ISSN: 0973-7510
E-ISSN: 2581-690X
This study was carried out to investigate the microbiological quality of cheddar cheese collected from markets and restaurants. A total of one hundred and fifty cheddar cheese samples were collected randomly in accordance with standard protocols from different markets and restaurants in Amman- Jordan. Pathogenic bacteria which include Salmonella, coliform bacteria, Staphylococcus aureus, and Listeria monocytogenes were considered as indicators of microbiological quality. All tests were performed according to official documented procedures. Listeria monocytogenes was found in 4% of market pre-sliced cheese and 8% pre-sliced restaurant samples. Packaged cheese samples that were sliced on request were free from Listeria monocytogenes. About 12% of pre-sliced restaurant cheese samples and about 8% of pre-sliced market samples were contaminated with S. aureus compared with only 4% of sliced on request cheese samples. Salmonella was not detected in packaged cheddar cheese that was sliced on request. However, about 10% of sliced cheddar cheese market samples and 8% of sliced cheddar cheese samples collected from restaurants were contaminated by Salmonella. About 10% of pre sliced market samples were polluted by coliform compared with 2% sliced on request cheddar cheese. The highest percentage of contaminated cheddar cheese by coliform was detected in restaurant samples (14%). The Jordanian food standards states that cheddar cheese samples must be free from pathogenic bacteria indicated above, therefore, a percentage of samples investigated herein are out of specifications. It is evident from the results presented above that the prevalence of different pathogenic organisms in the cheese samples studied was limited but mostly due to unhygienic handling of the product prior to presentation to the end customer.
Cheddar cheese, Contamination, Salmonella, Coliform, Staphylococcus, and L. monocytogenes.
Cheddar Cheese is made from pasteurized milk which leaves the product free from most pathogens. The typical characteristics of this cheese include; low pH, elevated salt concentration and low water activity (Pitt and Hocking, 1997). The finished products are usually packed as blocks, slices or shredded cheese. However, the components of cheddar cheese are expected to be hostile to microbial growth, proliferation of microorganisms can occur in and/ or on cheese during its ripening period or in the distribution chain even under refrigerated storage (Kwenda, 2015). The extent of microbial contamination of packaged processed cheese is affected by the preparation technology and the environment of storage (Oliveira et al, 2016). Type of spoilage microorganisms in dairy food differs widely because of the selective effects of practices followed in production, formulation, processing, packaging, storage, distribution, and handling (Ledenbach and Marshall,2009).
Pathogens such as coliform, Salmonella, Listeria monocytogenes and Staphylococcus aureus have been detected in cheese containing low and high moisture level as a result of poor pasteurization of milk. (U.S. FDA, 2003).
Cheddar cheese manufacturing includes pasteurization of milk and further processing using good manufacturing practices. Cullor (1997) suggested that if appropriate GMP conditions are observed while processing dairy products then occurrence and growth of pathogenic bacteria could be minimized. Hard and semi-soft cheese that developed during fermentation and aging create a hostile environment for bacterial pathogens (Bishop and Smukowski, 2006). Consumption of contaminated cheese with pathogenic microorganisms and their toxins can cause serious food borne problems to public health and big economic losses (Temelli, et al., 2006; Aygun, et al., 2005). Outbreaks of infections associated with the consumption of cheese were reported and the predominant organisms depicted in these infections included Salmonella, L. monocytogenes, verocytotoxin producing Escherichia coli (VTEC) and Staphylococcus aureus (Little, et al., 2008; Carvalho, et al., 2007).
Only few publications were released from Jordan concerning the microbial contamination of dairy products but none was related to cheddar cheese (Tahiri et al, 2008; Ibrahim et al, 1996). The objective of this paper was to investigate for the first time the microbiological quality of Cheddar cheese marketed in both Jordanian markets and restaurants.
Sample collection
A total of one hundred and fifty cheddar cheese samples (approximately 250g of each) were collected randomly from markets and restaurants in Amman. They included fifty already pre- sliced samples from market, 50 samples pre- sliced under request from market and 50 samples from restaurants. Each cheese sample was collected separately in a sterile plastic bag, preserved in an ice box then immediately delivered to the laboratory and refrigerated at 4oC. Analysis started after removal of samples from the refrigerator as follows: They were thawed at room temperature before 50 g from each sample were taken with a sterilized spatula, then transferred into sterile bag, diluted with 450 mL of 0.1% peptone water and finally homogenized in a stomacher for 2 min (BagMixer® 400 – Interscience International- France). Ten fold serial dilutions were prepared from the homogenized original dilution by transferring 1mL to a series of sterile test tubes containing 9 ml of 0.1% peptone water. These dilutions were used for the recovery of microorganisms given bellow.
Isolation of Listeria monocytogenes
Qualitative detection of L. monocytogenes from cheddar cheese samples was carried out through enrichment procedure as described in the U.S. Food and Drug Administration Bacteriological Analytical Manual (U.S., FDA, 2017). The media used included the followings: Fraser broth, Chromogenic Listeria agar, PALCAM agar, Trypose soya yeast extract agar, Trypose soya yeast extract broth and sheep blood agar. The count for this organism was performed by withdrawing 0.1 ml from the sample processed in the stomacher and plated on to Chromogenic Listeria agar. Colonies which appeared after incubation at 37oC for 48h were counted and expressed as count.
Coliform bacteria
Coliform count was determined by pour plate technique using violet red bile agar (VRBA) (Oxoid) as described in the International Commission on Microbiological Specifications for Foods (ICMSF, 2011) and it is summarized as follows: One ml from each of the previously prepared decimal dilutions was transferred aseptically into two sterile Petri dishes to which approximately 15 ml of sterile molten (45 ± 1oC) VRBA were added. Duplicate plates were run for each tested sample. After thorough mixing, the inoculated plates were allowed to solidify before being incubated at 35 – 37oC for 20 – 24 hours after which colony forming units were counted from plates containing 30-300 colonies. Confirmation of the identity of the isolates as coliform was achieved using a limited number of biochemical reactions such as Gram stain, indole production, methyl red reaction and voges- proskauer test.
Isolation of Salmonella
The isolation technique for Salmonella from samples was carried out in accordance with the guide lines indicated in the Food and Drug Administration Bacteriological Analytical Manual (FDA, 2012b) which involved: (1) Pre-enrichment for 24 hours using 10% pepton water (2) Selective enrichment on selenite cystine broth for 24h at 43oC to allow the growth of Salmonella whilst reducing the number of non-Salmonella organisms (3) Isolation of Salmonella on solidified media was achieved using selective agar plates (Desoxicholate citrate agar, Brilliant green agar and Bismuth sulphite agar). (4) Confirmation of Salmonella isolation was performed using the pattern of reaction in Triple sugar iron, Urease activity, lysine decarboxylation in addition to the examination for the following antigens: O antigen, VI antigen and H antigen. Antigenic reaction was done for confirmatory purpose and not to establish serotype as this is not a demand in the Jordanian Standards.
Staphylococcus aureus count
Staphylococcus aureus count was determined by spread plate technique using Baird- parker agar (Oxoid) as described in International Commission on Microbiological Specifications for Foods (ICMSF, 2011) and is summarized as follows: One ml from each of the previously prepared decimal dilutions was transferred aseptically over three sterile Petri dishes of Baird –parker agar. Plates were inverted and incubated at 35ºC for 48h before grown colonies were counted. Confirmatory tests included Gram reaction, catalase , coagulase test, anaerobic utilization of glucose and mannitol.
Pathogenic bacteria that included Salmonella, coliform, Staphylococcus, and L. monocytogenes were used in this study as indicators of hygienic quality and microbiological safety of cheddar cheese in both markets and restaurants. One hundred and fifty cheddar cheese samples were collected from nine markets as well as five restaurants in Amman area and were investigated for microbial contamination. The effect of handling of cheddar cheese on the microbial content of these products was also established. Figure 1 shows the colonial morphology of the 4 pathogenic bacteria isolated from the contaminated samples. The Jordanian food standards states that cheese samples must be free from the pathogenic bacteria studied. Therefore, samples that harbored these organisms in certain counts were considered out of specifications. It is important to note that 16 cheddar cheese samples collected were found to be contaminated (10.7%).
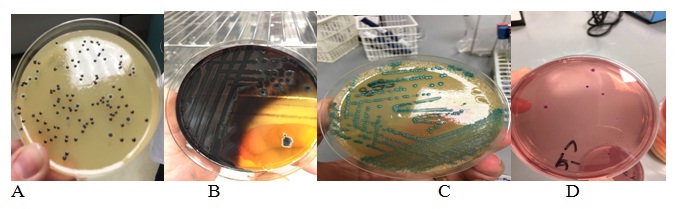
Fig. 1. Isolated Staphylococcus (A), Salmonella (B), L. monocytogenes (C), and coliform (D) that were isolated from contaminated cheddar cheese samples
In order to be fit for human consumption, cheese products must comply with specifications. The Jordanian specifications number 393/212 which is related to cheese products is shown in Table 1. It is important to note that total microbial count is not motioned as criterion for compliance and therefore, it is not given in this investigation. According to the above specification, the percentage occurrence of contaminated cheddar cheese samples by Salmonella, coliform, Staphylococcus, and L. monocytogenes collected from markets (pre- sliced and sliced on request) and restaurants (all were pre- sliced) is given in Table 2.
Table (1):
The Jordanian specification for Cheese products.
Total microbial count/ gram |
Not indicated |
|---|---|
Salmonella |
Absent |
L. monocytogenes |
Absent |
Coliform |
< 10/ gram |
S. aureus |
< 10/ gram |
Related food borne illnesses have been generally linked to soft cheese or cheese made from raw or unpasteurized milk, but rarely in hard cheese particularly cheddar. The reason for this is attributed to the low water activity which varies from 0.950 to 0.970 (CDC, 2013c). It is evident from Table 2 that the percentage occurrence of the out of specification samples was higher in those collected from restaurants.
Table (2):
Percentage occurrence of contaminated cheddar cheese samples collected from markets and restaurants.
| Bacterial species | Markets samples | Restaurants samples | |
|---|---|---|---|
| Pre- sliced cheddar cheese | Sliced on request cheddar cheese | All were pre sliced | |
| Salmonella | 10% | 0 | 8% |
| L. monocytogenes | 4% | 0 | 8% |
| Coliform | 10% | 2% | 14% |
| S. aureus | 8% | 4% | 12% |
Cheddar cheese available in the Jordanian market is all imported. When imported shipments are received, random samples are collected and sent to the laboratories of Jordan Food and drug administration. If analytical results indicate compliance with specification then product is allowed to be released into the market. Therefore, in this study it was prudently assumed that contamination detected must have been acquired in the market during transportation, storage and/ or handling. Several studies have examined the bacterial contamination that results from food handlers. Lambrechts et al., (2014) showed that hand hygiene may have serious implications for public health due to contamination of food from food handlers’ hands. Kamal et al., (2013) related the prevalence and counts of Staphylococcus aureus in samples of dairy products to neglected hygienic practices either in the production of raw milk and dairy products or in the personal hygiene.
Listeria monocytogenes
Listeria monocytogenes is a very serious food borne pathogen; cheddar cheese is susceptible to post pasteurization contamination and has been implicated in Listeria contamination and cases of listeriosis (Gianfranceschi, et al., 2006; Pagotto, et al., 2006; CDC, 2013a). The percentages of contaminated cheddar cheese samples from markets ( pre sliced and sliced on request) and restaurants samples by L. monocytogenes is shown in Table 2. L. monocytogenes presence in the collected samples was assessed quantitatively and qualitatively. The number of L. monocytogenes in contaminated cheese samples exceeded 10 CFU/g. L. monocytogenes was found in 4% of pre sliced cheese from markets and 8% pre sliced samples from restaurants. Packaged cheese samples that were sliced on request were free from L. monocytogenes. This observation is of interest as it indicates that handlers of cheese in these places maintain hygienic practices while slicing the cheese block in order to satisfy consumers.
Staphylococcus aureus
Food-borne illnesses related to S. aureus contamination of cheese have been attributed to the use of unpasteurized milk or to contamination due to improper handling. The presence of this organism at more than 5 Log CFU/mL produces heat-resistant enterotoxin (Ryser, 2001; Delbes, et al., 2006; CDC, 2010a). Intake of 20 to 1,000 ng of the enterotoxin can cause typical symptoms of S. aureus infection (Normanno, et al., 2007; Pelisser, et al., 2009).The percentages of contaminated cheddar cheese samples from markets (pre-sliced and sliced on request) and restaurants samples by S. aureus are seen in Table 2.
Cheddar cheese samples which were collected from both markets and restaurants were contaminated with S. aureus and were not in compliance with the Jordanian standards. About 12% of pre sliced restaurants cheese samples and about 8% of pre sliced market samples were contaminated with S. aurreus compared with only 4% of sliced on request cheese samples. The hard and extra-hard cheese types tested in Hungary (Varga, 2007) showed microbiological noncompliance rate of 33.3% and S. aureus was implicated as one of the contaminants of these products. This rate of noncompliance is far much higher than the one reported herein. On the other hand, Sospedra et al., (2009) tested 75 lots of dairy products collected from restaurants and could not detected S aureus in any of the samples collected.
Salmonella
Salmonella was not detected in packaged cheddar cheese that was sliced on request. However, about 10% of sliced cheddar cheese market samples and 8% of sliced cheddar cheese samples collected from restaurants were contaminated by Salmonella (Table 2). The antigenic reaction of the isolates with O, VI and H antigens was performed to confirm the identity of each isolate as Salmonella and not to serotype the isolates.
Salmonella is a well-recognized food-borne pathogen. All Salmonella strains were gastroenteritis-inducing pathogens (Ryser, 2001), and various Salmonella serotypes have been involved in cheese-borne outbreaks. Raw cheese was linked to outbreaks in different countries of the world including the United States of America (CDC, 2014c), but no fatalities were reported. Similar outbreak at a nationwide level was reported in France due to the consumption of goat cheese but again no fatalities occurred (Van, et al., 2009).
Coliform
Coliform bacteria are killed by pasteurization, therefore, their presence in cheese indicates post-pasteurization contamination (Hou, 2014). Presence of more than 10 detectable coliform colonies on the recovery medium rendered the product under test out of specifications. Coliform count was not given in the table because our interest was merely to establish the extent of compliance with specification. The relatively high numbers of contaminated restaurant samples by these bacteria are a reflection of the considerable amount of manual handling of cheese during food preparation. The percentages of contaminated cheddar cheese samples from markets ( pre-sliced and sliced on request) and (restaurants samples) by coliform are shown in Table 2. About 10% of pre-sliced market samples were polluted by coliform compared with 2% sliced on request cheddar cheese. The highest percentage of contaminated cheddar cheese by coliform was detected in restaurant samples (14%).
Coliforms refer to those members of the Enterobacteriaceae that ferment lactose such as Escherichia, Klebsiella and Enterobacter species (Quinn, et al., 2002). Coliforms are normal flora of the gastrointestinal tract which invade sterile parts of the body after consumption of defective foods and cause serious infections which are said to be “opportunistic pathogens” (Sharon, et al., 1994).
Presence of coliform bacteria in cheese made up of pasteurized milk indicates post pasteurization contamination. Many authors have reported the presence of these bacteria in marketed cheese. Asamenew et al., (2012) isolated coliform from 27.8% Ethiopian cottage cheese whereas Adenike et al., (2008) indicated 35% prevalence rate of coliforms from the samples they studied. The rate of coliform recovery established in this work was 8.7% which was a lot lower than those reported by others.
As discussed earlier, cheddar cheese blocks in the Jordanian market are assumed to be free from microbial contamination and this assumption was further confirmed from the files of the Jordan FDA laboratories. After the release of cheddar blocks from the customs, contamination may occur during transport, storage or handling. The first one is not likely to cause contamination as blocks are tightly wrapped and transportation is carried out in cooled vehicles. Storage is done in refrigerators while blocks are wrapped and thus contamination is unlikely to occur at this stage. Once blocks are opened, contamination is most likely. Thus, in this investigation, the source of contamination was attributed to handling rather than transport or storage. This conclusion is consistent with that made by Irkin (2010).
It is evident that a certain degree of association between contaminant. Whenever a sample was found to be out of microbiological limits, all of the assayed bacteria were in most cases isolated. Person correlation analysis was performed (though results are not shown) indicated a significant correlation (p<0.05) between the presence of Salmonella, coliform, Staphylococcus, and L. monocytogenes in contaminated cheddar cheese samples.
This investigation is to the best of our knowledge is the first to be published from Jordan to deal with the microbiological contamination of cheddar cheese in markets and restaurants. It is evident from the results presented above that the prevalence of different pathogenic organisms in the studied cheese samples were limited, but mostly due to un-hygienic handling of the product prior to presentation to the end customer.
- Adenike, A., O. Ogunshe and Kehinde, O. Microbial loads incidence of food- borne indicator bacteria in most popular indigenous fermented food contaminates from middle belt and southwestern Nigeria. African Journal Microbiology Research., 2008; 2: 332-339.
- Al-Tahiri, R., Omar, S., Rewashdeh, A. A study of the occurrence of Listeria species in raw sheep milk. International Journal of Dairy Technology., 2008; 61: 347–51.
- Asamenew, T.M., Mahendra, P., and Addisalem, H. Bacteriological Study on Coliform Organisms from Ethiopian Traditional Cheese West Showa Zone, Ethiopia. International Journal of Microbiological Research., 2012; 3(3); 188-191.
- Aygun, O., Aslantas, O., Oner, S. A survey on the microbiological quality of Carra, a traditional Turkish cheese. Journal Food Engneering, 2005; 66: 401–404.
- Bishop, J. R., and Smukowski, M. Storage temperatures necessary to maintain cheese safety. Food Protection Trends., 2006; 26: 714–724.
- Carvalho, J.D.G., Viotto, W.H., Kuaye, A.Y. The quality of Minas Frescal cheese produced by different technological processes. Food Contamination., 2007; 18: 262–267.
- CDC (Centers for Disease Control and Prevention). Staphylococcal food poisoning. http://www.cdc.gov/nczved/ divisions/dfbmd/diseases/staphylococcal/ Accessed January 6, 2015.
- CDC (Centers for Disease Control and Prevention). (2013a). Listeria (Listeriosis). http://www.cdc.gov/listeria/risk.html Accessed January 12, 2015.
- CDC (Centers for Disease Control and Prevention). (2013c). Listeria and food. http://www.cdc.gov/foodsafety/specificfoods/ listeria-and-food.html Accessed January 21, 2015.
- CDC (Centers for Disease Control and Prevention). (2014c). Multistate outbreak of Salmonella Stanley Infections linked to raw Cashew cheese (final update). http://www.cdc.gov/ salmonella/stanley-01-14/ Accessed January 8, 2015.
- Culler, J.S. HACCP ( Hazard Analysis Critical Control Points): is it coming to the dairy?. Journal of Dairy Science., 1997; 80(12): 3449-3452
- Delbes, C. J., Alomar, N., Chougui, J., Martin, F. and Montel, M. C. Staphylococcus aureus growth and enterotoxin production during the manufacture of uncooked, semihard cheese from cows’ raw milk. Journal Food Protection., 2006; 69: 2161-2167.
- FDA (Food and Drug Administration).( 2012b). Bad Bug Book: Handbook of foodborne pathogenic microorganisms and natural toxins. http://www.fda.gov/downloads/Food/FoodborneIllnessContaminants/
UCM297627.pdf Accessed January 12, 2015. - FDA/USDA (Food and Drug Administration/U.S. Department of Agriculture. (2017). Microbiological Methods & Bacteriological Analytical Manual (BAM). http://www.fda.gov/downloads/Food/FoodScienceResearch/labolatoriesmethods/
ucm2006949.htm. Pdf . Accessed January 5, 2015. - Gianfranceschi, M., M. C., D’Ottavio, A., Gattuso, M., Pourshaban, I., Bertoletti, R., Bignazzi, P., Manzoni, M., Marchetti, and Aureli, P . (2006). Listeriosis associated with Gorgonzola (Italian blue-veined cheese). Foodborne Pathogen Distribution. 3,190–195.
- Hou, J., Hannon, J. A., McSweeney, P. L. H., Beresford, T. P., and Guinee, T. P. Effect of curd washing on cheese proteolysis, texture, volatile compounds, and sensory grading in full fat Cheddar cheese. International Dairy Journal., 2014; 34: 190–198.
- Ibrahim, S. A., Khatib, K. A. A., Al-Haik, S. A., & Dagri, M. H. Labneh (Concentrated Yogurt): a traditional fermented dairy product in Jordan. Dairy, Food and Environmental Sanitation., 1996b; 16: 296-298.
- International Commission on Microbiological Specifications for Foods (ICMSF). Microorganisms in Foods (8): Their Significance and Methods of Enumeration, (1nd edition). University of Toronto Press, Toronto, Buffalo, London 2011.
- Irkin, R. Determination of microbial contamination sources for use in quality management of cheese industry: ‘‘Dil’’ cheese as an example. Journal Verbr. Lebensm., 2010; 5: 91–96.
- Kamal, R. M.; Bayomi, M.; Abd El Aal, S. MRSA detection in raw milk, some dairy products and hands of dairy workers in Egypt, a mini- survey. Food Control., 2013; 33: 49-53.
- Kwenda, A. An Investigation on the Causes of Escherichia Coli and Coliform Contamination of Cheddar Cheese and How to Reduce the Problem (A Case Study at Kefalos Cheese Products). Global Journal of Science Frontier Research: E Interdiciplinary, 2015; 15(2), 7-30.
- Lambrechts, A. A., Human, I.S., Doughari, J.H., and Lues J. F. R. Bacterial contamination of the hands of food handlers as indicator of hand washing efficacy in some convenient food industries in South Africa. Pakistan Journal Medical Sciences., 2014; 30(4): 755–758.
- Ledenbach, L. H. and Marchall, R.T. Microbiological spoilage of dairy products. Food Microbiology and Food Safety, 2009; pp. 41-67.
- Little, C., L., Rhoades, J., R., Sagoo, S., K., Haris, J., Greenwood, M., Mithani, V., Grant, K., McLauchlin, J. Microbiological quality of retail cheeses made from raw, thermized or pasteurized milk in the UK. Food Microbiology., 2008; 25: 304–312.
- Oliveira, R. B. A.; Margalho, L. P.;Nascimen, J. S.; Costa, L. E. O.; Portela, J. B.; Cruz, A. G.; Santana, A. S. Processed cheese contamination by spore-forming bacteria: A review of sources, routes, fate during processing and control. Trends in Food Science & Technology., 2016; 57: 11-19.
- Normanno, G., La Salandra, G., Dambrosio, A., Quaglia, N. C. , Corrente, M., Parisi, A., Santagada, G., Firinu, A., Crisetti, E., and Celano, G. V. Occurrence, characterization and antimicrobial resistance of enterotoxigenic Staphylococcus aureus isolated from meat and dairy products. International Journal Food Microbiology., 2007; 115: 290-296.
- Pagotto, F., L. K. Ng., Clark, C. , and Farber. J., and Canadian Public Health Laboratory Network. Canadian listeriosis reference service. Foodborne Pathogen Distribution., 2006; 3: 132–137.
- Pelisser, M. R., Klein, C. S. , Ascoli, K. R. , Zotti, T. R. , and Arisi, A. C. Occurrence of Staphylococcus aureus and multiplex PCR detection of classic enterotoxin genes in cheese and meat products. Brazalian Journal Microbiology., 2009; 40: 145-148.
- Pitt, J., I., and Hocking, A., D. Fungi and Food Spoilage. London: Blackie Academic and Professional 1997.
- Quinn, P.J., Carter, M.E., Markey, B. , and Carter, G.R. , Survey of retail. Enterobacteriaceae. In: Clinical Veterinary Microbiology, 8 ed. Wolf Pub. Spain, 2002; pp: 209-236.
- Ryser, E. T. Public Health Concerns. In: Applied dairy microbiology, 2nd ed. (Eds. E. H. Marth and J. L. Steele). Marcel Dekker, Inc., New York, NY, USA., 2001; pp. 397-546.
- Sharon, S., Sherril, R., Louise, D., and Amy, M. Microbiol., 25: 131-134. Pathogenic and clinical microbiology. A laboratory. 1 ed. Boston, New York, Toronto, London, 1994; pp: 71-107.
- Sospedra, I., Rubert, J. V., Soler, C., Soriano, J. M., Mañes, J. Microbial contamination of milk and dairy products from restaurants in Spain. Foodborne Pathogens and Disease., 2009; 6(10):1269-72.
- Temell, S., Anar, S., Sen, C., Akyuva, P. Determination of microbiological contamination sources during Turkish white cheese production. Food Contamination., 2006; 17: 856–861.
- U.S. Food and Drug Administration /Center for Food Safety and Applied Nutrition, U.S. Department of Agriculture /Food Safety and Inspection Service, and Centers for Disease Control and Prevention .(2003). Quantitative assessment of relative risk to public health from foodborne Listeria monocytogenes among selected categories of ready–to-eat foods. Available at: http://www.foodsafety.gov/;dms/ lmr2-toc.html. Accessed 18 March 2004.
- Van Cauteren, D., N., Jourdan-da Silva, F. X., Weill, L., King, A., Brisabois, G., Delmas, V., Vaillant, and de Valk, H. (2009). Outbreak of Salmonella Enterica serotype Muenster infections associated with goat’s cheese, France March 2008. Euro Surveill. 14:pii=19290.
- Varga, L. Microbiological quality of commercial dairy products. In: Communicating Current Research and Educational Topics and Trends in Applied Microbiology. 2007; Pp: 487- 494.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


