ISSN: 0973-7510
E-ISSN: 2581-690X
The current work was done to analyse the bioremediation and plant growth promotion (PGP) traits of endophytic bacteria isolated from Alternanthera philoxeroides from Bellandur Lake, Bangalore, India. Twenty-nine endophytic bacteria were isolated and tested for their PGP traits like indole acetic acid (IAA), ammonia, nitrogen fixation, 1-aminocyclopropane-1- carboxylate (ACC) deaminase production, phosphate solubilization etc. Endophytic bacterium, BEBAphL1 obtained from leaves of A. philoxeroides exhibited significant plant growth promotion properties and the isolate was identified as Bacillus velezensis OQ874364 using 16S rRNA sequencing. The bacterium showed potential IAA, ammonia production, nitrogen fixation, phosphate solubilization, and ACC deaminase production. The results indicate that this endophyte is promising as a growth-promoting inoculant, reducing the reliance on chemical inputs in conventional agricultural practices while enhancing nutrient uptake and stress resilience in plants. B. velezensis exhibited tolerance to high levels of chromium (500 mg/L) and NaCl (15%) and was also able to decolourize Congo red by 70% at 0.005% concentration of dye. Characterization of dye samples pre- and post-bacterial treatment was done using Fourier-transform infrared spectroscopy (FTIR) and Gas Chromatography-Mass Spectrometry (GC-MS) analysis. The findings of the study indicate that B. velezensis shows promise as a plant growth stimulator capable of withstanding heavy metal exposure and breaking down dyes.
Endophyte, Bellandur Lake, Congo Red, Chromium Tolerance, IAA production
Endophytes are microorganisms that reside inside plants, where they establish a symbiotic relationship with their hosts. They do not induce pathogenicity in host plants, and in return, plants provide nutrients to help them grow and survive.1 Endophytic bacteria can reduce the toxicity and evapotranspiration of pollutants by breaking them down. Endophytic bacteria have been found to exhibit good efficacy in breaking down pollutants within plants.2,3 In addition, they show plant growth-enhancing properties like production of IAA, siderophore, ammonia, nitrogen fixation, ACC deaminase, potassium solubilization, phosphate solubilization, etc. which further benefits bacterial-assisted plant growth promotion.4,5
The industrial revolution has led to significant advancements in meeting the needs of the growing population. The rapid growth of textile industries and the widespread use of artificial dyes have caused a concerning issue: the release of wastewater containing these dyes, leading to pollution. This pollution, arising from the discharge of dye-laden wastewater, has become a significant concern.6 Biological, physical and chemical techniques were used to eliminate dyes. Researchers have shown a great interest in biological methods for removing dyes, which are sustainable and economically viable. These methods encompass the utilization of microbial biomass for microbial cultivation, the adsorption of dyes by either living or non-living biomass, and enzymatic degradation. Fungi and bacteria are the most commonly studied microbes for dye removal because of their widespread occurrence and ability to adapt to different environments.7,8 Microorganisms and their enzymes have the ability to eliminate significant constituents like phenolics and aromatic amines found in dyes.9 Different types of microorganisms, including Bacillus, Enterobacter, Candida and Aspergillus etc. have been identified as capable of biologically removing colour from textile dyes. Bacillus sp., in particular, is well-known for its capacity to effectively decolorize dyes, especially those of the azo variety.10 The azoreductase enzyme derived from Bacillus velezensis has exhibited its ability to decolorize various azo dyes with different chemical structures, including Congo red, methyl orange, methyl red, and crystal violet. This enzyme shows promising potential in efficiently removing colour from a wide range of azo dyes.11 Bacteria isolated from locations contaminated with dyes have been found to be valuable resources for degradation, as indicated by various studies. Studies focused on phytoremediation have explored its application in treating wastewater from the textile industry. These studies have highlighted the significance of utilizing plants harbouring the highest number of endophytes, as they have the potential to enhance the level of degradation achieved.12 Naturally occurring heavy metals like zinc, copper, molybdenum, manganese, cobalt, and nickel play crucial roles in various biological processes. However, excessive levels of these elements, when combined with other harmful metals such as arsenic, lead, chromium, cadmium etc., can negatively impact plants and animals.13 Endophytic bacteria are studied their potential role in enhancing heavy metal tolerance in crops. By harnessing the beneficial properties of endophytic bacteria, it is possible to develop sustainable strategies for improving crop performance under heavy metal stress.14 To our current understanding, our research stands as a pioneering achievement in the isolation of endophytic Bacillus velezensis strains that possess remarkable traits such as promoting plant growth, tolerating high salt concentrations, exhibiting resistance to heavy metals, and displaying the ability to decolorize dyes.
Bellandur Lake, which is the biggest man-made water body in Bangalore City spanning over an area of 367 hectares, has been adversely affected by the discharge of untreated or poorly treated domestic and industrial wastewater from its surrounding areas. As a consequence, the water quality of this Lake has been severely impacted, posing a significant threat to the ecosystem and the human population residing in the vicinity.15 Alternanthera philoxeroides is one of the abundant plant species found in Bellandur Lake and recognised as invasive in the European Union Concern (EU Regulation 1143/2014).16 Currently, there is limited understanding regarding endophytic bacterial isolates present in A. philoxeroides plants. It is uncertain whether the endophytic bacteria found in A. philoxeroides, can promote plant growth as well as be utilized to facilitate the heavy metal absorbance and degradation of dyes. Further research is essential to explore this topic. Our current objective was to isolate endophytic bacteria from A. philoxeroides growing in contaminated Bellandur Lake and to determine their bioremediation and PGP properties.
Chemicals and Reagents
Sodium hypochlorite, Tween 80, tryptophan, nutrient broth, ammonium sulphate, Congo red, gelatin, IAA and Chromium sulphate were bought from HIMEDIA (India). All reagents like Salkowski reagent, Kovac’s reagent, Nessler’s reagent and HPLC grade solvents like ethyl acetate were purchased from HIMEDIA (India). Other chemicals and reagents used in this study were also bought from HIMEDIA (India). All reagents and chemicals used in this study were of analytical grade. For spectrometric readings, Shimadzu UV-1800UV/Visible Scanning Spectrophotometer (India) was used.
Isolation of Endophytic Bacteria
Aquatic plants were obtained from Bellandur Lake, Bangalore, India (Lat 12° 56’24.4824″ Long 77° 40’30.9144″), which is one of the largest and most polluted lakes in the region. The most abundant plant species found in the lake, Alternanthera philoxeroides (Mart.) Griseb, was selected for the isolation of endophytes (Figure 1). The plant parts were washed with running tap water, treated with Tween 80 for five minutes, and rinsed again using sterile distilled water. Surface sterilization of the plant parts was done inside a Laminar Air Flow (LAF) chamber. The plant samples were then sliced into small 1 to 2 mm pieces and immersed in ethanol (70%) for 30 seconds.17 Following several rinses with sterile water, the plant samples underwent a one-minute treatment with sodium hypochlorite containing 1% chlorine. After another round of washing with sterile water, the final rinse water, measuring 100 µl, was spread evenly onto a nutrient agar (beef extract 3 g, yeast extract 3 g, peptone 5 g, NaCl 5 g, agar 15 g, and dH2O 1 L with pH 7.0) as control.17 The sterilized plant parts were blot dried and inoculated onto nutrient agar, LB agar, and King’s B agar plates. The plates were incubated (37°C) for a week and examined for the growth of bacterial colonies. Unique colonies were chosen and purified for additional research.
Biochemical and Molecular Characterization of Endophytic Bacteria
The isolates were subjected to morphological characterization, and different biochemical tests, like Indole, Methyl Red, Citrate and Voges Proskauer tests, were conducted, as well as Gram staining and motility experiments. The endophytic bacterium that exhibited the most promising plant growth promotion and bioremediation properties was selected for molecular identification. In order to confirm the purity of the isolated DNA, agarose gel (1%) was used to evaluate it. The amplification of the 16S rRNA gene fragment was accomplished by utilizing 16S rRNA-F and R primers. The forward and reverse primers were utilized for sequencing reaction using the BDT v3.1 cycle sequencing kit on the ABI 3720xl Genetic Analyzer. The resulting consensus sequence was analysed through the NCBI GenBank database using BLAST analysis, based on the maximal identity score, and the initial ten sequences were aligned using ClustalW (http://www.clustal.org/clustal2),a software program for multiple sequence alignment. Distance matrix and phylogenetic tree were created using MEGA 10 (www.megasoftware.net). The accession number was obtained by submitting the sequence information to the NCBI database.18,19
Estimation of Ammonia and Indole Acetic Acid (IAA) Production
The isolated endophytic bacteria were analysed for the production of IAA on a qualitative and quantitative basis. The cultures were inoculated into nutrient broth enriched with tryptophan (0.5% w/v) and incubated at 37°C for 48 h. The cultures were centrifuged at 8000 rpm for 15 min. Salkowski reagent (0.5 M FeCl3:dH2O: H2SO4 in 1:50:30 ratio) (2 mL) was added to 1 mL supernatant. Positive isolates showed red colour formation and the quantity of IAA was assessed by measuring and estimating the absorbance of the sample at 530 nm by using a Shimadzu UV-Visible Spectrophotometer.20,21
Ammonia production by endophytes was determined using Nessler’s reagent test. The samples were grown in nutrient broth at a temperature of 37°C and 120 rpm. The cultures then underwent centrifugation at 10,000 rpm for 10 minutes. The supernatant was taken and treated with 0.5 mL Nessler’s reagent, followed by observation for the development of a yellowish-brown colour. Quantitative determination of ammonia production was estimated using a Shimadzu UV-Visible spectrophotometer at 430 nm.22
Estimation of Nitrogen Fixation, Phosphate and Potassium Solubilization by Endophytic Bacteria
The potential of isolates to solubilize phosphate was checked based on a modified method23. The endophytic isolates were inoculated to Pikovskaya medium (yeast extract 0.5 g, Ca3(PO4)2 5 g, dextrose 10 g, (NH4)2SO4 0.5 g, MgSO4.7H2O 0.1 g, KCl 0.2 g, FeSO4.7H2O 0.00001 g, MnSO4 0.0001 g, agar 15 g, dH2O 1 L; pH 7.0 containing 1mL 0.5% bromophenol blue). Incubation was done at 37°C for two days, and then checked for the formation of a yellow halo zone surrounding the colonies. This indicated that the phosphate had been solubilized. The Edi-Premono formula 24 was used to calculate the solubilization index.
Phosphate Solubilization Index (SI) = (Colony diameter + Halo zone diameter) / Colony diameter
Bacterial endophytes were inoculated onto Jensen’s medium to check nitrogen fixing ability.25 Isolates were incubated for seven d at 37 ! and observed for bacterial growth which was interpreted as a positive result. Solubilization of potassium by endophytic bacteria were tested in vitro using Aleksandrov media.26 Endophytes were inoculated to Aleksandrov media (glucose 5 g, FeCl3 0.1 g, MgSO4.7H2O 0.005 g, CaCO3 2 g, Ca3(PO4)2 2 g, mica powder 3 g, agar 20 g, dH2O 1 L, pH 7.2) and incubated for three to five days at 37 °C. The clearzone around the colonies was confirmed as positive for potassium solubilization.
Estimation of ACC Deaminase and Siderophore Production
ACC deaminase production by bacterial endophytes were screened in vitro using DF minimal salt media (KH2PO4 4 g, FeSO4.7H2O 0.1 g, Na2HPO4 6 g, MgSO4.7H2O 0.2 g, MnSO4 10 µg, H3BO3 10 µg, CuSO4 50 µg, ZnSO4 70 µg, gluconic acid 2 g, MoO3 10 µg, Glucose 2 g, citric acid 2 g, agar 12 g, d H2O 1 L).27 The media was supplemented with ammonium sulphate (2 g/L).28 The isolates were incubated at 37°C for 3 d, and the growth of bacteria were considered as a positive result.
Siderophore production by isolates was analysed using a modified method.29 The isolates were inoculated into nutrient broth and subjected to incubation at 37°C for 24 h, after which the culture underwent centrifugation at 10,000 rpm for 10 min. After adding 2 mL of 2% (w/v) FeCl3 to 5 mL of the supernatant, the solution was observed for the appearance of a red or purple colour, which was considered indicative of a positive result.
Screening of Extracellular Enzyme Production by Endophytic Bacteria
Protease production by endophytic isolates was screened using skim milk agar media (skim milk powder 28 g, tryptone 5 g, yeast extract 2.5 g, glucose 1 g, agar 18 g, dH2O 1 L, pH 7.2). Bacteria was inoculated to this media and incubated at 37°C for 48 h. The halozone around the colonies was considered as a positive result.30 Tributyrin media (yeast extract 3 g, peptone 5 g, tributyrin oil 4 mL, agar 18 g, dH2O 1 L, pH 6.8) was used to screen lipase producing bacterial isolates. Following incubation of the plates at 37°C for 48 h, a positive result was determined by the observation of a halo zone surrounding the colony.30
A modified medium was employed to assess the cellulose production capability of endophytic bacteria.31 The isolates were inoculated onto cellulose Congo red media, which consists of cellulose 2 g, MgSO4.7H2O 0.25 g, KH2PO4 0.5 g, Congo red 0.2 g, gelatin 2 g, agar 15 g, dH2O 1 L, pH 6.8. Subsequently, the media was incubated at 37 °C for 48 h, and identifying a clear zone encircling the colonies confirmed a positive result. Starch agar media (peptone 20 g, starch 20 g, agar 20 g, dH2O 1L, pH 6.8) was used to analyse the production of amylase by endophytes. The media was incubated at 37°C for 48 h, and after bacterial growth, 1% (v/v) Gram’s iodine was added to flood the colonies. A positive outcome was inferred from the existence of a distinct halo zone surrounding the colonies.32
Enzymatic index (EI) was calculated for these isolates.33
EI = (Halo zone diameter / Colony diameter (cm))
Halotolerance Properties of Endophytic Isolate
To assess the capacity of bacterial isolates to endure elevated salt levels, spot inoculation was conducted on nutrient agar plates supplemented with NaCl (5%, 7%, 10%, 12%, 15%, and 20% (w/v)). The media plates were incubated at 37°C for 5 d, and the growth of bacteria was observed every 24 h.34
Heavy Metal Tolerance of Endophytic Bacteria
Bioremediation properties of the best PGP bacterium were determined. The tolerance of the bacterium to chromium was evaluated using the agar-dilution method. Nutrient agar medium was supplemented with varying concentrations of chromium, spanning from 100 to 3000 mg/L. After autoclaving and cooling the media metal salts (Cr2(SO4)3) were added. The media was inoculated and cultured at 37°C for 48 h. Observation of bacterial growth was conducted to identify the metal concentration that completely halted the growth, which was subsequently designated as the Minimum Inhibitory Concentration (MIC).35
Estimation of Dye Degradation Potential of Endophytic Bacterium
The initial screening of the endophytic isolate’s dye degradation potential was conducted by spot-inoculating the bacterium on MSM agar plates containing different azo dyes. MSM media was composed of KH2PO4 4 g, K2HPO4 5 g, CaCl2·2H2O 0.5 g, MgSO4·7H2O 0.2 g, NH4Cl 0.4 g, dH2O 1 L, pH 7.2. The degradation potential of the isolated bacterium was evaluated against three azo dyes, namely Congo red (CR), crystal violet (CV), and reactive red (RR), at different concentrations (0.005, 0.01, 0.05 g /100 mL). The inoculated media was incubated at 37°C for 48 h and observed for the presence of a decolorization zone.36,37
Decolorization of CR Dye with Isolated Bacterium
To determine the extent of decolorization of CR by the isolated bacterium, a 1% overnight culture was inoculated into a medium composed of yeast extract 10 g and NaCl 5 g, along with different concentrations of the dye (0.005%, 0.01%, and 0.05%). Subsequently, the medium that had been inoculated was kept at incubator at 37°C and 120 rpm 24 to 48 h. Following incubation, the bacterial culture was centrifuged at 8000 rpm for 10 minutes, and the resultant supernatant was taken. The decolorization of the dye in the supernatant was assessed by measuring its absorbance at 497 nm using a Shimadzu UV-visible spectrophotometer. The percentage of dye decolourization was detected using the following equation.
Decolourization (%) = [ R1 – R2 / R1 ] x 100
where R1 is the initial absorbance, R2 is the final absorbance of CR dye decolorization.38
Characterization of treated and untreated dye samples: GC-MS and FTIR Analysis
Samples of untreated and bacterial-treated CR dye were obtained and centrifuged at 8000 rpm for 10 min sat 4°C.39 Treated samples are samples that consist of 0.005% Congo red and 1% bacterial culture which are then incubated for 48 h. Untreated samples do not contain bacterial culture. The obtained supernatant was utilized to extract the compounds from the samples using a liquid-liquid extraction technique, involving the use of an equal quantity of ethyl acetate and the sample. 39 The extract was evaporated to dryness and used for FT-IR whereas 5 µL extract was taken for GC-MS analysis.
GC-MS Analysis
The analysis conducted using the Clarus 680 GC involved a fused silica column packed with Elite-5MS, which is a mixture of 5% biphenyl and 95% dimethylpolysiloxane, and had dimensions of 30 m x 0.25 mm ID x 250µm df. A consistent flow rate of 1 mL/min of helium gas was employed as the carrier gas. Throughout the chromatographic process, the temperature of the injector was adjusted to 260°C, and a 1µL sample extracted was injected into the instrument. The temperature settings for the oven were programmed in the following manner: an initial temperature of 60°C was maintained for 2 min, after which it was increased at a rate of 10°C per minute until reaching 300°C. This temperature was then maintained for 6 min. The mass detector was utilized with specific temperature settings, including a transfer line and ion source temperatures of 240°C each. The electron impact ionization mode was employed with an energy of 70 eV. During the analysis, the scan time for each measurement was set to 0.2 seconds, with a scan interval of 0.1 sec. The mass fragments were recorded within the range of 40 to 600 Da. The obtained spectra were compared with the database of compounds saved in the GC-MS NIST (2008) library.
Fourier-transform infrared spectroscopy (FTIR) Analysis
The structural and functional groups in the degraded dye sample were analysed using FTIR-ATR spectra (ThermoScientific Nicolet iS50 spectrometer). The spectral data was captured within a range of wavelength of 400 to 4000 cm-1 with a resolution of 8 cm-1
Statistical Analysis
All tests were performed in triplicates, and the average value and standard deviation were calculated using MS Excel for data analysis.
Isolation of Endophytic Bacteria
In our study, we isolated bacterial endophytes from different plant parts of Alternanthera philoxeroides (Mart.) Griseb after surface sterilization. We used three media, including nutrient agar, LB agar, and King’s B agar for isolation. We have successfully obtained 29 bacterial endophytes with unique morphological features from leaves, shoots, and roots of A. philoxeroides. BEBAphL1 exhibited noteworthy abilities in promoting plant growth and bioremediation. Thus, we have discussed the test outcomes of this bacterial endophyte. BEBAphL1 was isolated from leaves of A. philoxeroides. The sampling site is shown in Figure 1. In a study, endophytic bacteria were isolated from specimens of Alternanthera philoxeroides that were grown in soil containing hydrocarbons. The bacteria were found to thrive in those conditions and could potentially be used for bioremediation.40 In another study, fungal endophytes were isolated from the same plant species that produced laccase enzymes.41
Figure 1. Sampling site for endophytic bacterial isolation from Bellandur Lake, Bangalore, India (Lat 12֯ 56’24.4824” Long 77֯ 40’30.9144”)
Biochemical and Molecular Characterization of Endophytic Isolate
Colony morphology and biochemical test results of the endophytic isolate were checked (Figure 2). The isolated bacterial colony was white in colour, medium sized and had mucoid consistency. The colony was circular with an entire margin and had umbonate elevation. The isolate was Gram-positive, rod-shaped and motile. Negative results were shown for indole and citrate tests. Voges Proskauer and Methyl red tests were negative.
Figure 2. Isolation of bacterial endophyte from Alternanthera philoxeroides (a) Bacterial growth from leaves of Alternanthera philoxeroides in nutrient agar media (b) pure colony isolation using quadrant streak method (c) Gram stain test of the isolated bacterial endophyte
The potential plant growth promoter with bioremediation properties has been identified through 16S rRNA sequencing, and the sequence data was deposited in Genbank (Figure 3). Based on BLAST analysis, BEBAphL1 showed a 99.64% sequence similarity to Bacillus velezensis strain FZB42 (NR_075005.2). The Genbank accession number for the sequenced isolate is OQ874364.
Figure 3. (a) Species identification of isolated endophytic bacteria BEBAphL1 isolated from leaves of Alternanthera philoxeroides: Genomic DNA isolation and comparison with DNA ladder (b) Phylogenetic tree of the isolated endophyte bacteria
IAA and Ammonia Production
The production of IAA is a significant characteristic of rhizospheric and endophytic bacteria, which play a crucial role in promoting and facilitating plant growth.42 IAA production of endophytic bacterium B. velezensis was 30.42±2.09 µg/mL in tryptophan-containing media. The plant growth promotion properties of endophytic Bacillus velezensis Lle-9, which was obtained from Liliumleucanthum, were assessed, and the bacterium produced IAA. Additionally, tryptophan present in the medium resulted in an increased rate of IAA production.43 Endophytic Bacillus velezensis D4 isolated from branches of apple and pear showed plant growth promotion properties but IAA production was not present in that particular strain.44 Endophyte B. velezensis was screened for its ability to produce ammonia, an essential metabolite that assists in meeting the nitrogen requirements of plants. This was done by adding Nessler’s reagent to peptone water to detect ammonia production activity.45 Quantitative estimation of ammonia produced was estimated based on the ammonium sulphate standard. It was found to be 0.89±0.08 µ/mol.A study was conducted to determine the potential of B. velezensis BACO3 to enhance plant growth and it was discovered to have the ability to generate ammonia. The production of ammonia is connected to the accumulation of nitrogen, elongation of plant roots, and increased biomass production.46
Nitrogen Fixation, Phosphate and Potassium Solubilization by Endophyte
Nitrogen is a vital component of nucleic acids, enzymes, proteins, and chlorophyll that enables photosynthesis in plants. Although nitrogen is present in large quantities in the Earth’s atmosphere it exists mainly in the inert form of dinitrogen (N2), which cannot be utilized by plants. Nitrogen fixation is the process by which Nitrogen gas is transformed into these usable forms, making it a crucial process for the survival of life on Earth. Few endophytic bacteria possess nitrogen-fixing abilities, which potentially promote the growth of plants. The nitrogen fixation ability of B. velezensis was assessed using Jensen’s media. The bacterium exhibited growth in this media, indicating a positive result for nitrogen fixation. B.velezensis strain OEE1, which was obtained from olive trees, was evaluated for its nitrogen fixation capacity in Jensen’s media that lacked nitrogen, and was observed to yield a positive result.47 Bacillus velezensis SR18 isolated from rhizospheric soils of Indo Gangetic plains (IGPs) showed a phosphate solubilization index of 8.5.48
The process of inorganic phosphate solubilization by bacteria and fungi associated with plants is a vital aspect of plant growth promotion.49 Our research involved examining the ability of endophytic B. velezensis to solubilize phosphate. The results showed that it had a phosphate solubilization index of 1.75±0.05. The plant growth promotion properties of Bacillus velezensis GL5, isolated from Gnetumgnemon L, was evaluated including its ability to solubilize phosphate. However, it showed negative results for phosphate solubilization.50
Potassium fertilizers make up 18% of the total consumption of fertilizers which contain nitrogen, phosphorus, and potassium (N, P, and K) worldwide. Although the soil contains substantial amounts of potassium (K), only a small portion of it is actually accessible for plants to absorb.51 It was found that the B. velezensis strain isolated in our study did not possess the capacity to solubilize potassium after testing it with Alexandrov’s media. Earlier research has also tried to depict the potassium solubilization capability of the endophytic strain Bacillus velezensis ERBS51. However, the outcome of the experiment showed that the strain could not solubilize potassium which was similar to our results.52
ACC Deaminase and Siderophore Production
ACC deaminase increases plant growth by decreasing levels of ethylene in plants. The enzyme converts ACC, a precursor involved in the synthesis of plant ethylene, into ammonia and a-ketobutyrate.53 Reduction in ethylene and ACC promotes root growth and development. Although recent research have suggested the possibility of endophytic bacteria to produce ACC deaminase, an area of study which is still being investigated and requires further exploration.54 The production or acquisition of siderophores is a characteristic that enables microorganisms to be effective competitors in various environments. This process involves the creation of small molecules with a high affinity for Fe (III) in conditions of iron deficiency, followed by the capture of iron-charged siderophores by the cell.55,56 Our research involved assessing the ability of endophytic B. velezensis to produce ACC deaminase and siderophores. Upon incubation, the isolate exhibited a positive outcome for ACC deaminase production, but a negative result for siderophore production. Bacillus velezensis Lle-9 isolated from Lilium leucanthum displayed PGP properties, such as siderophore production and ACC deaminase.57
Protease, Lipase, Cellulase and Amylase Production
Endophytic bacteria secrete extracellular enzymes, hydrolases, that help plants develop systemic resistance to prevent pathogen invasion. These enzymes have numerous applications in the food industry, pharmaceuticals, biofuels, biopesticides, detergents, and other industrial products. Over time, the methods for producing these metabolites and enzymes have been enhanced since their initial discovery.58,59 The results of our study indicate that B. velezensis exhibited lipolytic and cellulolytic characteristics, as evidenced by an enzymatic index of 2.22 ± 0.16 and 2.51 ± 0.10, respectively. In a study, endophytic Bacillus velezensis HNH7 and Bacillus altitudinis HNH9 were isolated which showed plant growth promotion properties as well as enzyme production. The endophyte exhibited proteolytic, amylolytic, lipolytic and cellulolytic activity.60
Halotolerance Properties of Endophytic Isolates
Soil salinity is recognized as a major abiotic stressor that can negatively affect agricultural output, microbial populations, and the economic viability of farming in impacted areas. This has become a significant issue in the agricultural sector worldwide, particularly over the past few decades. Nevertheless, there exists a possible remedy through salt-tolerant bacteria that exhibit various beneficial traits for plant growth. These bacteria can enhance the tolerance of plants to salinity stress and facilitate their growth in salinity-affected environments.61-63 In our study, it was observed that the endophytic isolate B. velezensis demonstrated the ability to withstand a high concentration of NaCl up to 15%. In a study, Bacillus velezensis FMH2 was found to be an efficient strain in safeguarding plants against fungal pathogens and enhancing the growth of tomato seedlings, in stressful environments with high levels of salt.64
Heavy Metal Tolerance of Endophytic Bacterium
One of the main concerns of industrialization is the build-up of heavy metals, particularly chromium in soils and lakes. The toxicity of heavy metals in plants can result in various detrimental effects such as inhibition of growth, chlorosis, necrosis, disruption in the balance of mineral nutrients leading to impaired enzymatic activities etc. Endophytes with the ability to tolerate or degrade heavy metals to protect the host plants are an area that has received limited research.65 Our study found that B. velezensis demonstrated the ability to withstand metal salt Cr2(SO4)3 at concentrations as high as 500 mg/L. Bacillus velezensis TW17, which was obtained from samples of sludge from a tannery, showed the ability to degrade chromium, with the highest rate of degradation occurring on the seventh day of observation.66 Many endophytic bacteria are also halotolerant and has high potential to be used as green inoculants.67
Dye Degradation
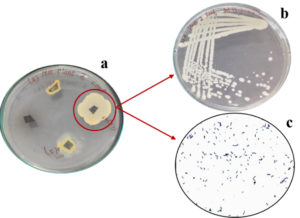
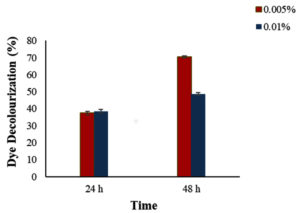
To assess the ability of B. velezensis in dye degradation, crystal violet, reactive red, and Congo red were used in the initial screening. The results revealed that the bacterium only exhibited a zone of clearance when grown in media containing Congo red, indicating its ability to degrade Congo red specifically (Figure 4). B. velezensis was found to have the ability to degrade Congo red dye, so we studied further. The amount of dye broken down by the bacterium at 24-h and 48-h intervals was measured and recorded the results as percentages of decolourization. Specifically, the decolourization percentages for dye concentrations of 0.005% and 0.01%, were done and the results are provided in figure 5. A study identified Bacillus velezensis as having the ability to remove azo dyes, and found out that this ability was linked to the presence of a gene called azoR2.11
Figure 4. Comparison of control and 48 h bacterial (Bacillus velezensis) incubated dye samples. (a) results obtained for dye samples with a concentration of 0.005% (b) results obtained for dye samples with a concentration of 0.01%
Figure 5. Decolourization of congo red by bacterial endophyte Bacillus velezensis isolated from Alternanthera philoxeroides at specific time intervals and different dye concentrations
FTIR
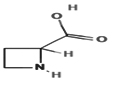
FTIR analysis described the functional groups present in bacterial-treated Congo red dye. Ethyl acetate extract of treated Congo red dye was analysed and several characteristic peaks were obtained near 849.69 cm-1, 941.11 cm-1, 1045.29 cm-1, 1097.69 cm-1, 1237.51 cm-1, 1373.01 cm-1, 1446.00 cm-1, 1738.86 cm-1, 2937.54 cm-1, 2984.03 cm-1, 3547.84 cm-1 (Figure 6a). The peak at 1045.29 cm-1 corresponds to stretching vibration of S=O, while peaks at 1237 cm-1, 1373 cm-1, 1446 cm-1 represent C–N stretching, C–N bending, and N=N stretching vibrations, respectively. Additionally, the peak at 2984.03 cm-1 arises from the symmetric stretching of C–H bonds.68-70 The chemical structure of Congo red dye is given in Figure 6b.
Figure 6. (a) FTIR spectrum of dye sample treated with endophytic Bacillus velezensis isolated from leaves of Alternanthera philoxeroides (b) Chemical structure of Congo red dye selected for decolourization study using Bacillus velezensis
GC-MS Analysis
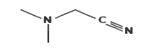
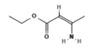
GC-MS analysis of treated and untreated dye samples were carried out and are given in Table. Major peaks observed in untreated dye samples are dimethyl aminoacetonitrile, aziridine, ethyl 3-aminocrotonate and 1,3-dioxane-2-propanol, 2-methyl at retention time (RT) 1.309. At RT 1.664, compounds like ethyl pentanoate, ethyl isovalerate and ethyl 5-methyl-5-hexenoate. 4-methylpentane-2,3-diol, Butyl 2-methylvalerate, 3-methoxypropanoic acid and acetylacetone were found at RT of 1.904. At RT 28.639, diethylborinic acid, ethyl isocyanoacetate, 2,2-dimethylundecane and 2-methyl-5-phenyl-thiazolidine were present. Compounds like hexanoate and methyl esters are utilized during the printing and dyeing process. However, dyes can have harmful effects on aquatic life, leading to a decrease in the amount of dissolved oxygen and negatively impacting both plants and animals.71 During the scouring stage of textile production, various axes, like undecane and dodecane were employed as phase changers and protective agents. These waxes serve to safeguard textile products from a range of potential threats, including water, high temperatures, nuclear radiation, biological agents and chemicals. Ethyl 3-aminocrotonate (EAC) is a crucial component in the production of numerous substances, including pharmaceuticals, dyes, and pesticides. It serves as a significant intermediate in their synthesis. 1,3-dioxane-2-propanol is a compound used as a solvent and extractor in the processing fats, waxes, and dyes. Following bacterial treatment, it was observed that the original compounds underwent mineralization while certain compounds were converted into novel metabolic products. After degradation at RT 1.909 1,1-dimethylsiletane, 4-methylpentane-2,3-diol and methoxycarbonyl isothiocyanate and 1,3:2,5-Dimethylene-4-methyl-d-rhamnitol were present. At RT 28.574, allylamine, cyclopentylamine, azetidine-2-carboxylic acidand2-methyl-1-tosyl aziridine were found.
Table :
Compounds present in untreated and treated dye samples analysed using GC-MS
Compounds |
Retention Time |
Untreated Dye Sample |
Treated Dye Sample |
2D Structure |
|---|---|---|---|---|
Dimethyl aminoacetonitrile |
1.309 |
+ |
– |
|
Aziridine |
1.309 |
+ |
– |
|
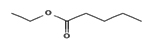
Ethyl 3-aminocrotonate |
1.309 |
+ |
– |
|
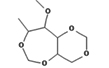
1,3-Dioxane-2-propanol, 2-methyl- |
1.309 |
+ |
– |
|
Ethyl pentanoate |
1.664 |
+ |
– |
|
Ethyl isovalerate |
1.664 |
+ |
– |
|
Ethyl 5-methyl-5-hexenoate |
1.664 |
+ |
– |
|
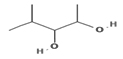
4-methylpentane-2,3-diol |
1.904 |
+ |
– |
|
Butyl 2-methylvalerate |
1.904 |
+ |
– |
|
3-Methoxypropanoic acid |
1.904 |
+ |
– |
|
Acetylacetone |
1.904 |
+ |
– |
|
Diethylborinic acid |
28.639 |
+ |
– |
|
Ethyl isocyanoacetate |
28.639 |
+ |
+ |
|
2,2- Dimethylundecane |
28.639 |
+ |
– |
|
2-Methyl-5-phenyl-thiazolidine |
28.639 |
+ |
– |
|
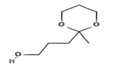
1,1-Dimethylsiletane |
1.909 |
– |
+ |
|
4-methylpentane-2,3-diol |
1.909 |
– |
+ |
|
Methoxycarbonyl isothiocyanate |
1.909 |
– |
+ |
|
1,3: 2,5-Dimethylene-4-methyl-d-rhamnitol |
1.909 |
– |
+ |
|
Allylamine |
28.574 |
– |
+ |
|
Cyclopentylamine |
28.574 |
– |
+ |
|
Azetidine-2-carboxylic acid |
28.574 |
– |
+ |
|
2-Methyl-1-Tosyl-Aziridine |
28.574 |
– |
+ |
Where, – is absent; + is present.
Overall, the study characterized bacterial endophyte Bacillus velezensis OQ874364 isolated from Alternanthera philoxeroides grown in Bellandur Lake, Bangalore, India. The isolate possessed potential PGP properties including IAA production, ammonia production, nitrogen fixation, phosphate solubilization and ACC deaminase production. Furthermore, the isolate showed bioremediation properties like heavy metal tolerance and Congo red dye degradation along with halotolerance properties. Within 48 hB. velezensis successfully decolorized 70% of Congo red dye at a concentration of 0.005%, and it exhibited chromium tolerance up to 500 mg/L. These results indicate the diverse roles of endophytic B. velezensis in plant growth promotion, dye degradation and heavy metal tolerance. The aforementioned bacterium exhibits considerable potential for future research endeavours and can be a valuable isolate in various studies and applications.
ACKNOWLEDGMENTS
The authors would like to thank University Grants Commission-Council of Scientific & Industrial Research (UGC-CSIR) for the fellowship granted. Authors are also thankful to the Department of Life Sciences, CHRIST (Deemed to be) University, Bangalore, India, for providing infrastructure for research. We are thankful to the School of Advanced Sciences (SAS), Vellore Institute of Technology (VIT), Tamil Nadu, India, for providing us with technical support for FTIR and GC-MS analysis.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SS and IP conceptualized the study. IP, SS, SB and SJ contributed to the study conception and design. IP, SB and SJ performed the experiments and wrote the manuscript. IP, SS, SB and SJ reviewed and approved the article for final publication.
FUNDING
This study was supported by University Grants Commission-Council of Scientific & Industrial Research (UGC-CSIR) with grant number UGC-Ref.No:911/CSIR-UGC NET JUNE 2018.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Afzal M, Khan S, Iqbal S, Mirza MS, Khan QM. Inoculation method affects colonization and activity of Burkholderia phytofirmans PsJN during phytoremediation of diesel-contaminated soil. Int Biodeterior Biodegrad. 2013;85:331-336.
Crossref - Khan S, Afzal M, Iqbal S, Khan QM. Plant-bacteria partnerships for the remediation of hydrocarbon contaminated soils. Chemosphere. 2013; 90(4):1317-32.
Crossref - Prakash J. Potential application of endophytes in bioremediation of heavy metals and organic pollutants and growth promotion: mechanism, challenges, and future prospects. Bioremediation for Environmental Sustainability. 2021; 1:91-121.
Crossref - Omomowo OI, Babalola OO. Bacterial and fungal endophytes: tiny giants with immense beneficial potential for plant growth and sustainable agricultural productivity. Microorganisms. 2019; 7(11):481.
Crossref - Chen C, Cao Z, Li J, Tao C, Feng Y, Han Y. A novel endophytic strain of Lactobacillus plantarum CM-3 with antagonistic activity against Botrytis cinerea on strawberry fruit. Biological Control. 2020;1:148:104306.
Crossref - Desore A, Narula SA. An overview on corporate response towards sustainability issues in textile industry. Environ Dev Sustain. 2018;1439-59.
Crossref - Chanzu HA, Onyari JM, Shiundu PM. Brewers’ spent grain in adsorption of aqueous Congo Red and malachite green dyes: Batch and continuous flow systems. J Hazard Mater. 2019;15:380:120897.
Crossref - Tara N, Siddiqui SI, Rathi G, Chaudhry SA, AsiriAM. Nano-engineered adsorbent for the removal of dyes from water: A review. Curr Anal Chem. 2020;6(1):14-40.
Crossref - Verma M, Lee I, Hong Y, Kumar V, Kim H. Multifunctional b-Cyclodextrin-EDTA-Chitosan polymer adsorbent synthesis for simultaneous removal of heavy metals and organic dyes from wastewater. Environ Pollut. 2022;292(Part B):118447.
Crossref - Ellafi A, Dali A, Mnif S, Ben Younes S. Microbial Enzymatic Degradation, Spectral Analysis and Phytotoxicity Assessment of Congo Red Removal by Bacillus spp. Catalysis Letters. 2023; 6:1-4.
Crossref - Bafana A. Identification and characterization of azoreductase enzyme AzoR2 from Bacillus velezensis for biodegradation of azo dyes. Int Biodeterior Biodegrad. 2022;167:105351.
Crossref - Shehzadi M, Fatima K, Imran A, Mirza MS, Khan QM, Afzal M. Ecology of bacterial endophytes associated with wetland plants growing in textile effluent for pollutant-degradation and plant growth-promotion potentials. Plant Biosystems-An International Journal Dealing with all Aspects of Plant Biology. 2016;150(6):1261-1270.
Crossref - Tiwari S, Lata C. Heavy metal stress, signalling, and tolerance due to plant-associated microbes: an overview. Front Plant Sci. 2018;9:452.
Crossref - Li Q, Fu Q, Zhu J, Sun Y, He H, Hu H. Endophytic Bacteria in Ricinuscommunis L.: Diversity of Bacterial Community, Plant” Growth Promoting Traits of the Isolates and Its Effect on Cu and Cd Speciation in Soil. Agronomy. 2023;13(2):333.
Crossref - Rajanna KB, Manjappa N, Nayak H, Sruthisree C. Assessment of water quality of Bellandur Lake in Bengaluru, Karnataka. The Pharma Innovation. 2023;12(3):3796-3800. https://www.thepharmajournal.com/archives/2023/vol12issue3/PartAO/12-3-406-323.pdf
- Bolpagni R. Towards global dominance of invasive alien plants in freshwater ecosystems: the dawn of the Exocene. Hydrobiologia. 2021;848(9):2259-2279.
Crossref - Gagne S, Richard C, Roussean H, Antoun H. Xylem-residing bacteria in alfalfa roots. Can J Microbiol. 1987;33(11):996-1000.
Crossref - Kimura M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111-120.
Crossref - Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol. 2018;35:1547-1549.
Crossref - Rahman A, Sitepu IR, Tang SY, Hashidoko Y. Salkowski’s reagent test as a primary screening index for functionalities of rhizobacteria isolated from wild dipterocarp saplings growing naturally on medium-strongly acidic tropical peat soil. Biosci Biotechnol Biochem. 2010;74(11):2202-2208.
Crossref - Gordon SA, Weber RP. Colorimetric estimation of indole-acetic acid. Plant Physiol. 1951;26(1):192-195.
Crossref - Cappuccino JC, Sherman N. In: Microbiology: A Laboratory Manual, New York. 1992. https://www.scirp.org/(S(lz5mqp453edsnp55rrgjct55))/reference/ReferencesPapers.aspx?ReferenceID=557184
- Pikovskaya RE. Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya. 1948;7:362-370. https://www.scirp.org/(S(351jmbntvnsjt1aadkposzje))/reference/ReferencesPapers.aspx?ReferenceID=1514468
- Edi-Premono MA, Moawad, Vleck PLG. Effect of phosphate solubilizing Pseudomonas putida on the growth of maize and its survival in the rhizosphere. Indonasian Journal of Crop Scienc (JERAMI). 1996;11:13-23 https://scirp.org/reference/referencespapers.aspx?referenceid=2239920
- Jensen HL. Nonsymbiotic nitrogen fixation. Soil Nitrogen. 1965;10:436-480.
Crossref - Aleksandrov VG, Blagodyr’ RN, Il’ev IP. Zvil’nennia sylikatnymy bakteriiamy fosfornoĭ kysloty z apatytu [Phosphorus acid isolation from apatite produced by silicate bacteria]. Mikrobiol Zh. 1967;29(2):111-4.
- Dworkin M, Foster J. Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol. 1958;75(5):592-603.
Crossref - Renuga G. Characterization of ACC Deaminase in plant growth promoting Pseudomonas from tannery sludge. I Control Pollution. 2005;21(1). https://www.icontrolpollution.com/articles/characterization-of-acc-deaminase-in-plant-growth-promoting-pseudomonas-from-tannery-sludge-.php?aid=45439
- Sebastian AM, Umesh M, Priyanka K, Preethi K. Isolation of plant growth-promoting Bacillus cereus from soil and its use as a microbial inoculant. Arab J Sci Eng. 2021;46:151-61.
Crossref - Senthilkumar M, Amaresan N, Sankaranarayanan A. Plant-Microbe Interactions: Laboratory Techniques, Springer Protocols Handbooks. 2021.
Crossref - Jabborova D, Annapurna K, Fayzullaeva M, et al. Isolation and characterization of endophytic bacteria from ginger (Zingiber officinale Rosc.). Ann Phytomed. 2020;9(1):116-21.
Crossref - Lal A, Cheeptham N. Starch agar protocol, American Society for Microbiology. 2012; 1:1-9.
- Hankin L, Anagnostakis SL. The Use of Solid Media for Detection of Enzyme Production by Fungi. Mycologia. 1975;67(3):597-607.
Crossref - Arora S, Vanza MJ, Mehta R, Bhuva C, Patel PN. Halophilic microbes for bio-remediation of salt affected soils. 2014;8(33):3070-3078.
Crossref - Govarthanan M, Mythili R, Selvankumar T, Kamala-Kannan S, Rajasekar A, Chang YC. Bioremediation of heavy metals using an endophytic bacterium Paenibacillus sp. RM isolated from the roots of Tridax procumbens. 3 Biotech. 2016;6(2):242.
Crossref - Khalid A, Arshad M, Crowley DE. Decolorization of azo dyes by Shewanella sp. under saline conditions. Appl Microbiol Biotechnol. 2008;79(6):1053-1059.
Crossref - Syed MA, Sim HK, Khalid A, Shukor MY. A simple method to screen for azo-dye-degrading bacteria. J Environ Biol. 2009;30(1):89-92. http: //www.jeb.co.in
- Kishor R, Purchase D, Saratale GD, et al. Environment friendly degradation and detoxification of Congo red dye and textile industry wastewater by a newly isolated Bacillus cohnni (RKS9). Environ Technol Innov. 2021;22:101425.
Crossref - Bharagava RN, Mani S, Mulla SI, Saratale GD. Degradation and decolourization potential of a ligninolytic enzyme producing Aeromonas hydrophila for crystal violet dye and its phytotoxicity evaluation. Ecotoxicol Environ Saf. 2018;156:166-175.
Crossref - Sheng X, Chen X, He L. Characteristics of an endophyticpyrene-degrading bacterium of Enterobacter sp. 12J1 from Allium macrostemon Bunge. Int Biodeterior Biodegrad. 2008.62(2):88-95.
Crossref - Jayaram S, Sarojini S. Bioprospecting of Fungal Endophytes in Hulimavu Lake for their Repertoire of Bioactive Compounds. ECS Transactions. 2021;107(1):10471.
Crossref - Mohite B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J Soil Sci Plant Nutr. 2013;13(3):638-649.
Crossref - Khan MA, Asaf S, Khan AL, et al. Plant growth promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biology. 2020;22(5):850-862.
Crossref - Liu R, Guo Z, Lu S. Genome-wide identification and expression analysis of the Aux/IAA and auxin response factor gene family in Medicago truncatula. Int J Mol Sci. 2021;22(19):10494.
Crossref - Kumar V, Pathak DV, Dudeja SS, et al. Legume nodule endophytes more diverse than endophytes from roots of legumes or non – legumes in soils of Haryana, India. J Microbiol Biotechnol Res. 2013;3(3):83-92.
- Meng Q, Jiang H, Hao JJ. Effects of Bacillus velezensis strain BAC03 in promoting plant growth. Biol Control. 2016;98:18-26.
Crossref - Cheffi M, Bouket AC, Alenezi FN, et al. Oleaeuropaea L. root endophyte Bacillus velezensis OEE1 counteracts oomycete and fungal harmful pathogens and harbours a large repertoire of secreted and volatile metabolites and beneficial functional genes. Microorganisms. 2019;7(9):314.
Crossref - Devi S, Sharma S, Tiwari A, et al. Screening for Multifarious Plant Growth Promoting and Biocontrol Attributes in Bacillus Strains Isolated from Indo Gangetic Soil for Enhancing Growth of Rice Crops. Microorganisms. 2023;11(4):1085.
Crossref - Varga T, Hixson KK, Ahkami AH, et al. Endophyte-promoted phosphorus solubilization in Populus. Front Plant Sci. 2020;11:567918.
Crossref - Agarwal H, Dowarah B, Baruah PM, Bordoloi KS, Krishnatreya DB, Agarwala N. Endophytes from Gnetumgnemon L. can protect seedlings against the infection of phytopathogenic bacterium Ralstonia solanacearum as well as promote plant growth in tomato. Microbiolog Res. 2020;238:126503.
Crossref - Shirale AO, Meena BP, Gurav PP, et al. Prospects and challenges in utilization of indigenous rocks and minerals as source of potassium in farming. J Plant Nutr. 2019;42(19):2682-701.
Crossref - Devi NO, Devi RKT, Debbarma M, Hajong M, Thokchom S. Effect of endophytic Bacillus and arbuscularmycorrhiza fungi (AMF) against Fusarium wilt of tomato caused by Fusariumoxysporum f. sp. lycopersici. Egypt J Biol Pest Control. 2022;32(1):1-4.
Crossref - Glick BR, Cheng Z, Czarny J, Duan J. Promotion of plant growth by ACC deaminase-producing soil bacteria. New perspectives and approaches in plant growth-promoting Rhizobacteria research. 2007:329-339.
Crossref - Rashid S, Charles TC, Glick BR. Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl Soil Ecol. 2012;61:217-24.
Crossref - Berg G. Plant-microbe interactions promoting plant growth and health: perspectives for controlled use of microorganisms in agriculture. Appl Microbiol Biotechnol. 2009;84(1):11-18.
Crossref - Li W, Lyte M, Freestone PP, Ajmal A, Colmer-Hamood JA, Hamood AN. Norepinephrine represses the expression of toxA and the siderophore genes in Pseudomonas aeruginosa. FEMS Microbiol Lett. 2009;299(1):100-109.
Crossref - Khan MS, Gao J, Chen X, et al. The Endophytic Bacteria Bacillus velezensis Lle-9, Isolated from Lilium leucanthum, Harbors Antifungal Activity and Plant Growth-Promoting Effects. J Microbiol Biotechnol. 2019;30(5):668-680.
Crossref - Singh M, Kumar A, Singh R, Pandey KD. Endophytic bacteria: a new source of bioactive compound. 3 Biotech. 2017;7(5):315.
Crossref - Subbulakshmi GK, Thalavaipandian A, Ramesh V, Rajendran A. Bioactive endophytic fungal isolates of Biota orientalis (L) Endl., Pinusexcelsa Wall. and Thuja occidentalis L. International Journal of Advanced Life Sciences (IJALS). 2012;4:9-15. https://www.academia.edu/11539699/Bioactive_endophytic_fungal_isolates_
of_Biota_orientalis_L_Endl_Pinus_excelsa_Wall_and_Thuja_occidentalis_L - Hasan N, Khan IU, Farzand A, et al. Bacillus altitudinis HNH7 and Bacillus velezensis HNH9 promote plant growth through upregulation of growth promoting genes in upland cotton. J Appl Microbiol. 2022;132(5):3812-3824.
Crossref - Etesami H, Beattie GA. Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front Microbiol. 2018;9:148.
Crossref - Etesami H, Noori F. Soil salinity as a challenge for sustainable agriculture and bacterial-mediated alleviation of salinity stress in crop plants, Saline soil-based agriculture by halotolerant. Microorganisms. 2019:1-22.
Crossref - Etesami H, Glick BR. Halotolerant plant growth-promoting bacteria: Prospects for alleviating salinity stress in plants. Environmental and Experimental Botany. 2020;178:104124.
Crossref - Masmoudi F, Tounsi S, Dunlap CA, Trigui M. Endophytic halotolerant Bacillus velezensis FMH2 alleviates salt stress on tomato plants by improving plant growth and altering physiological and antioxidant responses. Plant Physiol Biochem. 2021;165:217-27.
Crossref - Bibi N, Shah MH, Khan N, Mahmood Q, Aldosari AA, Abbasi AM. Analysis and health risk assessment of heavy metals in some onion varieties. Arab J Chem. 2021;14(10):103364.
Crossref - Roselin K, Rose JC. Biodegradation of Tannic Acid, Chromium and Cadmium Present in Leather Industrial Effluents Using Microorganisms Isolated from Leather Industrial Sludge. Int J Res Rev. 2021:1-10
Crossref - James N, Umesh M, Sarojini S, et al. Unravelling the potential plant growth activity of halotolerant Bacillus licheniformis NJ04 isolated from soil and its possible use as a green bioinoculant on Solanum lycopersicum L. Environmental Research, 2022: 114620.
Crossref - Olukanni OD, Osuntoki AA, Awotula AO, Kalyani DC, Gbenle GO, Govindwar SP. Decolorization of dyehouse effluent and biodegradation of Congo red by Bacillus thuringiensis RUN1. J Microbiol Biotechnol. 2013;23(6):843-849.
Crossref - Lade H, Govindwar S, Paul D. Low-cost biodegradation and detoxification of textile azo dye CI reactive blue 172 by Providencia rettgeri strain HSL1. J Chem. 2015;894109.
Crossref - Haque SU, Nasar A, Rahman MM. Applications of chitosan (CHI)-reduced graphene oxide (rGO)-polyaniline (PAni) conducting composite electrode for energy generation in glucose biofuel cell. Sci Rep. 2020;10(1):10428.
Crossref - Kaur K, Jindal R. Self-assembled GO incorporated CMC and Chitosan-based nanocomposites in the removal of cationic dyes. Carbohydrate polym. 2019;225:115245.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.