ISSN: 0973-7510
E-ISSN: 2581-690X
Begomoviruses are among the most damaging pathogens causing epidemics in economically important crops particularly in tropical and subtropical regions. During February 2015, 20 samples of Calendula with yellow vein disease were collected from the campus of S. V. Patel University of Agriculture & Technology, Meerut, Uttar Pradesh, India. Total genomic DNA was isolated from the symptomatic and asymptomatic leaf samples and subjected to PCR using coat protein gene specific primer of begomovirus. The PCR amplification of ~770 bp was obtained from the 13 plants out of 20 collected plants. The PCR amplicon from coat protein gene was cloned, sequenced and submitted to GenBank, with accession number KT833850. The sequence data was further analyzed by BLAST analysis and phylogenetic tree was constructed using MEGA5.0 software which revealed close similarity of sequences with coat protein gene (AV1) components of other potato begomoviruses, which are all tentative strains of Tomato Leaf Curl New Delhi Virus (ToLCNDV). The result also indicated that Calendula spp. plants infected with Tomato Leaf Curl New Delhi Virus may act as an alternate host (reservoir) for other economically important plants.
Begomovirus, PCR, ToLCNDV, Calendula officinalis.
Begomoviruses (genus Begomovirus, family Geminiviridae) are a group of plant viruses transmitted by the white ûy Bemisia tabaci (Aleyrodidae) to a large variety of cultivated and uncultivated plant species. They possess a circular single-stranded DNA genome encapsidated in twinned icosahedral virus particles (Rojas et al., 2005). Huge economic losses have been observed in India and other countries due to geminivirus infection in cotton (Briddon & Markham; 2000), tomato (Moffat; 1999), cassava (Thresh; 1998) and grain legumes (Verma; 1992). Begomovirus are an outsized varied family of plant viruses (Mansoor et al., 2003) which infects an expansive assortment of plants such as ornamentals, weeds and crops and causes a noteworthy loss to agriculture and horticulture worldwide (Lima et al., 2013). More than 80% of the known geminiviruses are transmitted by whiteflies and belong to the genus Begomovirus.Most of these viruses have bipartite genome designated as DNA-A and DNA-B and infect dicotyledenous plants. Ornamental plants are extensively scattered worldwide and have high environmental adaptability. Ornamental plants are considered as a foundation of new viruses and are considered as reservoirs of unidentified economically imperative viruses which are often neglected during virus diversity study (Urbino et al., 2013). Many scientific reports have demonstrated that ornamental plants serve as reservoir or alternative hosts for begomovirus survival (Raj et al., 2007) and spread in the absence of the main crops (Iiyas et al., 2013). Thus, there is a pressing need for additional information on the diversity and distribution of begomovirus in ornamental plants.
Calendula ofûcinalis L. (Asteraceae) is an important annual ornamental plant grown in gardens during the winter season and has an aesthetic beauty of bright yellow colored flower. It belongs to the family, Asteraceae, and is commonly known as Pot Marigold. The plant is native to Central and Southern Europe, Western Asia and USA. In addition, it has a considerable importance to the cosmetic/pharmaceutical industry because of it’s used in the manufacture of antiseptic creams.
Plant viruses affect the aesthetic value of ornamental Calendula by reducing its rate of growth as well as the quality and quantity of its ûowers. Calendula officinalis plants have been found to be affected by Cucumber mosaic virus (Lisa & Della-Valle, 1979; Naqvi & Samad, 1985), Turnip mosaic virus (Lisa et al. , 1979) and Tobacco mosaic virus (Hristova et al ., 1994). A rosette disease transmitted by whiteûies and grafting was recorded on C. ofûcinalis by Gupta & Verma (1983). In the present investigation PCR and nucleic acid sequence based molecular identification and characterization of begomovirus associated with leaf yellow vein disease of Calendula officinalis was done.
Sample Collection
The twenty Calendula officinalis plants showing vein yellowing, shortening of leaves and petioles, stunting of plants, reduction in growth, number and size of flowers and asymptomatic were randomly collected from the S. V. Patel University of Agriculture and Technology, Meerut, Uttar Pradesh during February 2015 – 2016. The samples were sealed in plastic zip bags and labelled to distinguish the identity of each sample and stored at -20ºC for further use.
DNA extraction, PCR and cloning
The total genomic DNA was extracted from symptomatic and asymptomatic leaves of calendula plant using CTAB method (Dellaporta et al., 1983). DNA extract was allowed for PCR based detection of virus using primers (TLCVAV1-F 5’CGAACCGACCAGCAGATATCA 3’ and TLCVAV1-R 5’TTTGATGCAT GAGTACAGGCCA 3’) from the CP region of leaf curl virus (Singh et al., 2013). PCR amplification were carried out using a MJ Mini, Bio- Rad thermal cycler in a 25µl reaction containing 2.5µl 10X PCR buffer, 0.5µl, 25mM MgCl2, 2.5mM each dNTPs, 20mM 1.25µl each primers, 0.1µl Taq DNA polymerase (Merk Bioscience Pvt. Ltd., Bengaluru, India) and 2µl template DNA. The DNA was amplified by initial denaturation of 94oC for 10min followed by 35 cycles of 94oC for 30 s denaturation, 67oC for 45 s, Primer annealing, 72oC for the 1 min. primer extension and final extension at 72oC for 10 min. The PCR amplicons obtained were electrophoresed through 0.8% (w/v) agarose gel in 1X TAE and visualized under UV light after staining with ethidium bromide (0.5ug mL-1). PCR amplified products of expected sizes were purified (GeneJET Gel Extration Kit, Lithuania) and ligated into the pTZ57R/T vector (Fermentas, Arlington, Canada). The recombinant vector was transformed into E.coli. strain DH5a. Selected recombinant clones were screened by PCR using same set of primers as described earlier. Restriction digestion of plasmid DNA of recombinant clones was carried out to further confirm the presence of insert in the vector.
Nucleic acid sequencing and data analysis
The selected positive clones were sequenced at the automated DNA sequencing facility, Department of Biochemistry, Delhi University, South campus, New Delhi using M13 and T7 primers (Chromus Biotech Pvt. Ltd., India). The sequence data obtained after sequencing was then validated by performing BLAST [www.ncbi.nih.gov/BLAST] (Altschul et.al., 1990) analysis. BLAST programme of NCBI was used to analyse the sequence data and to find related begomovirus sequences for in-silico analysis (Altschul et al., 1990). Nucleotide and amino acid sequence homologies were viewed using Bioedit software 7.3. (Hall, 1999). Sequence alignments were produced using Clustal W programme (Thompson et al., 1994). Phylogenetic trees were constructed based on matrices of aligned sequences using neighbour-joining algorithm of Mega 5.0 software (Tamura et al., 2011).
Detection of begomovirus
The symptoms of infected Calendula ofûcinalis plants observed during field survey in campus of S. V. Patel University of Agriculture & Technology, Meerut were vein yellowing, shortening of leaves and petioles, and stunting of plants, reduction in growth, number and size of flowers (Fig. 1 A & B). To identify the begomovirus associated with the disease, PCR was performed using the total DNA of infected and healthy samples with a pair of primers specific to the coat protein (CP) gene of the genus Begomovirus (Singh 2013). The electrophoresis of PCR products on 0.8% agarose gel showed the expected size (770 bp) amplicons in infected samples only (Fig. 2 and 3). However, no such amplicons were obtained in healthy samples. The PCR amplicon was cloned and sequenced and the data obtained from clones was submitted to GenBank and Accession number KT833850 was assigned.
 Fig. 1. A). Healthy Calendula plant; B). Naturally infected Calendula officinalis plant showing yellow vein net disease in field; a close view of leaf of infected plant showing severe yellow vein net symptoms
Fig. 1. A). Healthy Calendula plant; B). Naturally infected Calendula officinalis plant showing yellow vein net disease in field; a close view of leaf of infected plant showing severe yellow vein net symptoms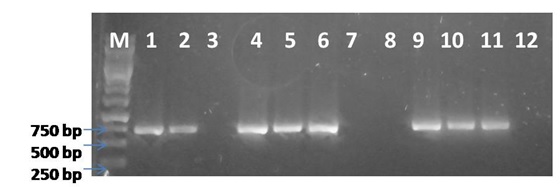 Fig. 2. Detection of ToLCV through PCR in infected calendula plants using TLCAV1-F/R. M- Molecular marker (1 Kb ladder), Lane – 1, 2, 4, 5, 6, 9, 10 & 11 : 770 bp DNA specific to coat protein region, Lane – 3, 7, 8, 12: no amplification
Fig. 2. Detection of ToLCV through PCR in infected calendula plants using TLCAV1-F/R. M- Molecular marker (1 Kb ladder), Lane – 1, 2, 4, 5, 6, 9, 10 & 11 : 770 bp DNA specific to coat protein region, Lane – 3, 7, 8, 12: no amplification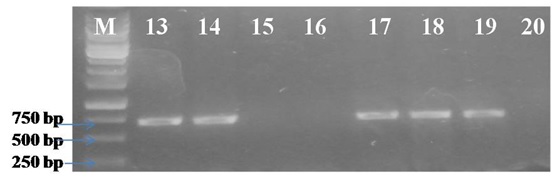 Fig. 3. Detection of ToLCV through PCR in infected calendula plants from different locations using TLCAV1-F/R. M- Molecular marker (1 Kb ladder), Lane – 13, 14, 17, 18 &19: 770 bp DNA specific to coat protein region , Lane – 15, 16 & 20- no amplification
Fig. 3. Detection of ToLCV through PCR in infected calendula plants from different locations using TLCAV1-F/R. M- Molecular marker (1 Kb ladder), Lane – 13, 14, 17, 18 &19: 770 bp DNA specific to coat protein region , Lane – 15, 16 & 20- no amplificationSequence Analysis
BLAST search analysis of nucleotide sequence KT833850 revealed 95–98% sequence identity with various strains of Tomato leaf curl New Delhi virus (ToLCNDV) infecting Tomato (AB976527), Chilli (DQ029202), Pumpkin (JN129254), Luffa (HM989845), Poppy (KC513822), Potato (KC205270) from India and Black nightshade ( AJ620187), Field bindweed (KC960492), Bitter gourd (AM747291) and Tomato (DQ116883) from Pakistan and KC207815 on Luffa from South Korea. Pair-wise alignment of nucleotide (nt) and deduced amino acid (aa) sequences of the CP gene of the virus isolate in study (Accession no KT833850) with CP gene sequences of selected ToLCNDV isolates from diverse plant species, and other begomoviruses was calculated using Bioedit programme. Pair-wise alignment of the virus isolate in study revealed the 95.3–98.4% identity at the nt level and 86.3 to 91.7% at aa level with various isolates of ToLCNDV (Table 1).
Table (1):
Coat protein gene sequence analysis of the virus isolate (EF123060) based on the Basic Local Alignment Search Tool (BLAST) and sequence identity..
Accession Number |
Virus |
Host Name |
Origin |
Nucleotide % |
Amino acid % |
|---|---|---|---|---|---|
AB976527 |
Tomato leaf curl New Delhi virus (AV1) |
Tomato |
India |
98.4 |
86.3 |
DQ029202 |
Tomato leaf curl virus coat protein gene |
Tomato |
India |
97 |
91 |
GU831539 |
Tomato leaf curl New Delhi virus isolate DHO1 |
Chilli |
India |
96.4 |
90.2 |
HM007120 |
Tomato leaf curl New Delhi virus pChTumB2 DNAA |
Chilli |
India |
96.3 |
91.4 |
AJ620187 |
Tomato leaf curl New Delhi virus segment A |
Black nightshade |
Pakistan |
96.3 |
90.2 |
KC960492 |
Tomato leaf curl New Delhi virus segment DNA A |
Field bindweed |
Pakistan |
96.1 |
91 |
JX232220 |
Tomato leaf curl virus segment DNA-A |
Tomato |
India |
96.1 |
91.7 |
JN129254 |
Tomato leaf curl New Delhi virus segment DNA A |
Pumpkin |
India |
96 |
90.6 |
AM286434 |
Tomato leaf curl New Delhi virus- segment DNA-A |
Pumpkin |
India |
96 |
91.4 |
DQ141676 |
Tomato leaf curl New Delhi virus – coat protein gene |
Chilli pepper |
India |
95.9 |
91 |
HM989845 |
Tomato leaf curl New Delhi virus-JLX10 |
Luffa |
India |
95.7 |
91.4 |
GQ284842 |
Tomato leaf curl New Delhi virus- cp (AV1) gene |
Chili pepper |
India |
95.7 |
90.6 |
KC207815 |
Tomato leaf curl New Delhi virus- at protein (AV1) gene |
Luffa |
South Korea |
95.6 |
91.4 |
AJ810365 |
Tomato leaf curl virus AV1 gene for cp-isolate 26 |
Tomato |
India |
96.3 |
91.4 |
JN208136 |
Tomato leaf curl New Delhi virus segment DNA-A |
Ash gourd |
India |
95.6 |
89.8 |
EF063145 |
Tomato leaf curl New delhi virus |
Cotton |
India |
95.6 |
91 |
KC513822 |
Tomato leaf curl New Delhi virus-segment DNA-A |
Poppy |
India |
95.4 |
91.7 |
AM747291 |
Tomato leaf curl New Delhi virus – complete sequence |
Bitter gourd |
Pakistan |
95.4 |
91.7 |
KC205274 |
Tomato leaf curl New Delhi virus-isolate KAL-6 (AV1) |
Potato |
India |
95.3 |
90.2 |
DQ339118 |
Whitefly transmitted Indian begomovirus (AV1) gene |
Datura metal |
India |
95.3 |
91 |
KC205270 |
Tomato leaf curl New Delhi virus- isolate JOR-2 (AV1) |
Potato |
India |
95.3 |
89.8 |
U15016 |
Tomato leaf curl New Delhi virus Mild coat protein |
Tomato |
USA |
96.1 |
91.7 |
AJ810340 |
Tomato leaf curl virus AV1 gene for cp |
Chilli |
UK |
95.7 |
90.6 |
DQ116883 |
Tomato leaf curl New Delhi virus |
Solanum |
Pakistan |
95.7 |
90.2 |
DQ169056 |
Tomato leaf curl New Delhi virus segment DNA A |
Tomato |
India |
95.4 |
91 |
KC205213 |
Tomato leaf curl New Delhi virus isolate HSM20 |
Potato |
India |
95.3 |
90.1 |
The highest identity was observed with several isolates of ToLCNDV reported from different geographical locations and different plant hosts such as tomato (AB976527), Chilli (GU831539), potato (KC205213), Tomato leaf curl New Delhi on Luffa (KC207815) from South Korea. Moreover, the phylogenetic analyses nucleotide sequences of the virus isolate showed a close relationship with various strains of ToLCNDV from tomato, poppy, luffa bitter guard etc. (Fig. 4).
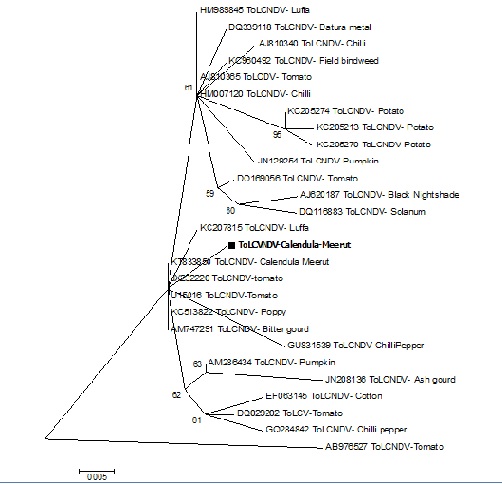
Fig. 4. Phylogenetic tree depicting the relationship of various begomoviruses based on alignment of coat protein gene nucleotide sequences. Tree was constructed using Mega 5 programme with 1000 bootstrap values.
Begomoviruses have been reported from different plant species in India. In the present study, initial survey for the incidence of yellow vein disease in calendula plants showed the 65% incidence (13 out of 20 plants) in PCR assay using coat protein (CP) gene primer pair (Fig. 2 and 3). The CP gene primers were reported in successfully detection of begomovirus by PCR in several plants species (Shorab et al. 2006; Singh et al., 2007; Tiwari et al., 2012, Singh et al, 2013). Similarly, Hallan (1998) reported begomovirus infection in tomato plants by extracting DNA followed by amplification of coat protein region using specific primer pair. The nucleotide sequence of virus isolate was BLAST searched to identify the homologous sequences in the NCBI databases and subjected to construct a phylogenetic tree tree using Mega 5 programme (Tamura et al., 2011). The coat protein gene of ToLCV isolates showed homology of more than 95-98% with all other reported geminiviruses coat protein gene sequences form different plat hosts (Table 1). The highest homology was observed with several isolates of ToLCNDV reported from different geographical locations and variety of plant hosts such as tomato (AB97657) and potato (KC205213).
The pairwise nucleotide sequence identity (Table 1) and phylogenetic study (Fig. 4) confirmed the causal agent of yellow vein net symptoms of Calendula officinalis as a strain of Tomato leaf curl New Delhi virus. Study also revealed that CP gene of present begomoviruses illustrates a high degree of sequence similarity and closeness with different important crop-infecting begomoviruses. However, virus isolate did not reveal a close identity to Cotton leaf curl Rajasthana virus (NC 003199) used earlier for cross hybridisation (Chatterjee et al. 2005) and with Mesta yellow vein mosaic virus (DQ298138) (Paul et al. 2006) (Raj et al. 2007). Yellow vein net disease of Calendula associated with Cucumber mosaic virus has been reported previously (Naqvi & Samad, 1985) which belongs to a plant pathogenic virus in the family Bromoviridae. The study of the CP gene of begomovirus complex isolates from northern India and their similarity with other important crop-infecting begomoviruses reveals the broadened host range in India.
Khan et al., 2005 observed yellow vein net disease on several Calendula officinalis plants in Aligarh and Lucknow, region of India with similar disease symptoms consisting of vein yellowing, shortening of leaves and petioles and stunting and the infecting virus isolate was identified as a Begomovirus. Khan et al., 2007 analyzed nucleotide sequence of coat protein gene from calendula which shared maximum identities of 96–97% with four strains of Tobacco curly shoot virus (ToCSV) and an Ageratum ernation virus (AgEV) during BLAST analysis of sequence data.
Similarily, Naturally infected plants showing yellow leaf vein netting symptoms accompanied by excessive yellowing and curling of leaves and stunting of the whole plant were reported on Ageratum houstonianum (Srivastava et al., 2015), Amaranthus cruentus (Raj et al., 2008), Hibiscus cannabinus L (Raj et al., 2007).
The geminivirus disease complexes have wide host range within dicots plants, including vegetables and fibre crops, ornamental plants and weeds indicating there is little, if any, natural resistance in their germplasm. Widespread distribution and diversity, coupled to the global movement of plant material and dissemination of the whitefly vector, suggest that geminivirus disease complexes pose a serious threat to tropical and sub-tropical agro-ecosystems worldwide (Mansoor et al., 2003). Hence, nucleotide sequence information of the viral genes can be used for developing suitable diagnostic techniques for detection of virus at early stage in plants. Moreover, cloned modified CP components can be used to develop transgenic potato against the virus disease.
ACKNOWLEDGMENTS
The authors acknowledge the Vice Chancellor, S. V. Patel University of Agriculture and Technology, Meerut-250110, Uttar Pradesh, Council of Science and Technology, Uttar Pradesh and Bioinformatics facility (DBT funded), India, for providing financial support and the facilities to carry out this research work.
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol, 1990; 215: 403-410
- Briddon RW, Markham PG. Cotton leaf curl virus disease. Virus Res, 2000; 71:151–159.
- Chatterjee A, Roy A, Padmalatha KV, Malathi VG, Ghosh SK. Occurrence of a Begomovirus with yellow vein mosaic disease of mesta (Hibiscus cannabinus and Hibiscus sabdariffa). Australas. Plant Pathol. 2005; 34: 609-610
- Dellaporta SL, Wood J, Hicks JB. A plant DNA minipreparation: version II. Plant Mol Biol Report, 1983; 1:19–21
- Gupta M, Verma VS. Calendula rosette disease. Gartenbauwissenschaft , 1983; 48: 106–107.
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. (Oxf.), 1999; 41:95–98
- Hallan VK. Genomic organization of geminivirus causing leaf curl on tomato. PhD Thesis. Lucknow University, Lucknow (IN) 1998.
- Hristova D, Barkerdzhieva N, Svrakov K. Tomato mosaic virus isolated from Calendula ofûcinalis. Conference on Plant Virology, 1994; 32: 153.
- Ilyas M, Nawaz K, Shafiq M, Haider MS, Shahid AA. Complete nucleotide sequences of two begomoviruses infecting Madagascar periwinkle (Catharanthus roseus) from Pakistan. Arch Virol, 2013; 158: 505–10.
- Khan AA, Naqvi QA, Singh R, Raj SK. First report of a begomovirus infecting Calendula in India. Plant Pathology 2005, 54(4). pp.569 ref.2 DOI: 10.1111/j.1365-3059.2005.01220.x
- Khan AA, Khan MS, Raj SK, Naqvi, QA. Molecular identification of a begomovirus causing yellow vein disease on Calendula officinalis in india. Bulletin, 2007; 37: 420-426.
- Lima AT, Sobrinho RR, González-Aguilera J, Rocha, CS, Silva, SJ, Xavier CA, Silva FN, Duffy S, Zerbini FM. Synonymous site variation due to recombination explains higher genetic variability in begomovirus populations infecting non-cultivated hosts. J Gen Virol, 2013; 94: 418–31.
- Lisa V, Della-Valle G. Isolation of two viruses from Calendula ofûcinalis. Informatore Fitopatologico, 1979; 29:11–12.
- Mansoor S, Briddon RW, Zafar Y, Stanley J. Geminivirus disease complexes: An emerging threat. Trends Plant Sci, 2003; 8: 128-134.
- Moffat AS. Geminiviruses emerge as serious crop threat. Science, 1999; 286: 1835.
- Naqvi QA, Samad A. Puriûcation and properties of Calendula yellow net virus. Indian Journal of Virology, 1985; 1: 143–146.
- Paul S, Ghosh R, Roy A, Mir JI, Ghosh SK. Occurrence of a DNA b -containing begomovirus associated with leaf curl disease of kenaf (Hibiscus cannabinus L.) in India. Australasian Plant Disease Notes, 2006; 1: 29-30.
- Raj SK, Khan MS, Snehi SK, Kumar S, Khan AA. Natural occurrence of a Begomovirus on Dimorphotheca sinuate in India. Australasian Plant Disease Notes, 2007; 2: 25–26.
- Raj SK, Snehi SK, Kumar S, Khan MS, Pathre U. First molecular identification of a begomovirus in India that is closely related to Cassava mosaic virus and causes mosaic and stunting of Jatropha curcas L. Australas Pant. Dis. Notes, 2008; 3: 69–72
- Rojas MR, Hagen C, Lucas WJ, Gilbertson RL. Exploiting chinks in the plant’s armor: evolution and emergence of geminiviruses. Annu Rev Phytopathol, 2005; 43: 361–394.
- Singh DK, Islam MN, Choudhury NR, Karjee S, Mukherjee SK. The 32 kDa subunit of replication protein A (RPA) participates in the DNA replication of Mung bean yellow mosaic India virus (MYMIV) by interacting with the viral Rep protein. Nucleic Acids Res, 2007; 35: 755–770
- Singh J, Singh A, Kumar P, Rani A, Barnwal VK, Sirohi A, Bhatnagar SK, Singh D, Evidence of association of a tomato leaf curl New Delhi virus with chilli leaf curl disease in western Uttar Pradesh, India. Vegetos, 2013; 26(2): 203-211
- Shorab SS, Mandal B, Ali A, Varma A. Molecular diagnosis of emerging begomovirus diseases in cucurbits occurring in northern India. Indian J Virol, 2006; 17:88–95.
- Srivastava A, Jaidi M, Kumar S. et al. Molecular identification of a new begomovirus associated with leaf crumple disease of Jatropha curcas L. in India Arch Virol, 2015; 160: 617.
- Thresh JM, Otim-Nape GW, Thankappan M, Muniyappa V. The mosaic diseases of cassava in Africa and India caused by whitefly-borne geminiviruses. Review of Plant Pathology, 1998; 77: 935-945.
- Tiwari AK, Snehi SK, Singh R, Raj SK, Rao GP, Sharma PK. Molecular identification and genetic diversity among six begomovirus isolates affecting cultivation of cucurbitaceous crops in Uttar Pradesh, India. Archives of Phytopathology and Plant Protection, 2012; 45(1):62-72
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance and Maximum Parsimony Methods. Molecular Biology and Evolution, 2011; 28(10): 2731-2739.
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res, 1994; 22(22): 4673-80.
- Urbino C, Gutiérrez S, Antolik A, Bouazza N, Doumayrou J, Granier M, Martin DP, Peterschmitt M. Within-host dynamics of the emergence of tomato yellow leaf curl virus recombinants. PLoS ONE., 2013; 8: e58375.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


