groups
Cultivation of clinical samples of RTI patients and preliminary identification
The clinical samples of RTI processed by standard microbiological culture methods revealed different colony morphologies on specific media as indicated in Figure 4. The clinical samples inoculated on MacConkey agar indicated dominant colonies with identical morphologies such as pink, cream, and colourless colonies. The lactose fermenters indicating pink coloured colonies belonging to Enterobacteriaceae family viz., E. coli, Klebsiella, Enterobacter sp., Citrobacter sp. while, colourless colonies were Pseudomonas sp. The colonies formed on Blood agar were analysed for haemolytic and non-haemolytic activity. Based on the colony characteristics, all the bacterial colonies were categorized into seven groups of isolates, i.e. K. pneumoniae, P. aeruginosa, E. coli, A. baumannii E. cloacae and S. aureus.
Figure 4. Colony morphologies of clinical isolates on differential media. (A). K. pneumoniae, (B). P. aeruginosa, (C). E. coli, (D). A. baumannii, (E). E. cloacae and (F). S. aureus
Identification of RTI pathogens by biochemical reactions and Vitek® 2 system
Following the cultural identification, all the positive clinical isolates of RTI (n = 228) were subjected to biochemical reactions, revealing majority of clinical isolates to be Gram-negative as compared to Gram-positive pathogens. The detailed results of biochemical reactions for 228 positive isolates are indicated in Table 3.
Table (3):
Biochemical tests performed for positive clinical pathogens (n = 228) of RTI
Biochemical parameters |
Isolate 1 |
Isolate 2 |
Isolate 3 |
Isolate 4 |
Isolate 5 |
Isolate 6 |
Isolate 7 |
|---|---|---|---|---|---|---|---|
Gram staining |
Gram-negative bacilli |
Gram- negative bacilli |
Gram-negative bacilli |
Gram- negative coccobacilli |
Gram- negative bacilli |
Gram- positive cocci |
Gram- positive cocci |
Motility |
Non-motile |
Motile |
Motile |
Motile |
Motile |
Non- Motile |
Non- Motile |
Colony colour on: MacConkey agar EMB Blood agar |
Pink Pink Haemolytic |
Colourless Cream Haemolytic |
Pink colour GMS Non-haemolytic |
Pale pink Pink Non-haemolytic |
Pink Pink Non-haemolytic |
No growth No growth Haemolytic |
No growth No growth Haemolytic |
Mannitol fermentation |
Negative |
Negative |
Negative |
Negative |
– |
Positive |
Positive |
Citrate utilization |
Positive |
Positive |
Negative |
Positive |
– |
– |
– |
Urease production |
Positive |
Negative |
Negative |
Negative |
– |
– |
– |
TSI |
Positive |
Positive |
Positive |
Negative |
– |
– |
– |
Coagulase test |
Negative |
Negative |
Negative |
Negative |
– |
Positive |
Negative |
RTI pathogen identified |
K. pneumoniae |
P. aeruginosa |
E. coli |
A. baumannii |
E. cloacae |
S. aureus |
CoNS |
Note: GMS-Green metallic sheen, TSI-Triple Sugar Iron, CoNS–Coagulase-negative Staphylococcus aureus, RTI-Respiratory tract infection
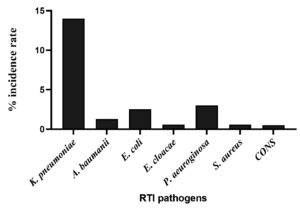
Of different etiological agents isolated from different RTI samples, K. pneumoniae was predominant with an incidence rate of 62.71%, followed by P. aeruginosa, E. coli, and A. baumannii with prevalence rate of 13.15%, 10.96%, and 5.70%, respectively. However, the incidence of E. cloacae was lowest with an incidence rate of 2.63%. Meanwhile, the Gram-positive pathogens such as S. aureus and CoNS were found to be lowest as compared to Gram-negative bacteria with 2.19%. The total number of positive isolates of RTI along with their % incidence and overall % incidence of each clinical isolate is as shown in detail in Table 4 and Figure 5.
Table (4):
Etiology of RTI pathogens collected from different clinical specimens from June 2020 – May 2021
Organisms |
No. of positive isolates (n = 228) |
% incidence rate (n = 228) |
Overall % incidence (n = 1016) |
|---|---|---|---|
K. pneumoniae |
143 |
62.7% |
14% |
A. baumanii |
13 |
5.7% |
1.28% |
E. coli |
25 |
10.9% |
2.5% |
E. cloacae |
06 |
2.6% |
0.6% |
P. aeuroginosa |
30 |
13.1% |
3% |
S. aureus |
06 |
2.6% |
0.59% |
CoNS |
05 |
2.1% |
0.5% |
Figure 5. Percentage incidence of RTI pathogens collected from different clinical specimens (June 2020 – May 2021)
Antibiotic resistance profiles of clinical pathogens of RTI
The positive isolates of RTI evaluated for AST using the disc diffusion method indicated various antibiotic resistance profiles as shown in Table 5. The isolates of K. pneumoniae showed a complete resistance to ampicillin (100%), followed by piperacillin/tazobactam (80.4%), ceftriaxone and ciprofloxacin with 74.8%. While, cephalosporin group of antibiotics revealed resistant rate varying between 69.9%-65.7%, for cefuroxime, cefuroxime axetil, cefepime, and cefoperazone/sulbactam. In contrast, P. aeruginosa isolates revealed a moderate resistance against most of the antibiotics used in the study. The highest resistance rate was observed against doripenem (66.6%), followed by amikacin, imipenem, and levofloxacin with 46.6%, 43.3% and 40%, respectively. However, the resistance rate towards remaining isolates was less than 30% and none of the isolates showed 100% resistance towards any selected antibiotics.
Table (5):
Antibiotic sensitivity assay of bacterial pathogens of RTI collected from June 2020 – May 2021
| Antimicrobials | K. pneumoniae (n=143) | P. aeruginosa (n =30) | A. baumannii (n = 13) | E. coli (n = 25) | E. cloacae (n = 6) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class of antibiotics | Name | Conc. (µg/disc) | No. of isolate; % Resistance | No. of isolate; % Sensitive | No. of isolate; % Resistance | No. of isolate; % Sensitive | No. of isolate; % Resistance | No. of isolate; % Sensitive | No. of isolate; % Resistance | No. of isolate; % Sensitive | No. of isolate; % Resistance | No. of isolate; % Sensitive |
| Penicillin & β-lactamase inhibitors | Ampicillin | 10 | 100 | 00; 00 | NA | NA | NA | NA | 23; 92 | 02; 08 | 05; 83.3 | 01; 16.6 |
| Piperacillin/tazobactam | 100/10 | 115; 80.4 | 28;19.6 | 05;16.6 | 25; 83.3 | 10; 76.9 | 03; 16.6 | 14; 56 | 11; 46 | 03; 50 | 03; 50 | |
| Ticarcillin/clavulanic acid | 75/10 | NA | NA | 12; 40 | 18; 60 | NA | NA | NA | NA | 06; 100 | 00; 00 | |
| 2nd Gen. Cephalosporins | Cefuroxime | 30 | 100; 69.9 | 43; 30.06 | NA | NA | NA | NA | 20; 80 | 05; 20 | 06; 100 | 00; 00 |
| Cefuroxime Axetil | 30 | 100; 69.9 | 43; 30.06 | NA | NA | NA | NA | 20; 80 | 05; 20 | 05; 83.3 | 01; 16.6 | |
| 3rd Gen. Cephalosporins | Ceftriaxone | 30 | 107;74.8 | 36; 25.1 | NA | NA | NA | NA | 20; 80 | 05; 20 | 05; 83.3 | 01; 16.6 |
| Ceftazidime | 30 | NA | NA | 09; 30 | 21; 70 | NA | NA | NA | NA | 05; 83.3 | 01; 16.6 | |
| Cefoperazone/Sulbactam | 75/10 | 94; 65.7 | 49; 34.3 | 06; 20 | 24; 80 | 09; 69.2 | 04; 30.8 | 06; 24 | 19; 76 | 05; 83.3 | 01; 16.6 | |
| 4th Gen. Cephalosporins | Cefepime | 30 | 100; 69.9 | 43; 30.06 | 08; 26.6 | 22; 73,4 | NA | NA | 19; 76 | 06; 24 | (5) 83.3 | 01; 16.6 |
| Carbapenems | Ertapenem | 10 | 71; 49.6 | 72; 50.4 | NA | NA | 09; 69.2 | 04; 30.8 | 07; 28 | 18; 72 | 00; 00 | 06; 100 |
| Imipenem | 10 | 71; 49.6 | 72; 50.4 | 13; 43.3 | 17; 56.7 | 09; 69.2 | 04; 30.8 | 07; 28 | 18; 72 | 00; 00 | 06; 100 | |
| Meropenem | 10 | 71;49.6 | 72; 50.4 | 14; 46.6 | 16; 53.4 | 09; 69.2 | 04; 30.8 | 07; 28 | 18; 72 | 00; 00 | 06; 100 | |
| Doripenem | 10 | NA | NA | 20; 66.6 | 10; 35.4 | NA | NA | NA | NA | 00; 00 | 06; 100 | |
| Aminoglycoside | Amikacin | 30 | 85; 59.4 | 58; 40.6 | 13; 43.3 | 17; 56.7 | NA | NA | 08; 32 | 17; 68 | 03; 50 | 03; 50 |
| Gentamicin | 10 | 85;59.4 | 58; 40.6 | 05; 16.6 | 25; 83.4 | NA | NA | 07; 28 | 18; 72 | 03; 50 | 03; 50 | |
| Fluoroquinolone | Ciprofloxacin | 05 | 107; 74.8 | 36;25.2 | 05; 16.6 | 25; 83.4 | 08; 61.5 | 05; 38.5 | 19; 76 | 06; 24 | 05; 83.3 | 01; 16.6 |
| Levofloxacin | 05 | NA | NA | 13; 43.3 | 17; 56.7 | NA | NA | NA | NA | NA | NA | |
| Polypeptide | Colistin | 10 | 00; 00 | 143; 100 | 00; 00 | 30; 100 | 00; 00 | 13;100 | 00; 00 | 25; 100 | 00; 00 | 06; 100 |
| Sulfonamides | Trimethoprim/Sulfamethoxazole | 1.25/23.75 | 86; 60.1 | 57; 39.9 | NA | NA | 08; 61.5 | 05; 38.5 | 05; 20 | 20; 80 | 05; 83.3 | 01; 16.6 |
Note: R: Resistant, S: Sensitive, NA: Not Applicable
A. baumannii exhibited highest resistance towards piperacillin/tazobactam (76.9%), followed by 3rd generation cephalosporins (Cefoperazone/sulbactam) and carbapenems (ertapenem, imipenem, and meropenem) with 69.2%. The following isolates also displayed 61.5% resistance against ciprofloxacin and trimethoprim/sulfamethoxazole. The isolates E. coli analysed had substantial resistance against ampicillin with 92%, followed by 2nd generation cephalosporins (80%), cefepime (76%), and ciprofloxacin (76%). Meanwhile, E. cloacae exhibited highest resistance rate to ticarcillin/clavulanic acid and cefuroxime (100%), followed by ampicillin and selected 2nd, 3rd, and 4th generation cephalosporins, ciprofloxacin (83.3%). Interestingly, the resistance rate towards antibiotics like amikacin and gentamicin were represented at 50%. Remarkably, all the six isolates of E. cloacae were found to be 100% sensitive to carbapenems. The most striking results in the present work highlights that all aetiological pathogens isolated were 0% resistant to colistin making it a remarkable choice of drug for treating these MDR strains associated with respiratory tract infections.
The AST was also analysed for Gram-positive pathogens, the antibacterial assay against S. aureus and CoNS are as recorded in Table 6. Following the total six S. aureus isolates, 5 isolates indicated highest resistance against penicillin, levofloxacin and ciprofloxacin (83.3%) followed by clindamycin (50%). Comparatively, low antibiotic resistance was observed in trimethoprim/sulfamethoxazole, rifampicin and gentamicin (16.6%). However, S. aureus was totally sensitive towards linezolid, teicoplanin, vancomycin, erythromycin, daptomycin, and tetracycline (100%). Among CoNS, three isolates exhibited higher resistance towards trimethoprim/sulfamethoxazole, penicillin, erythromycin, daptomycin (60%). While, two isolates designated resistance towards clindamycin, oxacillin, benzylpenicillin, levofloxacin, ciprofloxacin with 40%, and a single isolate exposed to gentamicin. Remarkably, all the isolates were found to be 100% sensitive to linezolid, teicoplanin, vancomycin rifampicin, and tetracycline used in the study.
Table (6):
Antibiotic sensitivity assay of S. aureus and CoNS causing RTI during June 2020 – May 2021
| Antibiotics | S. aureus (n = 6) | CoNS (n = 5) | ||||
|---|---|---|---|---|---|---|
| Class of antibiotics | Name | Conc. (µg/disc) | No. of isolate; % Resistance | No. of isolate; % Sensitive | No. of isolate; % Resistance | No. of isolate; % Sensitive |
| Oxazolidinone | Linezolid | 30 | 00; 00 | 06; 100 | 00; 00 | 05;100 |
| Glycopeptides | Teicoplanin | 30 | 00; 00 | 06; 100 | 00; 00 | 05;100 |
| Vancomycin | 30 | 00; 00 | 06; 100 | 00; 00 | 05;100 | |
| Lincosamides | Clindamycin | 02 | 03; 50 | 03; 50 | 02; 40 | 03; 60 |
| Sulphonamides | Trimethoprim/Sulfamethoxazole | 1.25/23.75 | 01;16.6 | 05; 83.3 | 03; 60 | 02; 40 |
| Macrolactams | Rifampicin | 05 | 01;16.6 | 05; 83.3 | 00; 00 | 05;100 |
| β-lactam antibiotics | Penicillin | 10 units | 05; 83.3 | 01;16.6 | 03; 60 | 02; 40 |
| Oxacillin | 01 | NA | NA | 02; 40 | 03; 60 | |
| Oxacillin Benzylpenicillin | 05 | NA | NA | 02; 40 | 03; 60 | |
| Fluoroquinolone | Levofloxacin | 05 | 05; 83.3 | 01;16.6 | 02; 40 | 03; 60 |
| Ciprofloxacin | 05 | 05; 83.3 | 01;16.6 | 02; 40 | 03; 60 | |
| Aminoglycoside | Gentamicin | 10 | 01;16.6 | 05; 83.3 | 01; 20 | 04; 80 |
| Macrolides | Erythromycin | 15 | 00; 00 | 06; 100 | 03; 60 | 02; 40 |
| Lipopeptide | Daptomycin | 05 | 00; 00 | 06; 100 | 03; 60 | 02; 40 |
| Tetracyclines | Tetracycline | 30 | 00; 00 | 06; 100 | 00; 00 | 05;100 |
Note: R: Resistant, S: Sensitive, CoNS: Coagulase-negative S. aureus
Production of extended spectrum β–lactamases
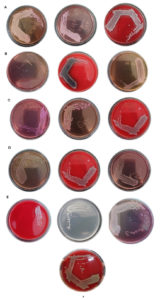
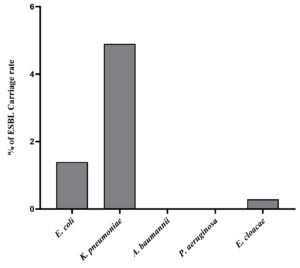
All antibiotic-resistant positive clinical isolates causing RTI were further assessed for phenotypic detection of ESBL production as indicated by Double Diffusion Synergy Test (DDST). After incubation of MHA plates inoculated with each positive isolate and placed with pairs of antibiotics, results revealed a significant formation of a zone of inhibition around cefotaxime/clavulanic acid and ceftazidime/clavulanic acid. While, no zone of inhibition was formed around cefotaxime and ceftazidime antibiotic discs indicating negative ESBL production. Of all the positive isolates analysed for the prevalence of ESBL producers, 29.8% were found to be ESBL producers with a carriage rate attributing with 6.6% (Table 7). The highest ESBL producer were K. pneumoniae contributing about 21.9% of the total 228 positive isolates. While, E. coli indicated ESBL production attributing to 6.5% and E. cloacae were found to be the least with 1.3% (with 3 isolates). None of A. baumannii and P. aeruginosa isolates were found to be ESBL producers. The details of clinical isolates of RTI-producing ESBL about total positive isolates and total clinical samples are shown in Figure 6 and Figure 7.
Table (7):
Incidence of ESBL-producing RTI pathogens isolated from June 2020 – May 2021
RTI pathogen |
No. of isolates |
% of ESBL producers (n = 228) |
% of ESBL Carriage rate (n = 1016) |
|---|---|---|---|
E. coli |
15 |
6.5 |
1.4 |
K. pneumoniae |
50 |
21.9 |
4.9 |
A. baumannii |
00 |
00 |
00 |
P. aeruginosa |
00 |
00 |
00 |
E. cloacae |
03 |
1.3 |
0.29 |
Total No. of ESBL isolates |
68 |
29.8 |
6.6 |
Figure 6. Phenotypic characterization of ESBL productions by clinical isolates of K. pneumoniae (A), E. coli (B), E. cloacae (C), and the negative control of ESBL (D)
One of the breakthrough pandemics of this era is COVID-19 which by the end of Jan-2021 claimed more than 2 million deaths all over the world.21 However, the specific cause associated with the mortality of COVID-19 patients is still not clear yet to be well established. A study conducted to establish the cause of death in COVID-19 and non-COVID patients that included 17,456,515 subjects revealed 17063 patients died due to COVID-19 while the remaining patients, i.e. 134,316 died due to other causes.22 Hence, deaths that occurred during the COVID pandemic were also associated with non-COVID causes and the present work is witnessing a similar instance of deaths due to non-COVID reasons.
Worldwide there is an increased prevalence to infectious diseases, in which RTIs stands in the fourth place globally. This statistically is a prominent cause of death worldwide which claimed to be 2,603,913 deaths in 2019 all over the world. During the COVID-19 pandemic, about 567 million confirmed respiratory tract diseases along with 6.3 million deaths have been recorded across the globe.23 The increased emergence of AMR in pathogens causing RTIs namely K. pneumoniae, A. baumannii, P. aeruginosa, E. coli, S. aureus, etc., has become more life-threatening and making it difficult to treat patients with respiratory tract diseases and challenging for need of significant antibiotic therapy.24-27 Hence, in the current investigation, clinical samples of patients with RTIs were collected, bacterial pathogens were identified and their antibiotic resistance profile along with ESBL production was studied.
The majority of clinical samples collected in the present study were from the suction tips of the ICU section and sputum samples of IP and OP wards in which the highest disease prevalence was observed in male patients. The high prevalence of RTI in ICU may be associated with healthcare-associated infections (HAIs) and it has been reported that about 30% of HAIs acquired by patients admitted to the ICU section. The High risk of HAIs in ICU may also be due to the high prevalence of devices, intensive procedures, the microflora of critically ill patients, nurses (source of cross-contamination), microbiological sampling, cross contamination, etc. along with the emergence of novel SARS-CoV-2 (COVID-19) pathogen has further intensified the ICU acquired infections.28
The percentage incidences of respiratory tract diseases were increased between the age of 5 to 35 years and was found declined in older age patients. In 2018, the naive out-patients with RTI in referral hospitals of Meru, County, Kenya also showed a high prevalence of respiratory tract diseases in male patients (71.4%) as compared to female patients (28.6%) with a ratio of 5:2. Further, the disease was increasing with increased age of the patients’ ages from 5 to 34 years which declined in the older patients of above 34 years.29 Following the microbiological culture and biochemical-based identification of respiratory bacterial pathogens in clinical samples of RTI indicated the predominant Gram-negative species such as K. pneumoniae, P. aeuroginosa, E. coli, A. baumanii and E. cloacae compared to Gram-positive S. aureus and CoNS pathogens. These etiological agents except E. cloacae and CoNS were also reported by other studies as commonly detected respiratory pathogens. However, percentage of antibiotic resistance against the selected antibiotics differs from in the following studies as compared to the others documented.30,31
The AST performed for K. pneumoniae in the present study showed highest resistance towards ampicillin (100%), followed by cephalosporin group (65.7-74.8%), fluoroquinolone (74.8%) aminoglycoside (59.4%), and carbapenems (49.6%) as compared to the reports of resistance rate with penicillin class of antibiotics of 57.6 to 80.4%, cephalosporins with 44.9 to 82%, carbapenems resistance 45.8 to 72.5%, aminoglycosides and tetracycline resistance with 44.1-64.4%, and fluoroquinolone resistance of 53.3 to 82% as reported five years in previous studies (2015-2019). Another study also reported 2.3-42.5% resistance to colistin, a polypeptide antibiotic, noteworthy that our study showed 100% sensitivity towards the same antibiotic.32 Among Gram-negative respiratory pathogens, K. pneumoniae is the 3rd most commonly detected pathogenic bacteria from patients with RTI (mean% of 10.9). The drug resistance rate worldwide for K. pneumoniae has been estimated to be more than 70% with rate of infection causing fatality rate of approximately 40-70%.33
P. aeruginosa strains from RTI patients in the present study showed the highest resistance to carbapenems (66.6%) compared to fluoroquinolone and aminoglycoside (43.3%), while, penicillin showed (16.6-40%). However, P. aeruginosa was reported as a major resistant pathogen as MDR, exclusively drug resistance (EDR), and difficult-to-treat.34,35 The global antibiotics resistance level of A. baumannii in respiratory tract diseases has been reported to be 45%, being MDR strains they have been reported with carbapenem resistant and colistin sensitive.36 However, high-rate resistance i.e., about 70% of MDR strains was noticed in low-income nations.37,38 The present study reports high resistance to antibiotics among E. coli isolates from RTIs as compared to earlier reports by Promite and Saha.39 Their study highlighted the resistance range of 72%, 56%, 54%, 48%, 32%, 28%, and 24% resistance to amoxicillin, cefixime, sulfamethoxazole/trimethoprim, ciprofloxacin, nalidixic acid, amikacin, and gentamycin, respectively in MDR E. coli of RTI as compared to varying antibiotics resistance of 50-92% in the present study.39
Majority of respiratory tract samples of E. cloacae isolated from 53 Chinese hospitals showed less than 8% resistance to meropenem, imipenem, polymyxin B, amikacin, and 10% resistance to carbapenem compared to high-level resistance to penicillin (100%), cephalosporin (83.3%), fluoroquinolone (83.3%), and sulphonamides (83.3%) antibiotics in our study indicating significant resistance level in the pathogens.40 However, in our study E. cloacae was found to be 100% sensitive to carbapenems and colistin although colistin-resistant E. cloacae accounted for high morbidity and mortality rate of ICU patients.41 Though, several studies have reported colistin resistance, our study significantly showed the inhibition of the growth of all respiratory pathogens indicating it as the choice of drug for the treatment of respiratory tract diseases. Among Gram-positive bacteria, the highest resistance was observed against β-lactam antibiotics, fluoroquinolone, and lincosamides in both S. aureus and CoNS and equivalent resistant against fluoroquinolone class of antibiotics, i.e. levofloxacin (80.4%) and ciprofloxacin (92.5%).42 The antibiotic resistance pattern of each isolate in the present study is supportive in the treatment of RTI patients and one can anticipate the possible future course of resistance in these pathogens. Hence, the use of antibiotics against which resistance is anticipated can be limited.
A high prevalence of antibiotic resistance was observed in Gram-negative pathogens as K. pneumoniae, P. aeruginosa, and E. coli, along with few species of A. baumannii and E. cloacae. These pathogens revealed resistance to more or least one class of antibiotics and were found to be carbapenem-resistant and ESBL producers. Following MDR strains, there is a need for continuous search towards novel antibiotics and alternative treatment options. The present study encased the levels of antibiotic resistance patterns that has been high values. The possibilities of resistance in pathogens are varied reasons such as inappropriate and incorrect administration of antimicrobial agents, lack of apt infection control strategies that are alarming situation with increased MDR strains. The problem can be addressed by performing the antibiotic susceptibility testing before empirical treatment. Surveillance of such infections and monitoring of antimicrobial resistant patterns needs to be carried out in both hospital and community-acquired infections. Further, studies need to be designed for molecular characterization and validated with accuracy for guiding empirical treatment options in RTI using antibiotics.
ACKNOWLEDGMENTS
The authors would like to thank Santosh Hospitals for providing clinical samples of RTI and facilitating the Microbiology Lab for processing.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
The study was approved by the Ethics Committee, Santosh Hospitals and Diagnostics, vide approval number SHIEC/ECAL/DSU/APR2020.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Hutchings MI, Truman AW, Wilkinson B. Antibiotics: past, present and future. Curr Opin Microbiol. 2019;51:72-80.
Crossref - Mayor A, Chesnay A, Desoubeaux G, Ternant D, Heuze-Vourc’h N, Secher T. Therapeutic antibodies for the treatment of respiratory tract infections-current overview and perspectives. Vaccines. 2021;9(2):151.
Crossref - Schluger NW, World Lung Foundation. Acute respiratory infections atlas. World Lung Foundation, 2010. https://search.worldcat.org/title/698129340. Accessed August 12, 2024.
- Schluger NW, Koppaka R. Lung disease in a global context. A call for public health action. Annals ATS. 2014;11(3):407-416.
Crossref - Dung TTN, Phat VV, Vinh C, et al. Development and validation of multiplex real-time PCR for simultaneous detection of six bacterial pathogens causing lower respiratory tract infections and antimicrobial resistance genes. BMC Infect Dis. 2024;24(1):164.
Crossref - Calderaro A, Buttrini M, Farina B, Montecchini S, De Conto F, Chezzi C. Respiratory tract infections and laboratory diagnostic methods: a review with a focus on syndromic panel-based assays. Microorganisms. 2022;10(9):1856.
Crossref - Global health estimates: Leading causes of DALYs. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/global-health-estimates-leading-causes-of-dalys. Accessed August 12, 2024.
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance. World Health Organization. 2014. https://iris.who.int/handle/10665/112642. Accessed August 12, 2024.
- Coque TM, Canton R, Perez-Cobas AE, Fernandez-de-Bobadilla MD, Baquero F. Antimicrobial resistance in the global health network: known unknowns and challenges for efficient responses in the 21st century. Microorganisms. 2023;11(4):1050.
Crossref - Patel MP, Hu L, Brown CA, et al. Synergistic effects of functionally distinct substitutions in b-lactamase variants shed light on the evolution of bacterial drug resistance. J Biol Chem. 2018;293(46):17971-17984.
Crossref - Ur Rahman S, Ali T, Ali I, Khan NA, Han B, Gao J. The growing genetic and functional diversity of extended spectrum beta-lactamases. BioMed Res Int. 2018;2018(1):1-14.
Crossref - Eiamphungporn W, Schaduangrat N, Malik AA, Nantasenamat C. Tackling the antibiotic resistance caused by class A b-lactamases through the use of b-lactamase inhibitory protein. Int J Mol Sci. 2018;19(8):2222.
Crossref - Ye Q, Wu Q, Zhang S, et al. Characterization of extended-spectrum b-lactamase-producing enterobacteriaceae from retail food in china. Front Microbiol. 2018;9:1709.
Crossref - Baziboroun M, Bayani M, Poormontaseri Z, Shokri M, Biazar T. Prevalence and antibiotic susceptibility pattern of extended spectrum beta lactamases producing Escherichia coli isolated from outpatients with urinary tract infections in babol, northern of iran. Curr Issues Pharm Med Sci. 2018;31(2):61-64.
Crossref - Naushad VA, Purayil NK, Wilson GJ, et al. Epidemiology of urinary tract infection in adults caused by extended-spectrum beta-lactamase (Esbl)-producing Enterobacteriaceae – a case-control study from Qatar. IJID Reg. 2022;3:278-286.
Crossref - Brown JH. Bergey’s manual of determinative bacteriology(5th ed.). Am J Public Health Nations Health. 1939;29(4):404-405.
Crossref - Wayne PA. Clinical and laboratory standards institute. Performance standards for antimicrobial susceptibility testing, 2011. Accessed August 12, 2024.
- Bauer AW, Kirby WMM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493-496.
Crossref - European Commitee on Antimicrobial Susceptibility Testing: Clinical breakpoints and dosing of antibiotics. https://www.eucast.org/clinical_breakpoints. Accessed August 12, 2024.
- Khan MKR, Thukral SS, Gaind R. Evaluation of a modified double-disc synergy test for detection of extended spectrum beta-lactamases in AMPC beta-lactamase-producing proteus mirabilis. Indian J Med Microbiol. 2008;26(1):58-61.
Crossref - Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533-534.
Crossref - Bhaskaran K, Bacon S, Evans SJW, et al. Factors associated with deaths due to COVID-19 versus other causes: population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet Reg Health Eur. 2021;6:100109.
Crossref - World Health Organization. The Global Health Observatory. Global Health Estimates: Leading Causes of Death. Cause-Specific Mortality, 2000-2019. https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death. Accessed August 12, 2024.
- Chakraborty A, Chanda DD, Choudhury N, Manjula NG, Shilpa BM. A retrospective study on the pyogenic pathogens and their antibiotic susceptibility patterns along with the ESβL production. Adv Microbiol. 2021;11(06):317-326.
Crossref - Nagshetty K, Shilpa BM, Patil SA, Shivannavar CT, Manjula NG. An overview of extended spectrum beta lactamases and metallo beta lactamases. Adv Microbiol. 2021;11(01):37-62.
Crossref - Shivannavar CT. Antibiotic susceptibility pattern of ESβL producing Klebsiella pneumoniae isolated from urine samples of pregnant women in karnataka. J Clin of Diagn Res. 2014;8(10):DC08-DC11.
Crossref - Georgiou G, Kotze A. Eradication of antibiotic-resistant E. coli, S. aureus, K. pneumoniae, S. pneumoniae, A. baumannii, and P. aeruginosa with chlorine dioxide in vitro. Med Res Arch. 2023;11(7.2):4218.
Crossref - Blot S, Ruppe E, Harbarth S, et al. Healthcare-associated infections in adult intensive care unit patients: Changes in epidemiology, diagnosis, prevention and contributions of new technologies. Intensive Crit Care Nurs. 2022;70:103227.
Crossref - Miriti DM, Muthini JM, Nyamache AK. Study of bacterial respiratory infections and antimicrobial susceptibility profile among antibiotics naive outpatients visiting Meru teaching and referral hospital, Meru County, Kenya in 2018. BMC Microbiol. 2023;23(1):172.
Crossref - Karakonstantis S, Kritsotakis EI, Gikas A. Treatment options for K. pneumoniae, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: an approach based on the mechanisms of resistance to carbapenems. Infection. 2020;48(6):835-851.
Crossref - Mayegowda SB, Ng M, Alghamdi S, Atwah B, Alhindi Z, Islam F. Role of antimicrobial drug in the development of potential therapeutics. Evid Based Complemen Alternat Med. 2022;2022(1):1-17.
Crossref - Singh S, Sharma A, Laxmi NV. Bacterial pathogens from lower respiratory tract infections: A study from Western Rajasthan. J Family Med Prim Care. 2020;9(3):1407-1412.
Crossref - Santella B, Serretiello E, De Filippis A, et al. Lower respiratory tract pathogens and their antimicrobial susceptibility pattern: a 5-year study. Antibiotics. 2021;10(7):851.
Crossref - Li Y, Kumar S, Zhang L, Wu H. Klebsiella pneumonia and its antibiotic resistance: a bibliometric analysis. BioMed Res Int. 2022;2022(1):1-10.
Crossref - Karruli A, Catalini C, D’Amore C, et al. Evidence-based treatment of Pseudomonas aeruginosa infections: a critical reappraisal. Antibiotics. 2023;12(2):399.
Crossref - Jadimurthy R, Mayegowda SB, Nayak SC, Mohan CD, Rangappa KS. Escaping mechanisms of ESKAPE pathogens from antibiotics and their targeting by natural compounds. Biotechnol Rep. 2022;34:e00728.
Crossref - Vazquez-Lopez R, Solano-Galvez SG, Vignon-Whaley JJJ, et al. Acinetobacter baumannii resistance: a real challenge for clinicians. Antibiotics. 2020;9(4):205.
Crossref - Manjula NG, Math GC, Patil A, Gaddad SM, Shivannavar CT. Incidence of urinary tract infections and its aetiological agents among pregnant women in karnataka region. Adv Microbiol. 2013;03(06):473-478.
Crossref - Promite S, Saha SK. Escherichia coli in respiratory tract infections: Evaluating antimicrobial resistance and prevalence of fimA, neuC and iutA virulence genes. Gene Rep. 2020;18:100576.
Crossref - Yan S, Sun Z, Yang Y, Zhu D, Chen Z. Changing antimicrobial resistance profile of Enterobacter spp. isolates in hospitals across China: a seven-year analysis from the CHINET antimicrobial resistance surveillance program (2015-2021). One Health Advances. 2024;2(1):11.
Crossref - Lima TB, Silva ON, De Almeida KC, et al. Antibiotic combinations for controlling colistin-resistant Enterobacter cloacae. J Antibiot. 2017;70(2):122-129.
Crossref - Gade ND, Qazi MS. Fluoroquinolone therapy in Staphylococcus aureus infections: where do we stand? J Lab Physicians. 2013;5(2):109-112.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.