ISSN: 0973-7510
E-ISSN: 2581-690X
In the context of sustainable bioremediation of Low-density polyethylene (LDPE), in this study, several strategies were explored to enhance the LDPE degradation by the bacterium Enterobacter cloacae AKS7. Initially, Mineral oil and Tween 80 were used to test whether they could modulate microbial colonization and polymer degradation by AKS7. Results indicated Mineral oil could increase microbial colonization and LDPE degradation whereas Tween 80 compromised the same. Since LDPE holds hydrophobic characteristics, the organism showing higher cell-surface hydrophobicity could adhere efficiently to the polymer. Thus, the organism AKS7 was grown in media with different concentrations of glucose and ammonium sulphate to exhibit differences in cell-surface hydrophobicity. We noticed that with increasing cell-surface hydrophobicity, the microbial colonization and LDPE degradation got enhanced considerably. The observations indicated that cell-surface hydrophobicity promoted microbial colonization to LDPE that increased the degree of biodegradation. Besides, LDPE films were photo-oxidized before microbial exposure which showed that AKS7 could degrade ultra-violet (UV) treated LDPE more proficiently compared to the UV-untreated polymer. Moreover, AKS7 could colonize more effectively to the UV-treated LDPE in contrast to the untreated LDPE. Furthermore, it was observed that UV exposure increased the carbonyl bond index of the polymer which got utilized by the organism efficiently thereby increasing the polymer degradation. Hence, the observations indicated that effective microbial colonization to UV-treated LDPE films exhibited a promising metabolic activity that could show an enhanced degradation of LDPE. Therefore, AKS7 warrants to be considered as a promising organism for enhanced degradation of LDPE.
LDPE, Enterobacter cloacae AKS7, Bioremediation, Microbial colonization, Cell-surface hydrophobicity, Carbonyl bond index
In 1970, Leo Baekeland coined the term “plastic” and introduced the world’s first synthetic plastic named “Bakelite”. In the following years, the versatility of plastic products made it a crucial ingredient of human existence. Plastics are malleable and its physicochemical properties such as light weight, flexibility, easy availability, and commercial viability have benefitted humans in diverse fields including manufacturing, packaging, healthcare, and agriculture1. As a result of which, worldwide production of plastic has increased to 381 million tons from 2 million tons in just 65 years2. Till 2017, more than 100 million tons of polyethylene (a thermoplastic polymer) were being made annually that covered nearly 34% of the total plastic market1. Thus, plastic production has become a major transboundary threat to both natural ecosystems as well as human health. Further studies have predicted that by 2030 there may be a twofold increase in plastic debris production (including both micro and nano-sized plastics)3. Considering the recent unfortunate times of the COVID-19 pandemic, the extent of plastic waste generation can increase significantly because of excessive consumption of one-time-use plastics which includes personal protective equipment (PPE) and masks3. Till 2015, nearly 6,300 metric tons of plastic wastes were generated out of which approximately 9% were recycled, 12% were incinerated, and 79% were dumped in landfills1. Among the different types of plastic molecules, LDPE happens to be a thermoplastic polymer that executes a range of applications in dispensing bottles, plastic bags, manufacturing toys, laboratory equipment, etc4. Since plastic-waste management methods are time-consuming, expensive, and labour intensive, the need of the hour is to look for various methods for effective polymer degradation. In this regard, it was reported that molecular weight, structure, crystallinity, density, mechanical, optical, and dielectric properties of plastic molecules could modulate the degree of degradation of LDPE considerably5-7. Towards the removal of LDPE plastics from the environment, bioremediation happens to be a sustainable option where the whole microorganism or a part of it could be involved in the degradation of recalcitrant molecules8. In this context, different types of microorganisms from various species like Bacillus, Pseudomonas, Arthrobacter, Staphylococcus, Penicillium, Aspergillus, etc. were reported to hold immense potential in breaking down polyethylene samples9-11. In our previous report, we stated that one of our soil isolates, Enterobacter cloacae AKS7, could degrade the LDPE polymer efficiently12. The said bacterium was found to degrade ~9% of the mentioned polymer (LDPE) under laboratory conditions12. Though in our previous report, a considerable level of LDPE biodegradation was spotted by Enterobacter cloacae AKS7, however, the extent of degradation needs to be increased to manage the huge accumulation of LDPE based polymers. Towards this direction, several strategies were undertaken to enhance the degree of LDPE degradation by Enterobacter cloacae AKS7. The results of the current report revealed that without being exposed to genetic manipulation, the bacteria Enterobacter cloacae AKS7 could modulate the degree of utilization of LDPE either by changing the surface hydrophobicity of the bacterial or by altering the structure of the polymer.
Sample collection
We approached a plastic bag manufacturing company, Hipack Systems Pvt. Limited, Kolkata, India for the collection of LDPE. After cutting these LDPE sheets into equal-sized (3×2 cm) pieces, the polymers were cleaned with sterile double distilled water. Thereafter, the small strips were sterilized by soaking them into 70% ethanol overnight. These sterilized LDPE strips were then dried in a dryer for an hour and subsequently used for biodegradation studies. In some experiments, the LDPE films were air-dried and exposed to UV radiation for 120 h inside a laminar airflow where a high-power UV bulb (15W) was used for illumination. The UV-absorbed LDPE films were sterilized using the same methods as discussed earlier.
Bacterial growth media and culture conditions
In this current study, the LDPE degradation efficiency of Enterobacter cloacae AKS7 was studied under varied conditions. To study the biodegradation of LDPE by the bacterium AKS7, the organism was inoculated (1×106 CFU/mL) in 100 mL of sterile basal medium. A 100 mL of basal medium was made by incorporating 10 mg yeast extract, 0.1 g (NH4)2SO4, 20 mg MgSO4•7H2O, 2 mg CaCl2•2H2O, 10 mg NaCl, 1 mg FeSO4•7H2O, .05 mg Na2WO4•2H2O, 0.16 g K2HPO4, 0.05 mg Na2MoO42H2O, 20 mg KH2PO4, 0.05 mg MnSO4 in 100 mL sterile double distilled water. After that, the basal media was autoclaved at 121°C for 15 min. To this broth, sterile LDPE films weighing 30 mg were added aseptically. A conical flask containing the sterile basal media along with the LDPE films was marked as control, as AKS7 was not added to the said experimental set. In some experiments, different chemicals (Mineral oil and Tween 80) were added separately or in combination to the growth media to examine the level of LDPE degradation by the organism AKS7 under different conditions. Tween 80 and Mineral oil was passed through a polycarbonate membrane filter (0.4 μm) before adding them to the growth media. However, in some experiments, varying amounts of glucose and ammonium sulphate were also introduced to examine any variation in the LDPE degradation by AKS7. Besides, UV-treated LDPE films (30 mg) were also added to the basal media (100 mL) to examine any increase in the degradation of LDPE by the organism AKS7. The organism was incubated at 30°C for different length of time for different experiments.
LDPE degradation studies by weight loss analysis
Weight loss analysis could be used to determine the quantitative estimation of LDPE degradation by microorganisms8. To do so, LDPE polymer samples were first divided into several equal pieces (3×2 cm) and then washed with 70% ethanol solution to make them microorganism-free. After that, 30 mg LDPE films were diligently added to 100 mL of sterile basal media which was augmented with either external agents (Mineral oil and/or Tween 80) or different concentrations of nutrients (glucose and/or ammonium sulphate). To it, 20 µL overnight culture of the organism (1×106 CFU/mL) was added and further incubated at 30°C for 30 days. In the control set, the basal media was incubated under similar conditions without the test organism. After the incubation, the LDPE films were individually collected from the growth media. Subsequently, the LDPE films were treated with 2% (v/v) sodium dodecyl sulphate (SDS) solution to remove the colonized microbial cells (if any)13. Afterward, the samples were washed, air-dried, and weighed. Differences in the initial weight and the final weight of LDPE films indicate the quantitative measurement of weight loss of the polymer by the organism.
Weight loss (in %) = {(Weight measured before microbial exposure-Weight measured after microbial exposure)/Weight measured before microbial exposure} X 100
Cell-surface hydrophobicity measurement
Bacterial adhesion to hydrocarbon (BATH) assay was followed to determine the cell-surface hydrophobicity (CSH) of AKS714. One (1) mL overnight culture of AKS7 (1X106 CFU/mL) was inoculated into different conical flasks comprising 100 mL of basal media augmented with different concentrations of glucose and ammonium sulphate. 30 mg sterile LDPE samples were added to each of the growth media under aseptic conditions. All the growth media were allowed to incubate at 30°C for 30 days. Post incubation, the culture media was collected from each conditioned media and subsequently centrifuged at 8000 rpm for 10 min. Afterward, the pellet was collected and suspended in sterile PUM {Phosphate (17g/L), Urea (1.8 g/L), and Magnesium (0.2 g/L)}buffer in such a manner so that the optical density (OD400) of the microbial suspensions could reach 1.0-1.2. Next, an organic solvent, n-hexadecane was distributed (0 to 0.2 mL) among the tubes carrying microbial suspensions. After vortexing all the experimental sets for 15 min, the tubes were allowed to stand for another 15 min at normal temperature to observe the separation of the aqueous and organic phases. Finally, OD400 of the aqueous suspensions was determined. A control set was also prepared wherein the OD was recorded for the PUM buffer only. Thereafter, by using the formula mentioned below, the CSH of the test bacteria was estimated:
CSH (in %) = 100 X {(Initial OD-Ultimate OD)/Initial OD}
Estimating the bacterial population on the LDPE film
The degree of adherence of AKS7 to the polymer films was determined by estimating the amount of extractable protein, as the extent of recovered protein is directly proportional to the degree of the attached microorganisms15. To extract proteins from the bacteria attached to either UV-treated or untreated LDPE film, each LDPE sample was separately taken from the growth media in which equal weight of either UV-treated or untreated LDPE films were added. The recovered LDPE films were boiled in 5 mL of 0.5 (N) NaOH for 30 min. In the next step, centrifugation of the boiled microbial suspension was carried out at 10000 rpm for 10 min. After that, the protein count of the centrifuged supernatant was estimated by following the Lowry method16.
Microscopic analysis of microbial colonization to LDPE film
To estimate the microbial association of AKS7 on the polymer surface, 30 mg of both UV-treated and untreated LDPE films were added to the basal media (100 mL). To it, 100 μL overnight culture of AKS7 was added. UV-treated and untreated LDPE films were individually recovered from the growth media post an incubation of 30 days at 30°C. The collected polymer films were subsequently stained with acridine orange solution (4 μg mL−1) and viewed under a fluorescence microscope15.
Analysing the metabolic activity
To estimate the metabolic activity of the attached bacterial population on the LDPE surface, Fluorescein diacetate (FDA) hydrolysis assay was performed15. To do the test, 30 mg of both UV-treated and the untreated polymer was independently introduced into the 100 mL of sterile basal media. To it, 100 µL of a saturated culture of AKS7 was introduced diligently. After an incubation of 30 days at 30°C, polymer films were recovered from all the growth media. After that, all the polymer films were individually dipped into 60 mM sodium phosphate buffer (pH 7.6) followed by incorporating FDA solution (10 μg mL−1) into the sodium phosphate buffer. After an incubation of 2 h at 30°C, the reaction mixture was centrifuged at 8000 rpm for 10 min. The supernatant was subsequently collected and subjected to the measurement of optical density at 494 nm10.
Fourier transformed infrared spectroscopy (FTIR) analysis of LDPE
To investigate the alterations in the bond profile of LDPE post UV treatment and microbial exposure, both the UV-exposed and unexposed LDPE samples (30 mg) were separately added to 100 mL sterile basal media. To it, a similar number of microorganisms (1×106 CFU/mL) were independently added. However, in the control set, LDPE sheets were added to the basal media but the said organism AKS7 was not inoculated. All the growth media was then incubated at 30°C for 30 days under similar conditions. Post incubation, both UV-exposed and unexposed LDPE sheets were recovered from the growth media and cleaned using 2% SDS solution to take away the attached microorganisms (if any). Then the LDPE films were washed twice and air-dried. Thereafter, the dried LDPE films were subjected to FTIR analysis (Jasco FT/IR-6300, Instrument ID: CP/QC/I/015, Cradel Pharmaceuticals Pvt. Ltd., India). LDPE samples were scanned at normal conditions under a range of wavelength starting from 400 to 4000 cm−1. The process was repeated three times for each sample to confirm the bond spectrum.
Statistical analysis
To evaluate the statistical difference in the experimental results, a one-way analysis of variance (ANOVA) was performed. To draw a contrast in the results with the control, the extent of the significance test was represented by using P-values. P-values obtained as < 0.05 were marked with (*), P values obtained as < 0.01 were marked with (**), and P values obtained as < 0.001 were marked with (***). N.S. (no statistical difference) was used to reveal the P values which were above 0.05. Each of the experimental sets was conducted three times to attain statistical confidence. The relation among the three variables namely cell-surface hydrophobicity, microbial colonization, and LDPE degradation was established by computing the contour plot using Minitab 19 software. However, the relation among microbial colonization, metabolic activity, and LDPE degradation was established by computing surface plots using NCSS software. The trial version of both Minitab 19 and NCSS software was used for the current study.
Mineral oil and Tween 80 exhibited considerable modulation in the microbial colonization and degradation of LDPE by Enterobacter cloacae AKS7
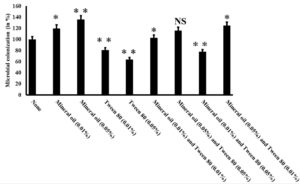
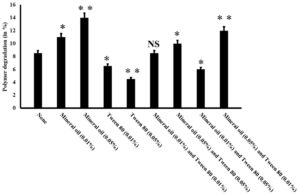
LDPE may have several applications in our day-to-day life but it also brings multiple drawbacks along with it. This polyethylene is highly resistant to natural degradation and persists in the environment for a very long time, thereby impacting the ecosystem adversely4,17. Different strategies including burning, recycling, and bioremediation are being employed to remove this LDPE-related wastes4. Among these, bioremediation is one of the eco-friendliest approaches as the process is cost-effective and its end products are nontoxic8,18. In the context of microorganism-based LDPE degradation, we had previously reported the isolation of an efficient organism namely Enterobacter cloacae AKS7 from soil that exhibited considerable degradation of LDPE12. The mentioned bacteria could exhibit nearly 9% degradation of LDPE for a period of 30 days12. Since bioremediation appears to be a sustainable approach to manage the LDPE-based plastic accumulation19, strategies were explored to enhance the extent of microbial degradation of LDPE. Microbial colonization to LDPE had been found to promote the extent of biodegradation considerably as the closer association between bacteria and polymer could lead to better utilization of the polymer by the bacteria4. Literature survey revealed that chemical compounds like Mineral oil and Tween 80 were found to influence the microbial colonization of LDPE noticeably10. In this regard, different concentrations of these compounds (Mineral oil and Tween 80) were used either separately or in combination to test whether they could modulate the microbial colonization of Enterobacter cloacae AKS7 to the LDPE surface. The observations indicated that Mineral oil and Tween 80 were found to increase or decrease the microbial colonization, respectively over the LDPE surface; in contrast, to control in which the cells were grown in the absence of the mentioned compounds (Fig. 1). We also experienced that with the gradual rise of the concentrations of Mineral oil and Tween 80, the level of microbial colonization to the LDPE surface got increased or decreased, respectively (Fig. 1). However, when these two agents were applied together, then the extent of microbial colonization of AKS7 to LDPE surface got changed considerably (Fig. 1). Microbial colonization got increased when the cells were treated with higher concentrations of Mineral oil and a lower concentration of Tween 80 (Fig. 1). However, the same got decreased on exposing the bacterial cells to a higher dose of Tween 80 and a lower dose of Mineral oil (Fig. 1). Taken together, the results demonstrated that both these agents either promote or compromise the AKS7 colonization to the LDPE surface, respectively. Further, to examine the effect of these compounds on the biodegradation of LDPE, weight loss of LDPE was compared between the Mineral oil and Tween 80 treated and untreated basal media supplemented with an equal weight of LDPE and a similar number of bacteria. A control set was also marked in which a similar number of cells were grown in basal media supplemented with LDPE. Mineral oil and Tween 80 were not added to the growth media for the control set. We noticed that with respect to the control, the extent of degradation of LDPE by Enterobacter cloacae AKS7 got changed considerably under the influence of Mineral oil and Tween 80 (Fig. 2). The result showed that with the increase of the concentrations of Tween 80, the extent of degradation of LDPE by Enterobacter cloacae AKS7 got reduced considerably (Fig. 2). On the contrary, the degradation of LDPE by the same bacteria got increased when exposed to Mineral oil (Fig. 2). The result further showed that when these two agents were applied together to the basal media containing LDPE and the test organism, then the extent of microbial degradation of LDPE got changed considerably (Fig. 2). Out of the two mentioned agents, higher concentrations of Mineral oil were estimated to accelerate LDPE degradation, whereas, higher concentrations of Tween 80 were found to effectively reduce the extent of LDPE degradation (Fig. 2). Thus, the results revealed that these two agents, Mineral oil and Tween 80 could enhance or reduce the microbial degradation of LDPE, respectively.
Fig. 1. Mineral oil and Tween 80 could efficiently modulate the microbial colonization to the LDPE surface. A saturated culture of Enterobacter cloacae AKS7 was inoculated in sterile basal media containing LDPE. After that, various concentrations of Mineral oil and Tween 80 were added to the growth media. All the culture media were then incubated at 30°C for 30 days. In the control set, Mineral oil and Tween 80 were not added to the growth media. Post incubation, LDPE films were collected and the total extractable protein from the LDPE films was calculated by following the Lowry method. The result demonstrated the average of three replicates. Error bars indicated mean ± standard error of the mean. Statistical analysis was performed using ANOVA and the P-value <0.05 (marked with *), <0.01 (marked with **) was mentioned in the figure. NS indicated that the observed result exhibited no significant statistical difference in respect to the control set.
Fig. 2. Mineral oil and Tween 80 could efficiently modulate polymer degradation. Different concentrations of Mineral oil and Tween 80 were added to the culture media to examine the effect of Mineral oil and Tween 80 on LDPE degradation by Enterobacter cloacae AKS7. In the control set, Mineral oil and Tween 80 were not added rather microorganism was incubated with LDPE only in sterile basal media. All the experiments were incubated at 30°C for 30 days. Weight loss analysis was performed to determine the extent of LDPE degradation by Enterobacter cloacae AKS7. Error bars indicated mean ± standard error of the mean. Statistical analysis was performed using ANOVA and the P-value <0.05 (marked with *), <0.01 (marked with **) was incorporated in the figure. NS indicated that the observed result exhibited no significant statistical difference in respect to the control set.
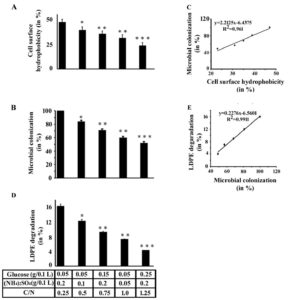
Glucose and ammonium sulphate in the growth media showed alteration in the cell-surface hydrophobicity and polymer degradation by Enterobacter cloacae AKS7
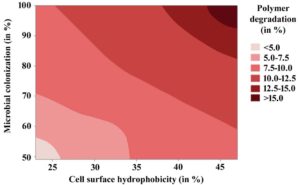
The results so far indicated that the increased bacterial colonization to the LDPE polymer surface could lead to exhibit the enhanced LDPE degradation by Enterobacter cloacae AKS7. Hence, in the present study, exploration of the underlying cause of bacterial colonization to the LDPE surface was investigated. In this regard, existing literature revealed that several factors including hydrophobic interaction could play a major role in microbial colonization to a hydrophobic surface20. The previous literature reported that the availability of the nutrients (glucose and ammonium sulphate) in the culture media was found to alter the cell-surface hydrophobicity of the microorganism that resulted in the variation in microbial colonization20. Thus, in the present study, different concentrations of glucose and ammonium sulphate were added to the basal media in which the polymer (LDPE) and the microorganism (Enterobacter cloacae AKS7) were added accordingly. Then, the cell-surface hydrophobicity of the organisms was measured by undertaking the BATH assay as mentioned in the methods and materials section. It was noticed that with the variation in the amount of glucose and ammonium sulphate, the microbial cell-surface hydrophobicity also got changed (Fig. 3A). The result further showed that with the increase of carbon to nitrogen ratio, the cell-surface hydrophobicity of AKS7 got decreased considerably (Fig. 3A). To understand the influence of cell-surface hydrophobicity over microbial colonization, the bacterial colonization to the LDPE surface was also measured under the conditions in which the cell-surface hydrophobicity of the bacteria was determined. In this connection, we observed that the bacterial colonization to the LDPE film surface got reduced with the increase of carbon to nitrogen ratio (Fig. 3B). As expected, the response of cell-surface hydrophobicity and microbial colonization followed the same pattern under the influence of varying concentrations of glucose and ammonium sulphate (See Fig. 3A and Fig. 3B). Thus, the result showed a directly proportional relationship between the cell-surface hydrophobicity and microbial colonization (Fig. 3C). Thus, the result showed that higher cell-surface hydrophobicity could lead to increased microbial colonization and vice versa. To comprehend the role of varying concentrations of glucose and ammonium sulphate over LDPE degradation by the test bacteria, microbial polymer degradation was determined under the tested conditions. The degree of LDPE degradation by the organism AKS7 got changed with the change in the concentrations of the applied nutrients in the growth media (Fig. 3D). The polymer degradation ability of the bacteria got reduced with the increase of carbon to nitrogen ratio in the growth media (Fig. 3D). Hence, the result revealed that the nutrient manipulation in the growth media was found to alter the LDPE degradation property of Enterobacter cloacae AKS7. To gain further confidence, the relationship between microbial colonization and LDPE degradation was determined. The result of the same revealed that a directly proportional relation prevailed between microbial colonization and LDPE degradation (Fig. 3E). The result suggested that efficient microbial colonization of AKS7 appeared to be the cause of increased LDPE degradation. To understand the relationship among the cell-surface hydrophobicity, microbial colonization, and polymer degradation, a contour plot was compiled in which we showed that the cell-surface hydrophobicity of the bacteria promoted increased bacterial colonization to the polymer surface that resulted in the enhanced degradation of the polymer by the bacteria, Enterobacter cloacae AKS7 (Fig. 4). Taken together, the results revealed that microbial colonization to the LDPE surface could be considered as a potential tool for the enhanced degradation of the polymer by the microorganism, Enterobacter cloacae AKS7.
Fig. 3. Different concentrations of glucose and ammonium sulphate could modify cell-surface hydrophobicity, microbial colonization, and LDPE degradation considerably. An overnight culture of Enterobacter cloacae AKS7 was added to the sterile basal media augmented with LDPE. To it, different concentrations of glucose and ammonium sulphate were added. All the growth media were then incubated for 30 days at 30°C. After the incubation, the following experiments were conducted. A. Cell-surface hydrophobicity profile. A similar number of cells were collected from all experimental sets and the BATH assay was followed to determine the cell-surface hydrophobicity of AKS7 under different conditions. B. Microbial colonization profile. LDPE films were collected from all experimental sets, boiled with NaOH solution and total proteins were then recovered. The recovered proteins were subsequently measured by the Lowry method. C. Plot of cell-surface hydrophobicity vs. microbial colonization on LDPE surface by AKS7. D. Polymer degradation profile. LDPE films were collected from all the experimental sets and the films were then air-dried. After that, the weight loss of LDPE films was recorded. Each experiment was performed thrice. Error bars indicated mean ± standard error of the mean. Statistical analysis was performed using ANOVA and the P-value <0.05 (marked with *), <0.01 (marked with **), and < 0.001 (marked with ***) were considered to express the significant difference. E. Plot of microbial colonization vs. LDPE degradation by AKS7.
Fig. 4. Relationship among cell-surface hydrophobicity, microbial colonization, and polymer degradation was established by constructing a contour plot using Minitab 19 software. Different colors represented different degrees of LDPE degradation.
Enterobacter cloacae AKS7 exhibited enhanced degradation of pre-oxidized LDPE
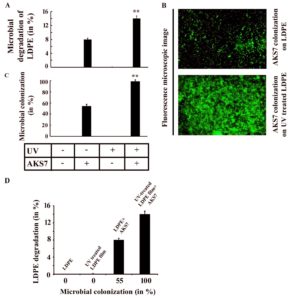
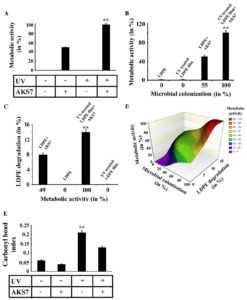
Previous literature reported that microorganisms could degrade pre-oxidized LDPE more efficiently than native LDPE film4. In this context, an effort was made to compare the bacterial degradation of both UV-treated and untreated polymer film under similar conditions. To examine the same, a similar number of AKS7 was inoculated to the basal media in which an equal weight of UV-treated and UV-untreated polymer films were added separately. In the control set, the basal media was augmented with polymer films but not inoculated with the bacteria, Enterobacter cloacae AKS7. All these experimental sets were put for incubation at 30°C for 30 days. After the incubation period, the LDPE films were collected from the tested growth media, and the level of polymer degradation was examined by following the weight loss analysis. The observations indicated that the test organism AKS7 could degrade UV-treated polymer films more proficiently than UV-untreated polymer film (Fig. 5A). Since microbial colonization was found to promote LDPE degradation considerably, here, in this report, the extent of bacterial colonization of AKS7 between UV-treated and untreated LDPE polymer films was studied. The outcome disclosed that AKS7 could colonize UV-treated polymer samples more proficiently than UV-untreated samples (Fig. 5B). The microbial associations on UV-treated LDPE films were found to be higher in contrast to the microbial associations noticed over UV-untreated LDPE film suggesting more microbial associations over the UV-treated LDPE polymer films. To validate the observation, the degree of microbial colonization of AKS7 on the polymer surface was verified by determining the protein from the adhered microbial population. The result showed that more protein was recovered from the UV-treated LDPE polymer film in contrast to the recovered protein from UV-untreated LDPE film (Fig. 5C). Thus, the results of microscopic analysis and protein recovery followed the same trend suggesting better microbial colonization of AKS7 to UV-treated polymer than UV-untreated LDPE. To examine the effect of microbial colonization over LDPE degradation, the relation between microbial colonization and LDPE degradation was determined. In this regard, we observed a directly proportional relation between microbial colonization and LDPE degradation (Fig. 5D). Next, to estimate the metabolic activity linked with the associated bacterial cells on the polymer surface, FDA assay was considered as mentioned before. Metabolically active bacteria secrete various kinds of enzymes that have been found to hydrolyze FDA resulting in the release of fluorescein that can be quantified through spectrophotometer15. The result showed that the colonized cells of AKS7 on UV-treated LDPE film exhibited increased metabolic activity in comparison to the metabolic activity of the cells adhered to the UV-untreated polymer films (Fig. 6A). To address the metabolic activity of the colonized cells of AKS7, a relation was established between microbial colonization and metabolic activity. The result of the same revealed that a directly proportional relation prevailed between them (Fig. 6B). This observation suggested that colonized cells of AKS7 on the polymer surface were metabolically active that could be responsible to attain the boosted degradation of UV-treated LDPE films. To confirm the observation, the relationship between metabolic activity and LDPE degradation was determined. The result of the same revealed that a directly proportional relation prevailed between metabolic activity and LDPE degradation (Fig. 6C). To understand the relationship between microbial colonization, metabolic activity, and polymer degradation, a surface plot was compiled. The result of the same revealed that along with the increase of microbial colonization, the degree of metabolic activity got amplified which resulted in the enhanced utilization of LDPE (Fig. 6D). To comprehend the underlined cause of this enhanced degradation of the polymer by Enterobacter cloacae AKS7, the carbonyl bond index was compared between UV-treated and untreated LDPE films as this bond index has been considered as a key factor for the utilization of LDPE by microorganisms4,21,22. The carbonyl bond index of LDPE can be measured by counting the ratio of the absorbance peaks of carbonyl (1712 cm−1) to that of CH2 (1462 cm−1)21,22. The result showed that UV exposure was able to increase the carbonyl bond index of LDPE considerably (Fig.6E). However, the degree of the carbonyl bond index of UV-treated LDPE film got reduced under the exposure of the microorganism, Enterobacter cloacae AKS7 (Fig. 6E). These results suggested an initial increase in the carbonyl bond index when the film was treated with UV. However, once these UV-treated polymer films were exposed to Enterobacter cloacae AKS7, the carbonyl bond index got decreased considerably which suggested that UV treatment made the LDPE films more accessible to the microorganisms. Taken together, the results indicated that prior oxidation of LDPE films by UV could make the polymer film more accessible to Enterobacter cloacae AKS7 that resulted in the increased degradation of LDPE.
Fig. 5. Enhanced degradation of pre-oxidized LDPE film by Enterobacter cloacae AKS7. A similar number of AKS7 were added to the sterile basal media in which either UV-treated or untreated LDPE films were separately added. All the growth media were then incubated for 30 days at 30°C. After the incubation, both types of LDPE films were recovered. A. Polymer degradation was carried out using weight loss measurement. B. Microbial colonization to LDPE films was analyzed under a fluorescence microscope. C. Degree of the microbial adherence to the polymer films was measured by estimating the total protein of the colonized microbial population following the Lowry method. Each experiment was performed thrice. Error bars indicated mean ± standard error of the mean. Statistical analysis was performed using ANOVA and the P-value <0.01 (marked with **) were considered to express the significant difference among the results. D. Plot of microbial colonization vs LDPE degradation.
Fig. 6. Enterobacter cloacae AKS7 could colonize and degrade UV-treated LDPE film more efficiently than UV-untreated LDPE film. An equal number of AKS7 was added to the sterile basal media in which either UV-treated or untreated LDPE films were independently added. All the growth media were then incubated at 30°C for 30 days. After the incubation, each type of LDPE film was recovered from the growth media. A. Metabolic activity of the microorganisms adhered to the LDPE films was determined by FDA hydrolysis assay. B. Plot of microbial colonization vs metabolic activity of the colonized cells. C. Plot of metabolic activity vs. LDPE degradation by AKS7. Each experiment was performed thrice. Error bars indicated mean ± standard error of the mean. Statistical analysis was performed using ANOVA and the P-value <0.01 (marked with **) was considered to express the significant difference among the results. D. Relation among microbial colonization, metabolic activity, and LDPE degradation was established by constructing a surface plot. E. Carbonyl bond index of different types of LDPE films was analyzed using an FTIR spectrophotometer. Each experiment was performed thrice. Error bars indicated mean ± standard error of the mean. Statistical analysis was performed using ANOVA and the P-value <0.01 (marked with **) were considered to express the significant difference among the results.
In conclusion, the current study deciphers the exploration of strategies where either the enhancement of microbial cell-surface hydrophobicity or pre-oxidation of LDPE could be pursued for enhanced bioremediation of LDPE molecule by Enterobacter cloacae AKS7.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
RKS and PP performed the experiments, analyzed the results, and wrote the manuscript. SD, SC and PC analyzed the results and framed the manuscript comprehensively. PT conceived the idea, designed the experiments, analyzed the results, and critically arranged the manuscript. All authors have read and approved the manuscript for publication.
FUNDING
None.
ETHICS STATEMENT
Not applicable .
AVAILABILITY OF DATA
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
- Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Sci Adv. 2017;3 (7):e1700782.
Crossref - Ritchie H, Roser M. Plastic pollution. Our World in Data. 2018. https://ourworldindata.org/plastic-pollution.
- Silva AL, Prata JC, Walker TR, et al. Rethinking and optimizing plastic waste management under COVID-19 pandemic: Policy solutions based on redesign and reduction of single-use plastics and personal protective equipment. Sci Total Environ. 2020;742:140565.
Crossref - Tribedi P, Dey S. Pre-oxidation of low-density polyethylene (LDPE) by ultraviolet light (UV) promotes enhanced degradation of LDPE in soil. Environ Monit Assess. 2017;189(12):624.
Crossref - Volke-Sepulveda T, Saucedo-Castaneda G, Gutierrez-Rojas M, Manzur A, Favela-Torres E. Thermally treated low density polyethylene biodegradation by Penicillium pinophilum and Aspergillus niger. J Appl Polym Sci. 2002;83(2):305-314.
Crossref - Yamada-Onodera K, Mukumoto H, Katsuyaya Y, Saiganji A, Tani Y. Degradation of polyethylene by a fungus, Penicillium simplicissimum YK. Polym Degrad Stab. 2001;72(2):323-327.
Crossref - Saha N, Zatloukal M, Saha P. Modification of polymers by protein hydrolysate-way to biodegradable materials. Polym Adv Technol. 2003;14(11-12):854-860.
Crossref - Tribedi P, Sarkar S, Mukherjee K, Sil AK. Isolation of a novel Pseudomonas sp. from soil that can efficiently degrade polyethylene succinate. Environ Sci Pollut Res Int. 2012;19(6):2115-2124.
Crossref - Chatterjee S, Roy B, Roy D, Banerjee R. Enzyme mediated biodegradation of heat treated commercial polyethylene by Staphylococcal species. Polym Degrad Stab., 2010;95(2):195-200.
Crossref - Tribedi P, Sil AK. Low-density polyethylene degradation by Pseudomonas sp. AKS2 biofilm. Environ Sci Pollut Res Int. 2013;20(6):4146-4153.
Crossref - Yang J, Yang Y, Wu WM, Zhao J, Jiang L. Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic eating waxworms. Environ Sci Technol. 2014;48(23):13776-13784.
Crossref - Sarker RK, Chakraborty P, Paul P, Chatterjee A, Tribedi P. Degradation of low density poly ethylene (LDPE) by Enterobacter cloacae AKS7: a potential step towards sustainable environmental remediation. Arch Microbiol. 2020;202(8):2117-2125.
Crossref - Gilan I, Hadar Y, Sivan A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl Microbiol Biotechnol. 2004;65(1):97-104.
Crossref - Rosenberg M, Perry A, Bayer EA, Gutnick DL, Rosenberg E, Ofek I. Adherence of Acinetobacter calcoaceticus RAG-1 to human epithelial cells and to hexadecane. Infect Immun. 1981;33(1):29-33.
Crossref - Tribedi P, Gupta AD, Sil AK. Adaptation of Pseudomonas sp. AKS2 in biofilm on low-density polyethylene surface: an effective strategy for efficient survival and polymer degradation. Bioresour Bioprocess. 2015;2(1):14.
Crossref - Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275.
Crossref - Zeng G, Chen M, Zeng Z. Risks of neonicotinoid pesticides. Science. 2013;340(6139):1403.
Crossref - Kawai F. Breakdown of plastics and polymers by microorganisms. Adv Biochem Eng Biotechnol. 1995;52:151-194.
Crossref - Pramila R, Ramesh KV. Biodegradation of low density polyethylene (LDPE) by fungi isolated from marine water a SEM analysis. Afr J Microbiol Res. 2011;5(28):5013-5018.
Crossref - Tribedi P, Sil AK. Cell surface hydrophobicity: a key component in the degradation of polyethylene succinate by Pseudomonas sp. AKS2. J Appl Microbiol. 2014;116(2):295-303.
Crossref - Hadad D, Geresh S, Sivan A. Biodegradation of polyethylene by the thermophilic bacterium Brevibacillus borstelensis. J Appl Microbiol. 2005;98(5):1093-1100.
Crossref - Balasubramanian V, Natarajan K, Hemambika B, et al. High-density polyethylene (HDPE)-degrading potential bacteria from marine ecosystem of Gulf of Mannar, India. Lett Appl Microbiol. 2010;51(2):205-211.
Crossref
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.