ISSN: 0973-7510
E-ISSN: 2581-690X
The growing consumer demand for preservative-free food products has intensified research into antimicrobial biopolymers for food packaging. This study explores the antimicrobial potential of chitosan by developing a novel biopolymer through blending chitosan and whey protein in varying ratios (1:1, 1:2, 1:3) and incorporating essential oils to enhance antimicrobial efficacy. Mint and ginger essential oils were added at concentrations of 0.5%, 1%, 1.5%, and 2%, with initial screenings identifying the optimal composition as a 1:1 chitosan–whey protein matrix supplemented with 1% mint essential oil. The optimized biopolymer exhibited broad-spectrum antibacterial activity against a Gram-positive (Staphylococcus aureus) and a Gram-negative bacteria (Salmonella enterica Serovar Typhimurium), as well as a yeast (Saccharomyces cerevisiae). Notably, the presence of essential oils significantly enhanced the polymer’s antimicrobial properties, with superior efficacy observed in the essential oils compared to the polymer alone. Structural and physicochemical analyses demonstrated that the addition of mint essential oil improved the polymer’s surface uniformity, elasticity, and viscosity. Fourier-transform infrared (FT-IR) spectroscopy confirmed that the functional groups of the biopolymer remained largely unaltered upon mint oil incorporation. Mechanical testing revealed an increase in tensile modulus and a decrease in cutting modulus, alongside a minor reduction (2.35%) in melting point. Additionally, both the untreated and mint-enriched biopolymers exhibited decreased brightness and a slight tendency toward yellowing. These findings underscore the potential of chitosan–whey protein-based biopolymers, reinforced with essential oils, as sustainable and effective antimicrobial packaging materials for food preservation.
Food, Bacteria, In vitro Study, Natural Products, Biodegradable Polymers
The belief that we are in the final stages of a period referred to as the post-antibiotic age is presently supported by the scientific community. The outlook for the future is deemed bleak unless our present strategy of producing synthetic antimicrobial agents, which quickly become ineffective against bacteria resistant to many drugs, is forsaken. It has been shown by numerous studies and investigations that natural products might be considered a viable alternative to insufficient drugs in the fight against infectious diseases.1
Significant interest in the packaging sector has been garnered by antimicrobial polymers due to their beneficial impact on food quality and safety, as well as their capacity to extend shelf life, offering an effective alternative for mitigating, inhibiting, or preventing the growth of bacteria that cause infections and spoilage in food products.2 Biopolymers, encompassing proteins, lipids, carbohydrates, and their composites, are regarded as the principal materials used for this purpose owing to their advantages, including biodegradability, abundant availability, and renewability.3
Antimicrobial chemical agents are integrated into the polymer matrix to create antimicrobial packaging barriers that help prevent the proliferation of certain microbes, which may result in food contamination.3 The food industry is now contending with many challenges, including regulating the natural ripening process, mitigating the restricted shelf life of perishable items, and minimizing processing levels.4 For many years, a major challenge in food science has been to mitigate the degradation of vitamins and minerals, prevent lipid oxidation, regulate enzymatic activity, and inhibit microbial growth through advancements in food packaging technologies. The goal is to enhance the stability and safety of food products, ensuring their quality is maintained until consumption.5 The most traditional packaging materials are derived from petroleum-based polymers, which provide significant environmental challenges upon disposal due to their biodegradability issues. Consequently, several researchers have shown interest in ecologically sustainable packaging membranes and coatings, which function as an environmentally responsible alternative for food packaging.6
Chitosan is an amino-polysaccharide biopolymer characterized by a distinctive chemical structure. It has a linear polycation structure with elevated charge density, reactive hydroxyl and amino groups, and robust hydrogen bonding capabilities. The material has exceptional biocompatibility, durability, and ease of manufacture. The name ‘chitosan’ denotes a diverse array of polymers that integrate various physical, chemical, and biological properties. This versatility enables a diverse array of applications, which are both intriguing and mostly unexplored.7 Chitosan is now considered a principal constituent in the formation of biodegradable food packaging, alongside carbohydrates, proteins, and lipids. The advantageous properties of membrane composition and its biological attributes, including antibacterial, antifungal, and antiviral activity, have augmented its use in food packaging.8 Whey protein is used as a dietary component owing to its nutritional content, gel-forming capacity, and viscosity.9 Whey protein membranes exhibit transparency, flexibility, and a pleasant odor and taste, making them appealing to customers.10
Historically, plants have served as the primary source of antimicrobial agents since ancient times.11 Essential oils obtained from medicinal plants exhibit significant antimicrobial and antioxidant properties. The antimicrobial properties of these oils are mainly linked to their phenolic compounds, as demonstrated in numerous studies on essential oils such as rosemary, thyme, ginger, peppermint, oregano, and others.12
Therefore, the current study aimed to focus on the synthesis of a biopolymer derived from chitosan and whey proteins, incorporating vegetable oils, and examined its physical, mechanical, structural, and antimicrobial properties.
Materials
The Chitosan was obtained from ACROS ORGANICS (Thermo Fisher Scientific, USA). It was derived from chitin (C6H1104N)n and has a molecular weight of (161n) and a density of 0.3 g/ml. The degree of deacetylation is 90%. Acetic acid was provided by Honeywell. The whey protein, which has a protein content of 35%, was acquired from Jarrow FORMULAS. Ginger oil (Zingiber officinale) and peppermint extract (Mentha piperita) were bought from a trusted local market in Buraydah, Qassim. Glycerol was provided by Panreac. The Department of Food Science and Human Nutrition at Qassim University donated samples of Staphylococcus aureus, Salmonella typhimurium (Salmonella enterica Serovar Typhimurium), and Saccharomyces cerevisiae strains sourced from contaminated foods.
Methods
Biopolymer preparation
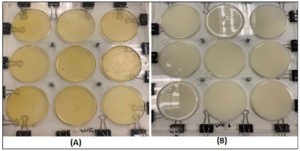
The biopolymer was prepared utilizing (chitosan/milk serum protein) (Figure 1) with the polymer loaded at different concentrations of extracts of both ginger oil and mint as antimicrobial agents (0.5, 1, 1.5, and 2%). The preparation of the biopolymer proceeded as follows: Chitosan at a concentration of one percent (w/w) was prepared by dissolving it in 1% acetic acid with vigorous stirring for 30 minutes until complete dissolution occurred. The chitosan solution was blended for 4 minutes to achieve homogeneity. The mixture was permitted to rest for two hours.13 A 12% (w/w) milk serum protein solution was prepared and mixed with a 6% glycerol aqueous solution at room temperature. The mixture was maintained at 74 °C for 20 minutes while being stirred.14 For the preparation of Biopolymer Blends, the chitosan solution was mixed with the milk serum protein solution for 4 minutes at different mixing ratios (1:3, 1:2, 1:1). Then, different concentrations of ginger oil or mint extract (0.5%, 1%, 1.5%, 2%) were added. The mixture was homogenized for 2 minutes at 5 °C, and then left for 30 minutes in an ultrasonic water bath to remove any air bubbles after homogenization. The biopolymer mixture, with or without the addition of ginger oil or mint, at different concentrations, was placed in special silicon dishes (dish area 9 cm2). The contents of the dishes were dried at 45 °C for 22 hours. The membranes were fixed for 48 hours at 25 °C and 58% humidity within a glass dryer containing calcium chloride. The optimal blending ratio was established through an initial experiment aimed at maximizing the antimicrobial capacity of the mixture. This was assessed by measuring the inhibition zone diameter (mm) surrounding the biopolymer disc (6 mm²) against the following microorganisms: Salmonella Typhimurium, Staphylococcus aureus, and Saccharomyces cerevisiae.
Antimicrobial properties of biopolymer
This study evaluated the antimicrobial effects of a biopolymer made from chitosan and milk serum proteins, both independently and in combination with varying concentrations (0.5%, 1%, 1.5%, and 2%) of ginger oil and mint extracts, on Gram-positive and Gram-negative bacteria as well as yeast. The disc diffusion technique was employed to achieve this outcome.15 Petri dishes with nutrient agar medium were coated with 0.1 ml of pathogenic bacteria, specifically Staphylococcus aureus and Salmonella Typhimurium, along with yeast. Yeast was placed into Petri dishes containing Potato Dextrose Agar medium. Composite membrane samples (6 mm2) were subsequently positioned on agar surfaces and incubated at 37 °C for 18 hours for pathogenic bacteria and at 25 °C for 3-5 days for yeast. The inhibition zones surrounding each membrane were identified and measured in millimeters (mm) using a ruler.
Morphological characterization of biopolymer
The morphological characteristics of the biopolymer consisting of chitosan and milk serum proteins, as well as the combination with 1% mint oil extract, were evaluated through Scanning Electron Microscopy (SEM) at a magnification of x103. The morphological characteristics of the membranes were analyzed using Scanning Electron Microscopy in accordance with established procedures.16 The membranes underwent pretreatment prior to analysis using Scanning Electron Microscopy. Samples were sectioned into small slices and immersed in a solution of 2% (v/v) glutaraldehyde and 0.1 M sodium cacodylate buffer (pH 7.2) for two hours. They were subsequently washed with 0.1 M sodium cacodylate buffer three times over ten minutes, then fixed in 0.2 M sodium cacodylate buffer (pH 7.2) and 0.4% (w/v) osmium tetroxide for two hours, followed by another series of washes with 0.1 M sodium cacodylate buffer three times in ten minutes. The samples underwent dehydration for 15 minutes using a graded ethanol series of 30%, 50%, 70%, and 90% (v/v), followed by three additional 15-minute treatments with 99.5% (v/v), and were subsequently dried overnight. The dried samples were affixed to aluminum stubs and coated with a gold layer via a “Baizer SCD SPUTTER” coater, facilitating surface visualization at an accelerating voltage of 5 kilovolts.
Estimation of rheological properties of the polymer
A stress-controlled rheometer (Discovery Hybrid Rheometer, DHR-2, TA Instruments, USA) was utilized to evaluate the rheological properties, specifically viscosity, of liquid polymer samples under four conditions: (i) chitosan alone; (ii) milk serum proteins alone; (iii) a 1:1 combination of chitosan and milk serum proteins; and (iv) the same 1:1 combination supplemented with 1% mint oil. The measurements were conducted using cone-plate geometry (40 mm diameter, 2° angle, 52 µm gap). The elastic and viscous characteristics of the polymer samples were analyzed across a temperature range of 5-35 °C (heating) and 35-5 °C (cooling) at a controlled rate of 1 °C per minute. An oscillation frequency of 1 Hz and an applied pressure of 3 Pa were maintained during the experiments. Temperature-dependent changes in the storage modulus (G’), loss modulus (G’’), and phase angle (tan δ) were systematically recorded.17
FTIR spectroscopy, ALPHA-Eco FT-IR spectrometer
Polymer Composition Characteristics was also studied using ATR-FTIR Spectroscopy, ALPHA-Eco FT-IR Spectrometer. ATR-FTIR spectroscopy was employed to analyze the spectra of powder samples of biopolymer blends comprising chitosan and milk serum proteins (1:1), with and without the addition of mint oil. The analysis was performed using an ALPHA-Eco FT-IR spectrometer equipped with an Eco-Zn Se sampling unit (Bruker Optics, Germany). Spectra were recorded over a wavenumber range of 4000–600 cm–¹.17
Estimation of pH
The pH value of chitosan/milk serum protein solutions and chitosan/milk serum protein solutions with 1% mint oil was measured using the Adwa-Romania pH Tester AD101 device. The measurements of the device ranged from 2.0 to 16.0 pH, with 0.01 pH accuracy and reading precision of ± 0.02 pH.18
Tensile, shear, and thickness properties
The tensile strength was measured using the Texture analyzer TAG XT2, UK, for samples of biopolymer membranes composed of chitosan/milk serum proteins (1:1) with or without 1% mint oil. Slices were made from each sample with an area of each slice measuring 2 × 5 cm. The polymer slices were mounted on the testing device and subjected to a load cell with a capacity of 5 kg. The experiments were conducted at a crosshead speed of 1 mm/s, utilizing a lower platen with a dimension of 0.05.19
Estimation of polymer thickness
The thickness of the biopolymer membranes was determined using a digital electronic thickness gauge with a precision of 0.01 mm. Measurements were taken at ten distinct points on each sample, and the average thickness was calculated to ensure accuracy and uniformity.20
Dissolution estimation
The water solubility of the biopolymer was evaluated by immersing it in distilled water at a ratio of 10:1 (water to polymer) for 48 hours. Following immersion, the polymer samples were dried in an oven at 50 °C for two days to remove residual moisture. It was then moved to a desiccator and left at ambient temperature for a period of two weeks until a stable weight was attained. The dissolution was assessed by measuring the starting and final weights of the polymer across three duplicates.21
Estimation of color
Color estimation was conducted using the Hunter Lab instrument.22 The calibration of the device was performed using black and white ceramic tiles. The measurements of the samples were taken using D-silluminant and observer, and color values were expressed through measuring three parameters: L – a – b. Where: L denotes lightness, ranging from 0 (black) to 100 (white); The parameter a denotes the intensity of redness, where positive values signify red tones and negative values indicate green tones; The parameter b denotes the extent of yellowness, where positive values signify yellow tones and negative values indicate blue tones.
Statistical analysis
Experimental data were analyzed using IBM SPSS Statistics (v. 28.0, IBM Corp., Armonk, NY, USA) with three independent replicates per treatment. One-way ANOVA was performed for each microbial strain (Saccharomyces cerevisiae, Staphylococcus aureus, Salmonella typhimurium) to assess the effects of biopolymer ratios (1:1, 1:2, 1:3) and essential oil concentrations (0-2%). Levene’s test confirmed variance homogeneity (p > 0.05). Tukey’s HSD post hoc test identified significant differences (p < 0.05), with results reported as mean ± SE and distinct superscripts indicating statistical significance within each strain.
The antimicrobial efficacies of essential oil-functionalized biopolymers (ginger and mint) are presented in Table 1 and 2, and Figure 1. Tukey’s HSD analysis revealed significant differences (p < 0.05) in the antimicrobial activity of chitosan-whey biopolymers functionalized with mint (MEO) or ginger essential oils (GEO) across all tested microbial strains (Saccharomyces cerevisiae, Staphylococcus aureus, Salmonella Typhimurium). For MEO-containing biopolymers (Table 1), the 1:1 chitosan:whey ratio with 1% MEO demonstrated optimal broad-spectrum inhibition, achieving zones of 21.5 ± 0.12 mm (yeast), 20.0 ± 0.42 mm (S. aureus), and 24.0 ± 0.08 mm (S. typhimurium). In contrast, GEO-enhanced biopolymers (Table 2) exhibited strain-specific efficacy: the 1:2 ratio with 2% GEO showed the strongest activity against S. Typhimurium (26.3 ± 0.33 mm), while the 1:3 ratio with 2% GEO was most effective against S. aureus (25.3 ± 0.04 mm). Regarding the dose-dependent antimicrobial effects, both MEO and GEO displayed non-linear dose-response relationships. For MEO, increasing concentrations beyond 1% reduced inhibitory effects against S. aureus (20.0 mm at 1% vs. 11.0 mm at 2%), likely due to oil volatility or matrix saturation. Similarly, GEO at 2% in the 1:3 ratio caused complete loss of activity against S. Typhimurium (0.0 mm), suggesting potential antagonistic interactions at higher doses. With reference to strain-specific sensitivity, the Gram-negative S. Typhimurium exhibited greater susceptibility to MEO (24.0 mm at 1%) than Gram-positive S. aureus (20.0 mm), aligning with chitosan’s ability to disrupt outer membrane integrity. Conversely, GEO showed stronger activity against S. aureus (25.3 mm at 2%) than S. Typhimurium (0.0 mm), likely due to gingerol’s affinity for Gram-positive peptidoglycan. Yeast inhibition was consistently lower than bacterial targets, reflecting fungal cell wall resilience.
Table (1):
Antimicrobial Activity of Chitosan-Whey Biopolymers with Mint Essential Oil (MEO)
| Mixing Ratio (Chitosan: Whey) | MEO (%) | Saccharomyces cerevisiae (mm) | Staphylococcus aureus (mm) | Salmonella Typhimurium (mm) |
|---|---|---|---|---|
| 1:1 | 0.0 | 18.0 ± 0.26a | 11.0 ± 0.10d | 14.0 ± 0.20c |
| 0.5 | 16.6 ± 0.13b | 23.0 ± 0.08a | 19.3 ± 0.43a | |
| 1.0 | 21.5 ± 0.12a | 20.0 ± 0.42b | 24.0 ± 0.08a | |
| 1.5 | 19.5 ± 0.12ab | 18.5 ± 0.12c | 15.0 ± 0.36b | |
| 2.0 | 19.0 ± 0.16b | 11.0 ± 0.08d | 14.0 ± 0.08c | |
| 1:2 | 0.0 | 12.3 ± 0.10c | 15.0 ± 0.00c | 10.3 ± 0.13e |
| 0.5 | 10.6 ± 0.04d | 12.3 ± 0.04d | 11.0 ± 0.08d | |
| 1.0 | 10.0 ± 0.08d | 14.6 ± 0.20c | 12.0 ± 0.08c | |
| 1.5 | 9.6 ± 0.04e | 17.3 ± 0.31b | 13.6 ± 0.12b | |
| 2.0 | 14.3 ± 0.04c | 18.6 ± 0.45a | 14.3 ± 0.17c | |
| 1:3 | 0.0 | 15.0 ± 0.31b | 17.0 ± 0.25b | 19.0 ± 0.15a |
| 0.5 | 18.0 ± 0.08a | 15.6 ± 0.40c | 0.0 ± 0.00f | |
| 1.0 | 16.5 ± 0.12ab | 16.0 ± 0.21c | 13.0 ± 0.08c | |
| 1.5 | 19.0 ± 0.21a | 15.0 ± 0.00c | 15.0 ± 0.14b | |
| 2.0 | 14.5 ± 0.12c | 18.3 ± 0.09a | 14.0 ± 0.35c |
Data expressed as mean ± standard error (n = 3); Superscript letters (a, b, c, etc.) denote statistically distinct groups (p < 0.05) within each microbial column, analyzed by Tukey’s HSD test; “0.0 ± 0.00” indicates no detectable inhibition
Table (2):
Antimicrobial Activity of Chitosan-Whey Biopolymers with Ginger Essential Oil (GEO)
| Mixing Ratio (Chitosan: Whey) | MEO (%) | Saccharomyces cerevisiae (mm) | Staphylococcus aureus (mm) | Salmonella Typhimurium (mm) |
|---|---|---|---|---|
| 1:1 | 0.0 | 18.0 ± 0.26a | 11.0 ± 0.10e | 14.0 ± 0.20c |
| 0.5 | 13.6 ± 0.12b | 11.6 ± 0.04e | 12.6 ± 0.20d | |
| 1.0 | 13.0 ± 0.00bc | 19.6 ± 0.04b | 16.6 ± 0.20b | |
| 1.5 | 14.3 ± 0.12b | 21.3 ± 0.04a | 11.0 ± 0.08e | |
| 2.0 | 14.3 ± 0.25b | 22.0 ± 0.21a | 11.3 ± 0.12e | |
| 1:2 | 0.0 | 12.3 ± 0.10c | 15.0 ± 0.00c | 10.3 ± 0.13f |
| 0.5 | 9.0 ± 0.08d | 12.3 ± 0.12d | 0.0 ± 0.00g | |
| 1.0 | 9.3 ± 0.04d | 14.0 ± 0.08c | 16.3 ± 0.09b | |
| 1.5 | 13.5 ± 0.12c | 17.0 ± 0.08b | 20.3 ± 0.04a | |
| 2.0 | 15.0 ± 0.16b | 20.0 ± 0.00a | 26.3 ± 0.33a | |
| 1:3 | 0.0 | 15.0 ± 0.31b | 17.0 ± 0.25b | 19.0 ± 0.15a |
| 0.5 | 12.6 ± 0.04c | 13.0 ± 0.14d | 18.0 ± 0.43a | |
| 1.0 | 12.0 ± 0.14c | 14.3 ± 0.09c | 16.6 ± 0.38b | |
| 1.5 | 12.3 ± 0.04c | 15.6 ± 0.09c | 19.0 ± 0.29a | |
| 2.0 | 17.0 ± 0.16a | 25.3 ± 0.04a | 0.0 ± 0.00g |
Data expressed as mean ± standard error (n = 3); Superscript letters (a, b, c, etc.) denote statistically distinct groups (p < 0.05) within each microbial column, analyzed by Tukey’s HSD test; “0.0 ± 0.00” indicates no detectable inhibition
Figure 1. The composition of the polymer blend 1:1 chitosan and milk serum proteins with 1% peppermint oil. The polymer before drying (A). The polymer after drying (B)
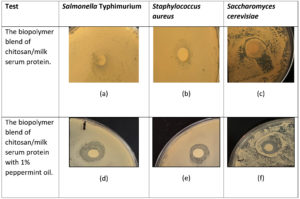
Table 3 and 4, together with Figure 2, depict the findings of the biopolymer’s antibacterial characteristics. The disc diffusion method was used for the biopolymer blend (chitosan and milk serum proteins in a 1:1 ratio with 1% peppermint oil), as well as for the control (Treatment 1) which lacked peppermint oil (chitosan and milk serum proteins 1:1). The findings demonstrated the inhibitory effect of the polymer infused with 1% peppermint oil on Gram-positive bacteria (Staphylococcus aureus), Gram-negative bacteria (Salmonella Typhimurium), and yeast (Saccharomyces cerevisiae), yielding inhibition zone diameters of 24.0 mm, 20.0 mm, and 21.5 mm, respectively. The diameters of the inhibition zones for Treatment 1 against the same bacteria and yeast were 14.0, 11.0, and 18.0 mm, respectively, as seen in Tables 3 and 4 and Figure 2 (a-f). The findings indicated that the incorporation of peppermint oil enhanced the polymer’s inhibitory impact on microorganisms, resulting in an increase in inhibition zone diameters of 10.0 mm for Salmonella Typhimurium, 9.0 mm for Staphylococcus aureus, and 3.5 mm for Saccharomyces cerevisiae. This rise may be elucidated as articulated by Sanchez-Gonzalez et al.,23 who proposed that antibacterial activity is enhanced in the presence of essential oils, which exhibit superior antimicrobial efficacy compared to the polymer.
Table (3):
Antimicrobial properties of the examined biopolymer
| Test | Mean zone of inhibition (mm) | ||
|---|---|---|---|
| Salmonella typhimurium | Staphylococcus aureu | Saccharomyces cerevisiae | |
| Treatment 1 | 14.0 | 11.0 | 18.0 |
| Treatment 2 | 24.0 | 20.0 | 21.5 |
Treatment 1: Biopolymer derived from chitosan and milk serum protein. Treatment 2: Biopolymer derived from chitosan and milk serum protein with the addition of 1% peppermint oil
Table (4):
Characteristics of the biopolymer with and without peppermint oil (1%) in terms of tensile strength, shear strength, solubility estimation, and acidity level
Biopolymer |
tensile strength |
shear strength |
solubility estimation |
pH level |
|---|---|---|---|---|
Treatment 1 |
0.055 |
121.008 % |
46.07% |
4.6 |
Treatment 2 |
0.071 |
113.490 % |
43.72% |
4.5 |
Treatment 1: Biopolymer resulting from chitosan and milk serum protein. Treatment 2: Biopolymer resulting from chitosan and milk serum protein with 1% peppermint oil addition
Figure 2. Images showcasing inhibition zones around the discs were captured to illustrate antimicrobial activity
Chitosan is a biopolymer with antibacterial and antifungal properties. The positively charged protons of the amino group in chitosan interact with the negatively charged surface of the microbe, resulting in cell membrane rupture, alterations in cell permeability, and finally cell death.24 Moreover, milk serum proteins include antibacterial characteristics via iron sequestration, including b-lactoglobulin, a-lactalbumin, lactoferrin, lactoperoxidase, serum albumin, and lysozyme. Milk serum proteins diminish the accessible iron for bacteria and also interact directly with microbial membranes. Lactoferrin disrupts the outer membranes of Gram-negative bacteria by binding to various fatty sugars and amplifying the efficacy of hydrophobic antimicrobials such as lysozyme.25
The integration of plant oils into polymers influences their physical and chemical characteristics, particularly augmenting their antibacterial and antioxidant effects. Incorporating plant oils into food packaging materials enhances the shelf life of packed goods, inhibits microbiological proliferation, and offers protection against oxidation. Plant oils also influence other characteristics of the polymer, including tensile strength and permeability.26 The inhibitory effect of plant oils is due to biologically active substances, including phenols, hydrocarbons, and mono/sesquiterpenes. These compounds interact with bacterial cell wall polysaccharides, phospholipids, and fatty acids, leading to ion loss, cellular content disruption, and cell death.27 Peppermint oil is utilized in gelatin-based biofilms to improve their antibacterial properties, particularly against Escherichia coli and Staphylococcus aureus, as well as fungi, owing to the strong antimicrobial activity of menthol, its primary component.28 Numerous studies have shown the substantial inhibitory effect of peppermint oil on Salmonella Typhimurium and Staphylococcus aureus.12 The results presented in the Table 3 confirm an enhanced inhibitory effect of the polymer containing peppermint oil relative to the polymer without oil (Treatment 1), as evidenced by the increased diameter of the inhibition zone against the examined microorganisms in the presence of peppermint oil. The results in the Table 3 demonstrate an increase in the sensitivity of Gram-negative bacteria relative to Gram-positive bacteria for both treatments (1 and 2). Some researchers have elucidate this point,29 and showed that the addition of Satureja khuzistanica Jamzad essential oils enhanced the properties of whey proteins composing packaging membranes led to an improvement in the antimicrobial membrane properties. The utilization of chitosan nanoparticles in oil capsules, such as peppermint, clove, and green tea essential oils, improved the antimicrobial properties of the oils compared to the control.30 A separate research focused on improving chitosan membrane characteristics showed that the incorporation of anise oil enhanced the antibacterial capabilities of the biopolymer.31
Figure 3. The morphological properties of the biopolymer using SEM
(A) Chitosan and milk serum protein with the addition of 1% peppermint oil; (B) Chitosan and milk serum protein without the addition of peppermint oil
Figure 3 displays the data obtained from the scanning electron microscope (SEM). The morphological qualities of polymers are essential for assessing their mechanical and physical attributes in packing membranes. Figure 4 shows a cross-sectional image of the polymer both with and without oil, using Scanning Electron Microscopy (SEM) at a magnification of x103. The findings reveal discrepancies in surface agglomerations among the analyzed polymer samples, with peppermint oil contributing to a decrease in surface agglomerations for polymer shape Figure 4(a), exhibiting some surface roughness. In contrast, treatment (1) resulted in more pronounced surface agglomeration and distinct roughness on the surface of shape Figure 4(b). Gohargani et al.32 clarified that membranes composed of a combination of chitosan and concentrated milk serum proteins have a rough surface and notable agglomerations, attributable to the polymer’s insufficient thermodynamic compatibility and the development of an inhomogeneous structure. This discrepancy may result from the uniform amalgamation of the included peppermint oil. The integration of oils into membranes often diminishes their gloss and transparency, attributable to heightened surface roughness of the composite membranes caused by droplet transfer or aggregation at the membrane’s surface during the drying process, resulting in surface imperfections.23 SEM research reveals that the polymer with 1% peppermint oil has a more uniform distribution and smoother surface than the untreated sample, which shows prominent and irregular clumping on its surface. The interaction between protein and chitosan may result in the establishment of both covalent and non-covalent interactions, hence enhancing the surface roughness of the polymer.33
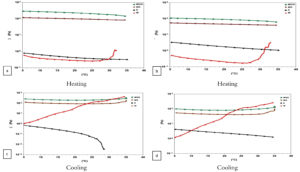
Figure 4. The rheological properties of the biopolymer.
Rheological properties of the biopolymer. (●) Chitosan, (▲) Whey protein, (▼) Chitosan/Whey protein, (■) Chitosan/Whey protein with the addition of 1% peppermint oil
The results in Figure 4 elucidate the rheological behavior of both milk serum protein and chitosan individually, as well as the mixture of chitosan with milk serum protein (1:1) with and without 1% peppermint oil. Upon heating the polymer (from 5-35 °C), it was found that milk serum proteins exhibited Shear thickening behavior while chitosan displayed Newtonian fluid behavior (Figure 4a and b). The combination of chitosan and milk serum proteins, with or without peppermint oil, resulted in a shift in rheological behavior to a pseudo-solid state upon heating, accompanied by an increase in elasticity values (G’) for the chitosan and milk serum protein mixture containing peppermint oil, in contrast to the mixture lacking peppermint oil (Figure 5a). Conversely, the shear modulus values (G”) diminished for the same mixtures (Figure 5b). This signifies that the mixes demonstrate elastic properties (similar to a solid substance) and underscores the effect of including peppermint oil on enhancing the elasticity of the mixture and, therefore, its viscosity. This may be ascribed to the augmentation of particle size in the presence of the oil.34 In the chitosan solution, the viscosity values (G”) exceeded the elasticity values (G’), whereas for the milk serum protein solution, both values (G”) and (G’) were equivalent (Figure 5a and b). The temperature of 27.5 °C was found to be the transition point for the milk serum protein solution to exhibit elastic behavior, where the elasticity value (G’) increased, with the highest elasticity and viscosity values observed for the milk serum protein solution at 32 °C. Upon cooling the polymers after heating (from 35-5 °C), a change in rheological behavior occurred for milk serum proteins, with an increase in elasticity (G’) and viscosity (G”) (Figure 4c and d). However, the rheological behavior of the mixture of chitosan and milk serum proteins with or without peppermint oil remained unchanged during the cooling process, as did that of chitosan.
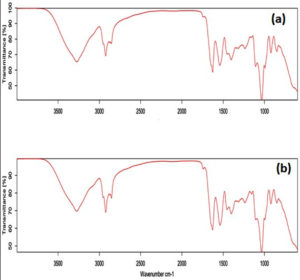
Figure 5. FTIR-ATR spectra for chitosan and milk serum protein with the addition of 1% peppermint oil (5a), FTIR-ATR spectra for chitosan and milk serum protein without peppermint oil (5b)
This analysis indicates that the bands around 3500 cm-1 are associated with the stretching vibrations of NH and OH groups, and those at 2900 cm-1 belong to the stretching vibrations of CH groups. The bands about 1640 cm-1 signify the stretching vibrations of C=O groups, whereas the vibrations between 1394-1332 cm-1 are ascribed to amino groups (-NH). The findings demonstrate that the incorporation of peppermint oil does not significantly influence the functional groups associated with the biopolymer’s structure, aligning with the conclusions of Gohargani et al.,32 The study demonstrated that the incorporation of Zataria multiflora oil does not significantly influence the functional groups of these compounds inside composite membranes containing 1% and 2% TiO2 nanoparticles. Nonetheless, these results are at odds with the conclusions of Alizadeh-Sani et al.,2 It was observed that the incorporation of essential oils from rosemary and gram-negative bacteria into milk protein/cellulose nanocomposite membranes and milk protein membranes resulted in substantial alterations in the functional groups associated with water repellency and hydrogen bonding interactions. This significant disagreement in the findings may be ascribed to variations in the biopolymers used in the preparation of membrane samples, together with changes in the content of the essential oils examined.
The pH values of the chitosan–whey protein biopolymer incorporating peppermint oil are presented in Table 4, showing values of 4.6 and 4.5, respectively. The addition of peppermint oil does not alter the biopolymer’s pH while enhancing its antibacterial properties. Mechanical resistance and shear strength are critical for the integrity of edible and biodegradable membranes, as interactions between water molecules and dispersed additives (e.g., lubricants, fats) influence their mechanical behavior. Tensile properties, key indicators of membrane performance, provide insights into yield strength, ultimate tensile strength, Young’s modulus, and elongation at yield and break. Ultimate tensile strength represents the maximum stress a membrane can withstand, while elongation at break reflects its flexibility. Young’s modulus serves as the primary measure of stiffness. The mechanical properties of hydrophilic membranes are highly dependent on environmental factors. Increased humidity (e.g., 50% RH) reduces membrane strength due to the plasticizing effect of water molecules, while rising temperatures generally decrease Young’s modulus, yield strength, and ultimate tensile strength but enhance elongation at yield and break. Additionally, additives and lubricants play a significant role in modulating mechanical behavior.
As lubricant content rises, tensile strength diminishes while elongation increases. Table 5 demonstrates the tensile strength (TS) characteristics of the polymer as assessed by a Texture Analyzer TAG. The findings indicated that the incorporation of peppermint oil into the polymer influences its tensile characteristics, resulting in enhanced elongation and reduced tensile strength relative to the oil-free polymer. This phenomenon may be ascribed to the plasticizing impact of essential oils, which partly disrupt the intra- and intermolecular hydrogen bonds among polymer molecules, hence diminishing the polymer’s strength. Molecular mobility thus rises, resulting in enhanced polymer flexibility.35
Furthermore, Table 5 illustrates the shear resistance of the biopolymer. The findings indicate that an increase in the tensile strength of the polymer correlates with a drop in the cutting point value, and vice versa.36 The findings demonstrate a reduction in cutting strength for the biopolymer with 1% peppermint oil (113.49%) relative to the biopolymer without it (121.008%). These findings align with those of AL-Hassan & Norziah36 despite the difference in polymer type.
Table (5):
The color estimation of the biopolymer produced from chitosan and whey protein with 1% added peppermint oil
Biopolymer |
brightness (L) |
Green-red (A) |
Yellow-blue (B) |
|---|---|---|---|
Treatment 1 |
28.54 |
0.34 |
7.83 |
Treatment 2 |
26.24 |
-0.07 |
6.16 |
Treatment 1: Biopolymer resulting from chitosan and milk serum protein. Treatment 2: Biopolymer resulting from chitosan and milk serum protein with 1% peppermint oil addition
All samples demonstrated membrane solubility with both chitosan/milk serum protein/peppermint oil formulations. The solubility of water is a critical criterion for evaluating the biodegradability potential of the membrane. Highly soluble membranes readily decompose, becoming into small particles. This behavior indicates that enhancing membrane solubility is advantageous, particularly for consumable applications. The enhanced solubility of protein membranes is due to diminished interactions between proteins and other constituents inside the polymer matrix.37 Elevated solubility is requisite when the membrane is designed for incorporation into a food product. Moreover, high solubility signifies a substance that is ecologically benign and readily biodegradable.38 Table 4 displays experimental data demonstrating that the interaction of chitosan, milk serum protein, and peppermint oil with the aqueous solution resulted in membrane solubility in water. The data clearly indicate that the incorporation of 1% peppermint oil led to a significant 2% reduction in the polymer’s solubility degree. Improving water resistance is advantageous for several polymer applications, particularly in packaging and coating sectors.39
Table 5 illustrates the color features of the biopolymer with 1% peppermint oil in contrast to the biopolymer without peppermint oil. The table demonstrates a variation in color values resulting from the application of oil. The values for L (brightness), A (green-red), and B (yellow-blue) for the polymer devoid of peppermint oil were elevated in comparison to the polymer infused with 1% peppermint oil. The polymer, both with and without peppermint oil, was characterized as having reduced glossiness and a propensity to yellow. The yellowness characteristic (B) of the biopolymer without peppermint oil was measured at 7.83, whereas the biopolymer with 1% peppermint oil recorded a ratio of 6.16. Conversely, regarding the greenness-redness characteristic (A), the biopolymer with 1% peppermint oil exhibited a ratio of (-0.07), but the biopolymer sample devoid of peppermint oil had a ratio of (0.34). The biopolymer devoid of peppermint oil exhibited a brightness ratio of 28.54, while the biopolymer infused with 1% peppermint oil had a ratio of 26.24. The incorporation of oil resulted in a reduction in the values of L, A, and B in comparison to the polymer devoid of peppermint oil. The color analysis reveals that the hue of polymer samples fluctuated with various additions.
The biopolymer’s antimicrobial qualities are defined by its capacity to impede the development and multiplication of microorganisms, such as bacteria and fungus. These attributes are crucial for food packing and preservation. The use of biopolymer materials has emerged as a potential trend in recent research and applications, owing to several benefits over packaging derived from chemical or petrochemical substances. Biopolymers are regarded as eco-friendly, readily biodegradable, and do not present environmental issues. They are considered safe since they originate from secondary by-products of some food items or from recycled food waste, ensuring their health safety. These polymers may include biologically active natural substances, such as plant oils, therefore augmenting their antibacterial efficacy against food spoilage microbes and foodborne pathogens. The present research illustrates the increasing interest in antimicrobial polymers within the food industry, driven by consumer demand for preservative-free products. The findings validate the potential of the newly developed biopolymer as an exceptionally effective packaging material with antibacterial characteristics in the food industry. This substance enhances food safety and quality, while catering to the increasing customer demand for preservative-free options.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Food Science and Human Nutrition, College of Agriculture and Food, Qassim University, for providing lab facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Abdallah EM, Alhatlani BY, de Paula MR, Martins CHG. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants. 2023;12(17):3077.
Crossref - Alizadeh-Sani M, Khezerlou A, Ehsani A. Fabrication and characterization of the bionanocomposite film based on whey protein biopolymer loaded with TiO2 nanoparticles, cellulose nanofibers and rosemary essential oil. Ind Crops Prod. 2018;124:300-315.
Crossref - Valenzuela C, Abugoch L, Tapia C. Quinoa protein-chitosan-sunflower oil edible film: Mechanical, barrier and structural properties. LWT-Food Sci Technol. 2013;50(2):531-537.
Crossref - Schudel S, Shoji K, Shrivastava C, Onwude D, Defraeye T. Solution roadmap to reduce food loss along your postharvest supply chain from farm to retail. Food Packag Shelf Life. 2023;36:101057.
Crossref - Gómez-Estaca J, López-de-Dicastillo C, Hernández-Muñoz P, Catalá R, Gavara R. Advances in antioxidant active food packaging. Trends in Food Science & Technology, 2014;35(1):42-51.
Crossref - Asgher M, Qamar SA, Bilal M, Iqbal HMN. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res Int. 2020;137:109625.
Crossref - Raafat D, Sahl HG. Chitosan and its antimicrobial potential-a critical literature survey. Microb Biotechnol. 2009;2(2):186-201.
Crossref - Diaz-Montes E, Castro-Munoz R. Trends in chitosan as a primary biopolymer for functional films and coatings manufacture for food and natural products. Polymers. 2021;13(5):767.
Crossref - Livney YD. Milk proteins as vehicles for bioactives. Curr Opin Colloid Interface Sci. 2010;15(1-2):73-83.
Crossref - Bourtoom T. Edible films and coatings: characteristics and properties. Int Food Res J. 2008;15(3):237-248.
- Abdallah EM. Plants: An alternative source for antimicrobials. J Appl Pharm Sci. 2011;1(6):16-20.
- Hyldgaard M, Mygind T, Meyer RL. Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. Front Microbiol. 2012;3:12.
Crossref - Coma V, Martial-Gros A, Garreau S, Copinet A, Salin F, Deschamps A. Edible antimicrobial films based on chitosan matrix. J Food Sci. 2002;67(3):1162-1169.
Crossref - Vasbinder, Astrid J, Cornelis G. De Kruif. Casein–whey protein interactions in heated milk: the influence of pH. International dairy journal. 2003;13(8): 669-677.
Crossref - Akhter R, Masoodi FA, Wani TA, Rather SA. Functional characterization of biopolymer based composite film: Incorporation of natural essential oils and antimicrobial agents. Int J Biol Macromol. 2019;137:1245-1255.
Crossref - Al-Hassan AA, Norziah MH. Starch-gelatin edible films: Water vapor permeability and mechanical properties as affected by plasticizers. Food Hydrocolloids. 2012;26(1):108-117.
Crossref - Al-Hassan AA, Abdel-Salam AM, Al Nasiri F, Mousa HM, Nafchi AM. Extraction and characterization of gelatin developed from camel bones. J Food Meas Charact. 2021;15:4542-4551.
Crossref - Arrieta A, Tuiran R, Montoya M. Influence of pH in mechanical properties of conductive polymers synthesized from cassava starch. Res J Appl Sci Eng Technol. 2017;14(4):155-160.
Crossref - Owczarz P, Ryl A, Wichlacz Z. Application of texture profile analysis to investigate the mechanical properties of thermosensitive injectable chitosan hydrogels. Prog Chem Appl Chitin Deriv. 2019;(24):151-163.
Crossref - Moghadam M, Salami M, Mohammadian M, Khodadadi M, Emam-Djomeh Z. Development of antioxidant edible films based on mung bean protein enriched with pomegranate peel. Food Hydrocolloids. 2020;104:105735.
Crossref - Kowalczyk P, Hippert F, Bernier N, et al. Impact of Stoichiometry on the Structure of van der Waals Layered GeTe/Sb2Te3 Superlattices Used in Interfacial Phase Change Memory (iPCM) Devices. Small. 2018;14(24):1704514.
Crossref - Committee AAoCCAM. Approved methods of the American association of cereal chemists. vol 1. American Association of Cereal Chemists. 2000.
- Sanchez-Gonzalez L, Vargas M, Gonzalez-Martinez C, Chiralt A, Chafer M. Use of essential oils in bioactive edible coatings: a review. Food Eng Rev. 2011;3(1):1-16.
Crossref - Hasan M, Gopakumar DA, Olaiya NG, et al. Evaluation of the thermomechanical properties and biodegradation of brown rice starch-based chitosan biodegradable composite films. Int J Biol Macromol. 2020;156:896-905.
Crossref - Jimenez XT, Cuenca AA, Jurado AT, Corona AA, Urista CRM. Traditional methods for whey protein isolation and concentration: effects on nutritional properties and biological activity. J Mex Chem Soc. 2012;56(4):369-377.
Crossref - Zubair M, Shahzad S, Hussain A, Pradhan RA, Arshad M, Ullah A. Current trends in the utilization of essential oils for polysaccharide-and protein-derived food packaging materials. Polymers. 2022;14(6):1146.
Crossref - Burt S. Essential oils: their antibacterial properties and potential applications in foods-a review. Int J Food Microbiol. 2004;94(3):223-253.
Crossref - Tang M, Hou D, Ding C, Wang K, Wang D, Wang J. Anti-oil-fouling hydrophobic-superoleophobic composite membranes for robust membrane distillation performance. Sci Total Environ. 2019;696:133883.
Crossref - Kouravand F, Jooyandeh H, Barzegar H, Hojjati M. Characterization of cross linked whey protein isolate based films containing Satureja khuzistanica Jamzad essential oil. J Food Process Preserv. 2018;42(3):e13557.
Crossref - Shetta A, Kegere J, Mamdouh W. Comparative study of encapsulated peppermint and green tea essential oils in chitosan nanoparticles: Encapsulation, thermal stability, in-vitro release, antioxidant and antibacterial activities. Int J Biol Macromol. 2019;126:731-742.
Crossref - Mahdavi V, Hosseini SE, Sharifan A. Effect of edible chitosan film enriched with anise (Pimpinella anisum L.) essential oil on shelf life and quality of the chicken burger. Food Sci Nutr. 2018;6(2):269-279.
Crossref - Gohargani M, Lashkari H, Shirazinejad A. Study on biodegradable chitosan-whey protein-based film containing bionanocomposite TiO2 and Zataria multiflora essential oil. J Food Qual. 2020;2020(1):1-11.
Crossref - Hosseini SF, Rezaei M, Zandi M, Farahmandghavi F. Fabrication of bio-nanocomposite films based on fish gelatin reinforced with chitosan nanoparticles. Food Hydrocoll. 2015;44:172-182.
Crossref - Moakes RJA, Sullo A, Norton IT. Preparation and characterisation of whey protein fluid gels: The effects of shear and thermal history. Food Hydrocoll. 2015;45:227-235.
Crossref - Socaciu M-I, Fogarasi M, Semeniuc CA, et al. Formulation and characterization of antimicrobial edible films based on whey protein isolate and tarragon essential oil. Polymers. 2020;12(8):1748.
Crossref - Al-Hassan AA, Norziah MH. Effect of transglutaminase induced crosslinking on the properties of starch/gelatin films. Food Packag Shelf Life. 2017;13:15-19.
Crossref - Mahmoud R, Savello PA. Solubility and hydrolyzability of films produced by transglutaminase catalytic crosslinking of whey protein. J Dairy Sci. 1993;76(1):29-35.
Crossref - Romani VP, Olsen B, Collares MP, Oliveira JRM, Prentice-Hernandez C, Martins VG. Improvement of fish protein films properties for food packaging through glow discharge plasma application. Food Hydrocolloids. 2019;87:970-976.
Crossref - El-Sayed HS, El-Sayed SM, Mabrouk AMM, Nawwar GA, Youssef AM. Development of eco-friendly probiotic edible coatings based on chitosan, alginate and carboxymethyl cellulose for improving the shelf life of UF soft cheese. J Polym Environ. 2021;29:1941-1953.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.