ISSN: 0973-7510
E-ISSN: 2581-690X
Polyhydroxyalkanoates (PHA) production from diverse group of microorganisms has been a topic for extensive research for several decades. Despite this extensive research explorations, commercialization of PHA is still facing major hurdles, mainly due to the high cost involved in PHA production and recovery. This study was designed to determine a sustainable approach to produce PHA using an underutilized fruit extract. The major novelty of this research work is the use of starfruit (Averrhoa carambola L.), a tropical fruit, as a substrate for PHA production employing Bacillus licheniformis NJ04. Commercialization of PHA production can help to tackle global issues like raising microplastic pollution and biomagnification. The maximum PHA production reported in this work was 3.8 g/L under optimized conditions like temperature of 37 °C, pH 7 under shaking conditions (120 rpm) with 2% glycerol and starfruit extract as a carbon source after 72 h of incubation. The extracted PHA was further characterized through (FTIR) Fourier-transform infrared radiation, differential scanning calorimetry (DSC), Thermogravimetric analysis (TGA), X-Ray diffraction (XRD), and Proton Nuclear magnetic resonance (1H NMR). Thus, the present work highlights a novel strategy for using starfruit waste as a cost-effective substrate for PHA production.
Biopolymer, Bacillus licheniformis, Polyhydroxyalkanoates, Star Fruit, Sustainable
Plastic production has exponentially increased in the past few decades due to their versatile nature thereby serving every area of human life and this in turn has accelerated more than a million tons of plastics going unrecycled, unused, or single used most of which are discarded carelessly ending up contributing towards water and land pollution. The increasing concern related to the evidence of microplastic entry into the otherwise sterile human placenta for the first time (both maternal and fetal) and their persistence in the least expected places has created an alertness about the long-term impact of plastic pollution to the ecosystem.1 Cost effectiveness and enhanced material properties of petroleum-based plastics made them ideal candidates for packaging and other applications. But the issues associated with their biodegradability and toxicity of breakdown products made the research focus on biopolymers like PHA.2 PHAs are the only class of biopolymers that are both completely biodegradable and biocompatible. They are linear polyester that contain 3-hydroxy fatty acid monomers. The R group in the structure represents an alkyl group if the R group contain methyl (CH3). The polymer obtained is (3-hydroxy-butyrate) and if it contains 4 monomeric unit it is poly (3-hydroxyhexanoate).3 The chemical modification of PHAs depends on various compositions which are useful for different applications.4 PHAs are polyesters that are produced in nature by many microorganisms. Many bacteria produce PHA through utilization of wide variety of sugars and lipids. When PHA is produced from bacteria under stress conditions they serve as both a carbon source and as an energy source. More than 150 monomers can be combined with PHA to obtain materials that have different properties such plastics have biodegradable properties and are used to produce bioplastics. These plastics can be thermoplastics or elastomeric materials which have a melting point of about 40 to 180.5
PHAs have a major use in biomedical field as a targeted drug delivery system that can travels to a particular organ with the given drug. PHAs are also used in skin tissue engineering technology that aids in regeneration of new tissue to replace the damaged or wounded tissue. PHAs are used in 3D printing and tissue regeneration studies.6 The major use of PHAs currently is in the agricultural field as mulch films, nets to cover the crop field that protects the crops and as grow bags, agricultural foils. PHA beads are also used as a tuberculin skin reagent for diagnosis of bovine tuberculosis.7 PHAs are gaining popularity in the aquaculture field as their supplementation was reported to enhance growth and pathogen resistance in prawns and fishes.8 Star fruit is a common tropical fruit extensively seen in South-East Asia and has extensive nutritional and medicinal properties. It is reported to have anti-inflammatory, anti-tumor activities and is traditionally used to treat fungal skin infections, eczema, and many other diseases in Ayurvedic and Chinese medicinal systems.9 The bacteria used Bacillus licheniformis is majorly found in soil and it is a gram-positive rod-shaped bacterium and assigned to the phylum Firmicutes and has high capacity of producing commercially important enzymes like amylases and proteases. The major aim of this study was to explore the possibility of using star fruit extract as a cost-effective medium for biopolymer production.
Microorganism and chemicals
All the media components used in this study were procured from Hi Media Laboratories Pvt. Ltd. (Mumbai, India). All the media components were of analytical grade and solvents were purchased from Future Labs (Bangalore, India). The strain Bacillus licheniformis NJ04 used in the study was previously isolated from soil samples and maintained in nutrient agar through periodic subculturing in the Microbiology Lab, Department of Life Sciences, Christ University, Bangalore India.10
Inoculum and hydrolysate (substrate) preparation
The Bacillus licheniformis NJ04 strain was cultured in basic nutrient broth media, pH 7.0 ± 0.02 for 24 h at 120 rpm in a shaker incubator and this served as the inoculum for PHA production.6 Star fruits were purchased from KR Market, Bangalore, India. The fruits were washed thoroughly with distilled water to remove all the dirt and grinded using a laboratory blender to extract the fruit pulp. The pulp was then strained using multiple layers of muslin cloth and 10 ml of the fruit extract (pH 3.2) was mixed with distilled water and made up to 100 ml. The extract was centrifuged at 7000 rpm for 10 min to separate all the materials that has density greater than water. The supernatant was collected and made up to 100 ml using distilled water, autoclaved and stored at -2 °C until further use. This served as a substrate for PHA production.
Media preparation
The PHA production media contained NaCl (10 g/L), Na2HPO4(3.7 g/L), (NH4)2HPO4 (0.5 g/L), MgSO4.7H2O (0.2 g/L), glycerol 2% (v/v), tryptone (5 g/L), yeast extract (0.5 g/L), and KH2PO4 (1 g/L) dissolved in fruit extract. Glycerol and MgSO4.7H2O should be autoclaved separately to avoid precipitation during autoclave and then added to the media. The pH of the fermentation media was adjusted to 7 using 1N NaOH solution and autoclaved. After sterilization and 1% (v/v) of inoculum (1.2 x 106 CFU/ml) was added to the modified media and incubated at 37 °C (120 rpm) for 72 h in a shaker incubator.11,12
Extraction and recovery of PHA
Recovery of PHA from bacterial biomass was done using solvent extraction method as suggested by Santhanam and Sasidharan.13,14 Bacterial cells were harvested through centrifugation at 10000 rpm for 15 min at 4 °C. The supernatant was discarded and the pellet was dried in a hot air oven for at 40 °C. Cell digestion was carried out by adding 5 ml of 4% sodium hypochlorite to the bacterial pellets in the tube followed by incubating it in a shaker incubator for 1 h for complete digestion of cell components. PHA extraction was carried out by centrifuging this mixture at 10000 rpm for 10 mins. The supernatant was discarded and the mixture was washed initially with distilled water followed by a solvent mixture consisting of diethyl ether, methanol, and acetone (1:1:1 ratio) by centrifuging at 10000 rpm for 5 mins.10 Precipitation of PHA is done adding 20 ml of hot chloroform into the tubes and emptying the mixture into pre weighed petri plates. The dry weight of the extracted PHA was measured after complete drying at room temperature.6
Characterization of PHA
Fourier-transform infrared characterization of PHA
The biopolymer’s ability is to produce peaks as the product of their functional group serves as the basic principle for the characterization of PHA by FTIR. The extracted sample was characterized using 2000 Perkins-Elmer spectrophotometer.10
Differential scanning calorimetry and Thermogravimetric analysis of PHA
DSC is used to study the melting temperature and glass transition temperature of PHA by observing the pattern of the absorption or release of heat during the polymer. This specific analysis was done at by selective heating of extracted PHA from room temperature to around 400 °C (Netzsch DSC 204 F1 Heat flux DSC) and the TGA was done to understand the pattern of thermal degradation of the polymer and its stability using Perkin ElmerSTA6000 thermal analyzer.11
X-ray diffraction analysis of PHA
This analysis uses X-rays to determine the physical and chemical composition of the material. The basic principle of this analysis is that monochromatic radiation is directed towards the sample that produces a diffraction pattern from this the intensity and function can be studied.15 The sample was scanned at the 2θ range between of 10-80° (Philips Xpert PRO, Netherlands).
1H NMR analysis of PHA
1H NMR technique analyses the molecular structure of the biopolymers by using a strong magnetic field that interacts with the nuclei of the sample and the resulting magnetic spin is used to produce the resonance images that can predict the three-dimensional structure of the polymers.16 The instrument used for this study is Bruker Avance III Spectrometer.
Screening for PHA production
In this study, the maximum production of PHA was observed in the modified production media (3.8 ± 0.05 g/L) prepared using start fruit extract as compared to the control media (2.81 ± 0.02 g/L) that is prepared with distilled water instead of fruit extract after 72 h of incubation. This obtained PHA was further used to characterized using FTIR, DSC, TGA, XRD and 1H NMR.
Characterization of PHA
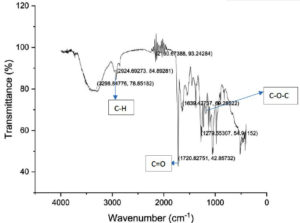
In this study, the IR spectra of the extracted polymer (Figure 1) show absorption bands at 1720.43 and 1377.17 cm-1 corresponding to the bonds C=O and C-O-C vibrations of the polymer. This IR peaks are attributed to compounds containing carbonyl group which is a distinguishing factor to confirm the presence of PHA.17,18 The results reported in this study agreed with a previous study on PHA production from bread manufacturing waste using bacteria isolated from soil. The FTIR peaks of the PHA extracted in that study was at 1718.3 cm-1 and 1080-1176 cm-1 and suggested that the extracted product as PHA.19,20 In another study dealing with evaluation of PHA synthesis by Pichia sp. TSLS24 yeast isolate in Vietnam the FTIR analysis indicated characteristic peaks at 1720 cm-1 (C=O of ester group), 1379 cm-1 (-CH(CH2)2 group) and 1100 cm-1 (C-O-C group) confirming the presence of PHA.21,22 In another study reporting production and characterization of PHA from lignin derivatives by Pandoraea sp. ISTKB, similar peaks were reported confirming the extracted molecule to be PHA.23
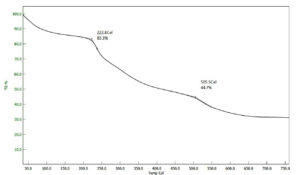
TGA studies of PHA extracted for Bacillus spp. shows that their thermal decomposition takes place in the range between 250-300 °C. The TGA plot of PHA extracted from B. licheniformis NJ04 revealed thermal degradation at 222.8 °C (Figure 2) which is in correlation with some of the available literature.11,12 Since PHA undergo degradation at a particular temperature the thermogram obtained shows the water loss, depolymerization and combustion in water loss.24 TGA curve spikes at 100 °C indicating the evaporation of water molecules. The second depolymerization happens in two steps, initial degradation happens breaking the ester bonds and second complete depolymerization happens when all the CO2, H2O and other small molecules are lost and finally the residual mass only 30% which may be due to the impurities present and can be improved through more precisely controlled purification processes.25,26 In this graph the initial weight loss of 16.7% was observed at 222.8 °C and further reduction can be seen at 55.3% was observed at 505.5 °C this does not correlate with other hence, graph shows a lower melting point that can be associated with impurities or the property of PHA produced by this bacterium.19 In a study reporting the biosynthesis and characterization of PHA copolymers produced by Pseudomonas putida Bet001 it was reported that increased thermal degradation occurred from 264.6 to 318.8 °C and suggested that long monomers are incorporated in the extracted PHA.27 The thermal degradation of the biopolymer extracted Bacillus megaterium strain JHA was observed at 277 °C and 98.04% loss is at 296.10 °C.28
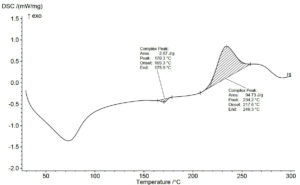
DSC analysis of the polymer is used to detect the melting temperature of the polymers. The melting temperature of Bacillus sp., was reported to be ranging from 130-170 °C.17 In this study, DSC curve of PHA shows the melting temperature as a complex peak at 234 °C (Figure 3). This is higher than the usual range probably due to the impurities present in the polymer or the property of PHA by this bacterium if a PHA sample has high degree of crystallinity indicates the tightly packed polymer chain, which requires more energy to break apart during melting.20 The DSC analysis of PHA obtained from Bacillus megaterium strain JHA shows the peaks corresponding to melting temperature of 158.04 °C.28 In another study dealing with the utilization of oxidized polyethylene wax as a potential carbon source for PHA production the melting temperature was observed at 171 °C and correlated with the result obtained in this study.29 In another research work reporting PHA production in Pseudomonas sp. phDV1 strain grown on phenol as carbon sources the DSC analysis revealed the complex peak at 172.8 °C indicating the presence of 3-HHx monomer.30
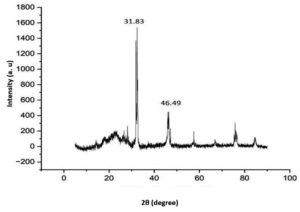
XRD pattern of PHA showed dominant peaks at 31.83° and 46.49° (Figure 4) and the minor peaks can be seen at 25.34° suggesting the semicrystalline nature of extracted PHA. Another literature data analysis showed characteristic peaks at 19.98°, 22.15°, 25.41° and 30.57° for PHB which is similar to the results obtained.24 In another study dealing with synthesis, characterization and PHA-based nanoparticles the XRD pattern peaks are observed at 13.5°, 19.35°, 22.88°, 25.37° and 27.73 °C respectively representing the crystalline nature of PHA.26 The larger and wider peaks indicate the crystalline structure of PHA and the minor peaks indicate the partial crystalline property of the extracted PHA.22
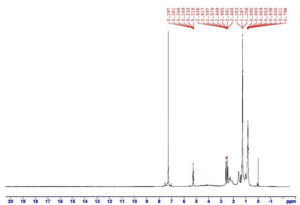
The 1H NMR spectrum of PHA typically indicates the signals from the proton which are the backbone of the polymer for crystalline PHA. In this study the 1H NMR spectrum of PHA (Figure 5) shows characteristic peaks at 1.2 ppm and 1.5 ppm indicate the presence of methyl proton in the polymer and the peaks at 2.5 ppm to 3 ppm indicates methylene protons adjacent to the carbonyl group in the polymer.18 In another study with a Bacillus spp. the extracted PHA showed characteristic peaks at 1.30 ppm and 2.57 ppm which corresponds to methylene and methyne groups.20 In 1H NMR analysis of PHA from Paraburkholderia sp. PFN 29 characteristic peaks were observed at 1.27 ppm (methyl groups) and multiple peaks at 1.57-1.61 ppm which indicated the presence of methylene protons.22 In another study dealing with characterization of PHA produced by Halomonas venusta KT832796, the resonance peaks at 1.2 ppm (attributed to methyl group) and the peaks from 2.4 to 2.6 ppm (for CH2 group) confirmed the polymer to be PHA.24 PHA produced from Pseudomonas sp. phDV1 Strain grown on phenol as carbon sources produced major peak at 1.27 ppm indicating the presence of methyl group.30
In summary, traditional fossil-based plastic materials lack biodegradability and leave carbon footprints; henceforth, PHA are the most promising microbial bioplastic that can replace conventional plastics, particularly for one-time usage, as they have similar performance. The global increase in demand for sustainable plastics is a major driver for developing this research work. The study focused on producing a sustainable biopolymer through microbial fermentation and using a cost-effective substrate like fruit extract. This is a cost-effective and economically attractive approach, but it is still a time-consuming process; thus, further studies are required to find affordable and fast ways for production. Further research can enhance the scalability and commercial viability of this bioproduction method. Hence, the current research work focuses on the concept of low-cost and abundant substrate. This is a simple and adaptable way for other fruit or fruit waste biopolymer production.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Life Sciences, CHRIST (Deemed to be University) for providing the necessary facilities and support for carrying out the research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
GR performed investigation, data analysis and wrote the manuscript. MU supervised the study, reviewed and edited the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Koller M, Rodriguez Contreras A. Techniques for tracing PHA producing organisms and for qualitative and quantitative analysis of intra and extracellular PHA. Eng Life Sci. 2015;15(6):558-581.
Crossref - Chavan S, Yadav B, Tyagi RD, Drogui P. A review on production of polyhydroxyalkanoate (PHA) bio polyesters by thermophilic microbes using waste feedstocks. Bioresour Technol. 2021;341:125900.
Crossref - Ray S, Kalia VC. Biomedical applications of polyhydroxyalkanoates. Indian J Microbiol. 2017;57(3):261-269.
Crossref - Ludevese-Pascual G, Laranja JLQ, Amar EC, Sorgeloos P, Bossier P, De Schryver P. Poly- beta-hydroxybutyrate-enriched Artemiasp. for giant tiger prawn Penaeus monodon larviculture. Aquaculture Nutrition. 2016;23(2):422-429.
Crossref - Castilho LR, Mitchell DA, Freire DMG. Production of polyhydroxyalkanoates (PHAs) from waste materials and by-products by submerged and solid-state fermentation. Bioresour Technol. 2009;100(23):5996-6009.
Crossref - Shah S, Kumar A. Production, and characterization of polyhydroxyalkanoates from industrial waste using soil bacterial isolates. Braz J Microbiol. 2021;52(2):715-726.
Crossref - Zhu J. Bioactive modification of poly (ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31(17):4639-4656.
Crossref - Amaro TMMM, Rosa D, Comi G, Iacumin L. Prospects for the use of whey for polyhydroxyalkanoate (PHA) production. Front Microbiol. 2019;10:992.
Crossref - Lakmal K, Yasawardene P, Jayarajah U, Seneviratne SL. Nutritional and medicinal properties of Star fruit (Averrhoa carambola): A review. Food Sci Nutr. 2021;9(3):1810-1823.
Crossref - James N, Umesh M, Sarojini, S, et al. Unravelling the potential plant growth activity of halotolerant Bacillus licheniformis NJ04 isolated from soil and its possible use as a green bioinoculant on Solanum lycopersicum L. Environ Res. 2023;216(Pt 2):114620.
Crossref - Bhuwal AK, Singh G, Aggarwal NK, Goyal V, Yadav A. Isolation and screening of polyhydroxyalkanoates producing bacteria from pulp, paper, and cardboard industry wastes. Int J Biomater 2013;2013(1):752821.
Crossref - Valdez-Calderon A, Barraza-Salas M, Quezada-Cruz M, et al. Production of polyhydroxybutyrate (PHB) by a novel Klebsiella pneumoniae strain using low-cost media from fruit peel residues. Biomass Convers Bioref. 2020;12(11):1-14.
Crossref - Sabapathy PC, Devaraj S, Parthipan A, Kathirvel P. Polyhydroxyalkanoate production from statistically optimized media using rice mill effluent as sustainable substrate with an analysis on the biopolymer’s degradation potential. Int J Biol Macromol. 2019;126:977-986.
Crossref - Santhanam A, Sasidharan S. Microbial production of PHA from Alcaligens spp, and Pseudomonas oleovorans using different carbon source. Afr J Biotechnol. 2010;9(1):3144-3150.
- Bunaciu AA, Udristioiu EG, Aboul-Enein HY. X-Ray diffraction: instrumentation and applications. Crit Rev Anal Chem. 2015;45(4):289-299.
Crossref - Gerothanassis IP, Troganis A, Exarchou V, Barbarossou K. Nuclear Magnetic Resonance (NMR) Spectroscopy: Basic Principles and Phenomena, and Their Appilcations to Chemistry, Biology and Medicine. Chem Educ Res Pract. 2002;3(2):229-252.
Crossref - Jacob GS, Garbow JR, Schaefer J. Direct measurement of poly(b-hydroxybutyrate) in a pseudomonad by solid-state 13C NMR. J Biol Chem. 1986;261:16785- 16787.
Crossref - Thirumala M, Reddy SV, Mahmood SK. Production and characterization of PHB from two novel strains of Bacillus spp. isolated from soil and activated sludge. J Ind Microbiol Biotechnol. 2010;37(3):271-278.
Crossref - Valappil SP, Peiris D, Langley GJ, et al. Polyhydroxyalkanoate (PHA) biosynthesis from structurally unrelated carbon sources by a newly characterized Bacillus spp. J Biotechnol. 2007;127(3):475-487.
Crossref - Sandhya M, Aravind J, Kanmani P. Production of polyhydroxyalkanoates from Ralstonia eutropha using paddy straw as cheap substrate. Int J Environ Sci Technol. 2013;10(1):47-54.
Crossref - Thu NTT, Hoang LH, Cuong PK, et al. Evaluation of polyhydroxyalkanoate (PHA) synthesis by Pichia sp. TSLS24 yeast isolated in Vietnam. Sci Rep. 2023;13(1):3137.
Crossref - Sriyapai T, Chuarung T, Kimbara K, Samosorn S, Sriyapai P. Production and optimization of polyhydroxyalkanoates (PHAs) from Paraburkholderia sp. PFN 29 under submerged fermentation. Electron J Biotechnol. 2021;56:1-11.
Crossref - Kumar M, Singhal A, Verma PK, Thakur IS. Production and Characterization of Polyhydroxyalkanoate from Lignin Derivatives by Pandoraea sp. ISTKB. ACS Omega. 2017;2(12):9156-9163.
Crossref - Stanley A, Murthy PSK, Vijayendra SVN. Characterization of Polyhydroxyalkanoate Produced by Halomonas venusta KT832796. J Polym Environ. 2020;28(3):973-983.
Crossref - Nanaki S, Barmpalexis P, Iatrou A, Christodoulou E, Kostoglou M, Bikiaris DN. Risperidone controlled release microspheres based on Poly(Lactic Acid)-Poly(Propylene adipate) novel polymer blends appropriate for long acting injectable formulations. Pharmaceutics. 2018;10(3):130.
Crossref - Samrot AV, Samanvitha SK, Shobana N, et al. The synthesis, characterization and applications of polyhydroxyalkanoates (PHAs) and PHA-Based nanoparticles. Polymers. 2021;13(19):3302.
Crossref - Gumel AM, Annuar MSM, Heidelberg T. Biosynthesis and Characterization of Polyhydroxyalkanoates Copolymers Produced by Pseudomonas putida Bet001 Isolated from Palm Oil Mill Effluent. PLoS ONE. 2012;7(9):e45214.
Crossref - IJRSR Journal-UGC guidelines journal |. http://www.recentscientific.com/
- Radecka I, Irorere V, Jiang G, et al. Oxidized polyethylene wax as a potential carbon source for PHA production. Materials. 2016;9(5):367.
Crossref - Kanavaki I, Drakonaki A, Geladas ED, Spyros A, Xie H, Tsiotis G. Polyhydroxyalkanoate (PHA) Production in Pseudomonas sp. phDV1 Strain Grown on Phenol as Carbon Sources. Microorganisms. 2021;9(8):1636.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.