ISSN: 0973-7510
E-ISSN: 2581-690X
Staphylococcus aureus is responsible for most bacterial wound infections. Antibiotics are the first-line treatment; however, their indiscriminate use led to the emergence of resistance. Alternative therapeutic options beyond antibiotic treatment are required. Our study aimed to evaluate and compare the healing parameters and antibacterial activity of Jojoba and Citrullus colocynthis oil extracts in the treatment of Staphylococcus aureus wound infections. In-vivo assessment of inflammatory biomarkers, matrix metalloproteinase and histopathological examination of Staphylococcus aureus induced wound lesions were conducted in mice. Levels of interleukin 1 and interleukin 6 were reduced, while matrix metalloproteinases ratio; MMP-1 /MMP-9 was increased after topical application of both essential oils. Citrullus colocynthis oil showed optimum wound healing compared to the other treated groups in histopathological examination. In conclusion, topical Citrullus colocynthis preparation may be a promising alternative natural dermatological application with enhanced antibacterial activity.
Antibacterial Activity, Citrullus colocynthis , Jojoba, Staphylococcus aureus, Wound
Wound infection, mainly attributed to Staphylococcus aureus (S. aureus) is one of the most common infections that cause significant morbidity and mortality. It also causes other skin and soft tissue infections. S. aureus wound infections may progressively invade the bloodstream resulting in, abscesses, pneumonia, bacteremia and septicemia.1
Antibiotics and immunotherapy are therapeutic options used to combat S. aureus infections. Antibiotics are highly effective and comprise the first-line treatment.1,2 However, the misuse of antibiotics has led to the development of multiple resistant S. aureus strains as methicillin resistant S. aureus (MRSA). Therefore, alternative therapeutic options beyond antibiotic treatment are required.2,3
Jojoba plant (Simmondsia chinensis) has been cultivated for many years in several countries as the United States, Mexico, South America, Tunisia, Saudi Arabia, and Egypt.4 Jojoba seeds contain about 65% of a light golden liquid-oil that is odorless with a high-viscosity. This liquid is formed of wax-like unsaturated esters, consisting of fatty acids and higher alcohols, rendering it different from other plant oils. It has a similar texture to sebum, where its consistent application to skin could prevent excessive oil production. Jojoba oil has been used as a home remedy for common colds, warts, and wounds.5 It possesses an anti-inflammatory, antimicrobial, and antifungal activity.4,5
Citrullus colocynthis , or the bitter apple is a medicinal plant that has been used for many years in the treatment of diseases such as ulcers, wound infections, bronchial asthma, bronchitis, urinary tract and throat infections. The medical importance of Citrullus colocynthis oil lies in its anti-inflammatory, antioxidative, anti-fungal, and antihelminthic effect.6
Our study aimed to evaluate and compare the healing parameters and antibacterial activity of Jojoba and Citrullus colocynthis oils in the treatment of S. aureus wound infections.
In vivo experimental testing
Essential oil ointment preparation
Citrullus colocynthis and Jojoba pure oil extracts were commercially purchased from EL Captain Company ( CAP PHARM, Cairo, Egypt). The two ointments were prepared by melting together both white beeswax (7 gm) and cocoa butter (14 gm) on a hot plate stirrer at 70°C. Then 4 drops from each oil were separately added to each of the molten bases while stirring. The two mixtures were stirred while cooling. A vitamin E capsule was then added to each mixture as a preservative to form each ointment.
Induction of wound infection and treatment design in mice
Male mice weighing 23-25 gm were used in our study. The mice were retained within a temperature range of 22-25°C and at a 12-hour light/dark cycle with free access to a standard chew diet and water ad libitum. Ethical clearance was granted from the Ethics Committee, Faculty of Medicine, Alexandria University, Alexandria, Egypt [IRB No.: 00007555-FWA No.:00018699].
The dorsal fur of mice (middle back area of the mice) was removed by depilatory cream. The next day, mice were anesthetized with 2% (vol/vol) isoflurane in oxygen and the skin was swabbed with 70% ethanol. Full-thickness wounds were excised (5 mm in diameter) from the dorsal surface of each mouse by using a sterile biopsy punch.
S. aureus reference strain NCTC 10788/ATCC ® 6538 (Selectrol, TCS Biosciences Ltd, UK) pellet was rehydrated and prepared according to the manufacturer’s instructions. A 0.05 ml (50 ul) of the prepared strain was injected as 2x 106 colony forming units (CFUs/ml) per 10 ul of S. aureus, intradermally into the middle back area of the mice.7 The lesions were subsequently covered with a square of parafilm, gauze and microspore surgical tape. After surgery, the infected-wound mice were housed individually.8
Twenty-five mice were used in this study. They were divided into five groups. Each group consisted of five mice. The control group; the infected wound group (untreated); the Jojoba group (infected wound and treated daily with Jojoba ointment); the Citrullus colocynthis group (infected wound and treated daily with Citrullus colocynthis ointment, and the Fucidin group (infected wound and treated daily with Fucidin® ointment 2% LEO Pharma Inc., Denmark). Treatment with Jojoba, Citrullus colocynthis, and Fucidin ointments (0.1 ml volume) were applied and spread over the infected wounds after 1 hour of bacterial inoculation and was continued for 10 days until the wounds healed. On day 11, mice were sacrificed, where mice were anesthetized using ether and euthanized by cervical dislocation. Samples of skin including the wounds were excised and divided into two parts; the first part was stored at -80°C for Reverse Transcriptase – Polymerase Chain Reaction (RT-PCR) and Enzyme-Linked Immunosorbent Assay (ELISA) analysis. The second part of skin samples was kept in 10% formalin for histological investigation.
Histopathology of wound tissue
Skin tissue samples were trimmed, and were subjected to serial passages in ethyl alcohol, then cleared in xylene. These samples were then embedded in paraffin, and sectioned using a microtome at 5 um slices (Microtome, Leica RM2155, Leica Inc, Nussloch, Germany). The sections were stained by hematoxylin and eosin (H&E) and Masson’s Trichrome (MT) dyes. Histolopathological examination of slides were performed (Olympus Light Microscope, Japan).
Evaluation of biomarkers
The biomarkers of wound healing that were measured in skin tissue homogenates included interleukin 1β (IL-1β), interleukin 6 (IL-6), and matrix metalloproteinase 1 and 9 (MMP-1 and MMP-9). Levels of IL-1β and IL-6 were measured by ELISA kits and according to the manufacturer’s instructions (mouse IL-1β, CUSABIO, CSB-E08054m; Mouse IL-6, CUSABIO, CSB-E04639m).
Quantitative analysis of gene expression of MMP-1 and MMP-9 were analyzed using qRT-PCR (QuantiTectR 205311, Germany). Total RNA was isolated from the tissues using miRNeasy kit as per the producer’s guidelines. A reverse transcriptase enzyme was used to reverse the isolated RNA into complementary DNA (cDNA), then amplified and identified using precise primers for MMP-1 and MMP-9 genes by qRT-PCR. The forward and reverse primer sequences for MMP-1 gene were as follows: AGGAAGGCGATATTGTGCTCTCC and GGCTGGAAAGTGTGAGCAAGC. Regarding MMP-9 gene, the forward primer was: GCTGACTACGATAAGGACGGCA and the reverse was: TAGTGGTGCAGGCAGAGTAGGA. Gene expression analysis was normalized to the housekeeping gene glyceraldehyde-3- phosphate dehydrogenase (GAPDH); CATCACTGCCACCCAGAAGACTG (forward), ATGCCAGTGAGCTTCCCGTTCAG (reverse). MiScript II RT Kit (Qiagen, Germany) was used to transcriptionally reverse all RNA species into cDNA according to the producer’s guidelines. The skin expression of MMP-1 and MMP- 9 genes was quantified utilizing the cDNA by Rotor-Gene Q qPCR using a Master Mix of QuantiTect SYBR Green PCR. An initial denaturation at 95°C for 10 min was started for the PCR amplification. This was followed by 40 cycles of PCR denaturation for 15 sec at 95°C, annealing for 15 sec at 58°C, and extension for 15 sec at 60°C. Rotor-Gene Q-Pure Detection version 2.1.0 (build 9) was used to determine the values of the threshold cycle. The 2-ΔΔCt method was used to determine the alteration of mRNA levels in the samples for each gene and standardized to the reference gene.
Statistical analysis
Data were expressed as means ± Standard Error of Mean (S.E.M). Statistical analysis of the results was performed using One-way analysis of variance (ANOVA), and after that post-hoc analysis was performed by Tukey Kramer test. P < 0.05 was considered as the significance limit for all comparisons. Statistical analysis were performed using the software package Prism® 7 (GraphPad Software, Inc., CA, USA).
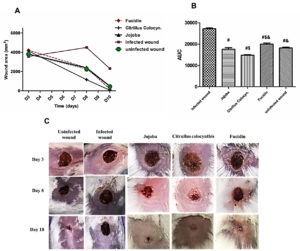
Post-treatment healing of wound lesions
Figure 1 A and B show that S. aureus significantly delayed wound healing in the infected wound group compared to other experimental groups (P < 0.0001). The wound area in the infected wound mice was increased 2-fold on day 8 and 5-fold on day 10. The mice group treated with Citrullus colocynthis has the smallest area under the curve (AUC), where P< 0.05. compared to other groups proving a faster healing process of S. aureus-infected wounds (Figure 1; B). Figure 1; C shows redness, swelling, foamy granulation tissue, tissue breakdown, and epithelial bridging that was observed on the skin of the infected control mice indicating characteristic signs of wound infection. Mice were subjected to topical application of two essential oil ointment preparations (Jojoba and Citrullus colocynthis) and were compared with the conventional Fucidin ointment used in treatment of S. aureus wound infections. Wound healing was observed after the intervals of 3, 8, and 10 days of treatment. Citrullus colocynthis showed improved wound healing compared to the other treated groups.
Figure 1. Effect of different treatments on wound healing against controls. Panel A line graph showing the changes in wound area (mm2) 3-, 8-, and 10 days following treatments. Panel B shows the (AUC) of the wound area. Values are means ± SEM of 3 mice. #P<0.05 vs. infected wound group, $P<0.05 vs. Jojoba group, &P<0.05 vs. Citrillus colocynthis. Panel C shows representative photographs in different animal groups showing wound size on days 3, 8, and 10 for the uninfected and infected wound groups and the 3 treated groups with the different ointments; Jojoba, Citrullus colocynthis, and Fucidin. On day 3 wound lesions showed an amber serous exudate appearance. On day 8 they displayed healthy red moist granulation tissue formation. On day 10 the surface of the wound lesions became covered by new pinkish almost white epithelium with approximation of wound edges.
Histopathological examination of wound tissue
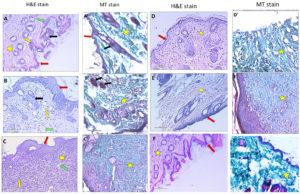
Figure 2 denotes the histopathological changes in skin wound infection stained with H & E and MT stain. The normal control together with the uninfected wound groups displayed normal epidermis thickness and normal dermis with skin appendages and collagen formation. Wound infection in the untreated infected mice group showed incomplete epidermal epithelialization and differentiation. The dermis displayed a marked decrease in collagen and skin appendages with a remarkable increase in granulation tissue and inflammatory cell infiltration. Jojoba ointment treated group revealed incomplete epidermal epithelialization, moderate decrease in collagen, fibroblast formation, and skin appendages with a moderate increase in granulation tissue and inflammatory cells. Citrullus colocynthis treated mice group revealed mature epidermis with complete epithelialization in the form of closure of the basal layer with spinous and granular epidermal differentiation. There was a remarkable increase in collagen fiber and fibroblast formation with a mild decrease in inflammatory and granulation tissue compared to the Jojoba group. The Fucidin group showed incomplete epithelialization in the form of incomplete closure of the basal layer of the epidermis and incomplete epidermal differentiation. The dermis shows a marked decrease in collagen compared to other treated groups. However, mice treated with Citrullus colocynthis demonstrated mature epidermis with complete epithelialization and epidermal differentiation that mimicked normal skin tissue.
Figure 2. Representative histolopathology photomicrograph of skin tissues of the normal, infected wound, Jojoba ointment, Citrullus colocynthis ointment, Fucidin ointment treated groups and uninfected wound group using H&E and MT (X200).
A. Normal Group: normal skin showing normal epidermis (red arrow) showing spinous and granular epidermal layer consisting of stratum corneum (green arrow), stratum granulosum (yellow arrow), stratum spinosum and stratum basale. Dermis shows normal skin appendages (black arrow) and collagen formation (yellow star). B. Infected Wound Group: skin shows incomplete epidermal (red arrow) epithelialization and differentiation. Dermis shows a markedly decrease in collagen, fibroblast formation, and skin appendages (black arrow) with a markedly increase in granulation tissue (green arrow) and inflammatory cells (yellow arrow). C. Jojoba Group: skin showing incomplete epidermal (red arrow) epithelialization and differentiation. Dermis shows a moderate decrease in collagen (yellow star), fibroblast formation, and skin appendages with a moderate increase in granulation tissue (green arrow) and inflammatory cells (yellow arrow). D. Citrullus colocynthyis Group: skin represents mature epidermis (red arrow) showing complete epithelialization in the form of closure of the basal layer with spinous and granular epidermal differentiation. There is a remarkable increase in collagen fiber (yellow star) and fibroblast formation with a mild decrease in inflammatory and granulation tissue. E. Fucidin Group: skin shows incomplete epithelialization in the form of incomplete closure of the basal layer of the epidermis (red arrow) and incomplete epidermal differentiation. The dermis shows a marked decrease in collagen (yellow star). F. Uninfected Group: skin shows mature epidermis (red arrow) with nearly complete epitheliazation of the basal layer with spinous and epidermal differentiation. An increase in collagen fiber (yellow star) and fibroblast formation with a decrease in inflammatory and granulation tissue is noticed
Evaluation of biomarkers
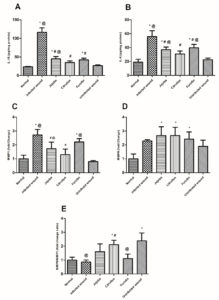
Figure 3 demonstrates the levels of inflammatory markers measured in the tissue homogenates of mice (IL-1β, IL-6, MMP-1, and MMP-9). Both IL-1β and IL-6 were measured by ELISA technique, while MMP-1 and MMP-9 were measured by qRT-PCR.
Regarding IL-1β (pg/mg protein) measurements; the normal level was 23.43±0.693; 116.4 ±6.78 pg/mg in the infected wound; 44.9±3.479 pg/mg in Jojoba oil treated group; 34.77±2.689 pg/mg in Citrullus colocynthis oil-treated group; 42.1±2.572 pg/mg in Fucidin treated group and 26.7±1.124 in the uninfected wound group. Results were found to be statistically significant between the treated groups and the untreated infected wound group (p < 0.0001). However, the Jojoba (p < 0.001, p < 0.01) and Fucidin (p < 0.01) groups showed a significantly elevated level of IL-1β compared to the normal and the uninfected ones and the normal mice, respectively (p < 0.05) (Figure 3; A).
As for IL-6 (pg/mg protein) measurements; the normal level was 19.1±2.31 pg/mg; 55.7±4.91 pg/mg in the infected wound; 37.1±2.15 pg/mg in Jojoba oil treated group; 30.9±2.49 pg/mg in Citrullus colocynthis oil-treated group, 39.8±2.75 in Fucidin treated group and 22.6±1.31 in the uninfected wound group. These results were also found to be statistically significant between Citrullus colocynthis-, Jojoba-, and Fucidin-treated groups and the untreated infected wound group (p < 0.0001, p < 0.001, p < 0.01, respectively). The levels of IL-6 in both Jojoba oil and Fucidin groups were significantly higher than that in the normal skin and the uninfected wound groups (p < 0.001, p < 0.01). Skin homogenate concentration of IL-6 in the Citrullus colocynthis oil-treated group approached normal level (Figure 3; B).
Concerning MMP- 1 expression, the following results were revealed; the normal gene expression: 1.00±0.151; infected wound: 2.71±0.229; Jojoba oil treated group: 1.73±0.275; Citrullus colocynthis oil treated group: 1.3±0.229; Fucidin treated group: 2.21±0.139 and uninfected wound: 0.795±0.0464. The infected wound (p < 0.0001) and Fucidin (p < 0.001) groups showed significantly higher MMP-1 gene expression than the normal and uninfected groups. Gene expression of MMP-1 was upregulated in Jojoba (p < 0.01) and Fucidin (p < 0.001) compared with the uninfected wound mice. Moreover, Jojoba and Citrullus colocynthis oil results compared to the untreated group were statistically significant with p-values (<0.01, <0.001), respectively (Figure 3; C).
As for MMP- 9 expressions; the results revealed the significant upregulation of the gene in the Jojoba oil treated group: (2.67±0.367, p <0.001); Citrullus colocynthis oil treated group: (2.67±0.34, p<0.0001) and Fucidin treated group (2.42±0.295, p<0.01) compared with the normal gene expression 1.00±0.2 (Figure 3; D).
MMP-9/MMP-1 (fold change ratio) was 1.003±0.1197 for the normal mice; whereas the ratio in the infected wounds was 0.857±0.09, Jojoba oil treated group was 1.605±0.325; Citrullus colocynthis oil treated group was 2.113±0.179; Fucidin treated group was 1.113±0.182 and the uninfected group was 2.39±0.323 (Figure 3; E). The fold change ratio in the uninfected wound was significantly elevated compared to the normal (p < 0.0016), infected wound (p < 0.0016), and Fucidin (p < 0.01) groups. The fold change ratio in Citrullus colocynthis oil group was statistically significantly higher compared with the normal and untreated infected groups (p<0.01).
Figure 3. Evaluation of IL-1 β (panel A) and IL-6 (panel B) levels, and gene expression of MMP-1 (panel C) and MMP-9 (panel D) on normal, untreated infected, other treated infected excised wounds and uninfected wounds in male mice. Excision of the wound was induced and inoculated with S. aureus reference strain (NCTC 10788/ATCC ® 6538), after which all mice were treated for 10 days, then sacrificed on day 11. Data are presented as mean ± SEM (n = 5). Comparisons between groups were analyzed using one-way ANOVA followed by Tukey post-hoc test. Data are compared at p < 0.05 with normal (*), Infected wound (#), and uninfected wound(@)
Wound healing is a complex regulated process that includes four phases: haemostasis, inflammation, proliferation, and remodelling. Following a wound injury, a temporary wound matrix is created, in which the clotting cascade is immediately activated in the haemostasis phase.9,10 The inflammatory phase comprises the recruitment of phagocytic polymorphonuclear neutrophils at the site of injury to remove tissue debris. Polymorphs initiate phagocytosis.11,12
These phases are regulated by crosstalk between molecules and the components of the extracellular matrix (ECM) as integrins, growth factors, and metalloproteinases (MMPs). As a result, high levels of collagenase, elastase, and MMPs are released, which in turn lead to the degradation of the damaged cells and ECM. Hence, MMPs are key elements of wound repair.13
Topical antimicrobial therapy is used to control microbial colonization, thus preventing the development of infections. They are also used as a prophylaxis and treatment of wound infections.14 They enhance wound healing in infections caused by S. aureus which is regarded as a major cause of delayed wound healing, especially with an underlying pathology such as diabetic neuropathy or vasculopathy.15 The conventional prophylactic and therapeutic options include fusidic acid16,17; or antiseptics such as alcohol, chlorhexidine, or triclosan.18,19 Topical antimicrobials possess the advantage of producing a higher concentration at the target site and fewer adverse effects.16,17,20
However, the widespread misuse of topical therapies such as fusidic acid has led to an increased rate of bacterial resistance to S. aureus, thus limiting its therapeutic options. The racial use of topical agents to prevent further resistance is crucial.16,17 Medicinal plants are a fundamental source of natural products that are used to treat infections.21,22 Hence the concept of applying natural plant oils as an alternative therapeutic option has been postulated.
In our study, we evaluated the antibacterial activity of two essential oils; Jojoba and Citrullus colocynthis. These oils possess antimicrobial and antifungal activities against several pathogens.4 Jojoba is composed of oil sterols and different tocopherols. It comprises up to 50% wax esters rendering it resistant to degradation, and it also possesses high oxidative stability.23,24 It has been used in the cosmetics and skincare industry, for skin repair in conditions with defective and altered sebaceous barriers, such as atopic dermatitis, psoriasis, seborrheic dermatitis, rosacea, acne, and wounds.25
To our knowledge, there are limited studies regarding the antibacterial activity of these two natural oils. We studied the effect of both oils against S. aureus reference strain NCTC 10788/ATCC ® 6538 by in vivo induction of Staphylococcus wound infection in mice. Histopathological examination of wound tissue sections for control groups and tested oils were performed. IL-1β, IL-6, MMP-1, and MMP-9 levels were estimated from these tissue sections.
Regarding, histopathological examination of wound tissue, our study revealed that treatment by Jojoba and Citrullus colocynthis displayed an improvement in wound healing parameters compared to the untreated infected mice. However, optimum wound healing was best observed with Citrullus colocynthis topical treatment from 8 to 10 days, compared to Jojoba and Fucidin.
In acute wound infection, the inflammatory response is proportionate to the intensity and duration of inflammation.9,10 Extreme inflammation and duration are associated with increased number of macrophages, resulting in compromised wound healing.9,26
Pro-inflammatory macrophages play a role in wound healing by producing inflammatory mediators, such as IL-1, IL-6, and TNF-α, as well as MMPs.26,27 The levels of IL-1β and IL-6 in wound tissues in this study were significantly elevated in the infected untreated mice as compared to the normal group, but were significantly decreased in the three treatment groups compared to the infected untreated mice. However, IL-6 levels in both Jojoba and Fucidin groups were significantly higher than in the normal mice group. This denotes a decreased antibacterial activity and incomplete wound healing for both Jojoba and Fucidin.
IL-1β promotes the recruitment of neutrophils in deep intradermal S. aureus infection.27 They are produced by macrophages, dendritic, and Langerhans cells. IL-1β is an upregulator of inflammasome activity in wound macrophages, preventing their polarization towards an anti-inflammatory phenotype.26
IL-6 is released early in response to injury, thus induces the release of proinflammatory cytokines from tissue macrophages, keratinocytes, endothelial cells, and stromal cells. It also induces chemotaxis of leukocytes into wounds.28,29 As inflammation progresses, IL-6 signalling is responsible for the switch to a reparative milieu. In normal wound repair, the expression of IL-6 is significantly decreased during the remodelling phase. This may be due to apoptosis of infiltrating leukocytes and the subsequent reduction in cytokine signalling.29
MMPs play a significant role in regulating ECM degradation and deposition that is essential for wound re-epithelialization. Excessive levels of MMPs that are released from polymorphonuclear neutrophils and macrophages, may result in extensive damage to ECM. This in turn will interfere with the migration and proliferation of new cells in the area of wound injury leading to chronic non-healing wounds. The time of expression and activation of MMPs in response to tissue injury are vital for successful wound healing. Therefore, the regulation of MMP levels in wounds could lead to improved wound healing.13
In the present study, MMP-1 was significantly induced in the untreated infected mice, while Citrullus colocynthis and Jojoba groups showed significant down-regulation of MMP-1 expression. The Fucidin group showed significant upregulation of MMP-1 compared with the normal and oil-treated groups. Moreover, MMP-9 gene expression qRT-PCR results revealed significant upregulation in the untreated and the three treated groups compared with the normal rats. Jojoba group showed a slight increase in MMP-9 gene expression compared to Citrullus colocynthis group. MMP-9/ MMP-1 ratio levels were significantly higher in Jojoba and Citrullus colocynthis . However, MMP-9/ MMP-1 ratio levels in the Fucidin group showed no significant changes.
MMP-1 and MMP-9 are major chemokine regulators during wound healing. MMP-1 promotes human keratinocyte migration on fibrillar collagen.30 They are rapidly expressed in basal keratinocytes at the migrating front epithelial membrane edge in wounds with the loss of ECM during wound healing.31 Overexpression in keratinocytes delays re-epithelialization. High levels of MMP-1 are found in chronic non-healing wounds. Down-regulation of MMP-1 is essential for normal tissue remodeling. MMP-9 is expressed in several injured epithelia, as the skin where it plays a role in wound healing and cell signaling. It also plays a pivotal role in keratinocyte migration, as it is expressed at the leading edges of migrating keratinocytes during wound closure; as well as in regulating angiogenesis during wound healing.13
Anti-inflammatory and antibacterial activities of Citrullus colocynthis are due to their constitution of tannins and phenolic compounds, of which flavonoids and terpenoids play a pivotal role against bacterial infections. Tannins interfere with protein synthesis. Flavonoids are widely known and used to hinder the synthesis of prostaglandins and produce a therapeutic effect on inflammation.6
Topical fusidic acid can be used in the form of an ointment, cream, lotion, and gel. Fusidic acid is primarily active against staphylococci with a susceptible MICs ranging from 0.016 to 0.5 gm/ml.16,32 The prevalence of fusidic acid resistance in Staphylococci is influenced by the patient population, specimen type, and geographic region.33 Previous studies have reported the possible relation between the use of topical fusidic acid agents and the development of resistance to Staphylococci.32,34 Williamson et al.16 revealed that 96% of patients who were infected with fusidic acid-resistant S. aureus isolates, had used topical fusidic acid therapy within the previous 6 months, suggesting an association between prior use and resistance.
In conclusion, the optimum antibacterial activity against S. aureus infection and wound healing was found best using Citrullus colocynthis oil application. Jojoba oil showed a potential, however, less efficacy against S. aureus. The conventional topical fusidic acid revealed a relatively decreased activity compared to the two tested oils. Topical Citrullus colocynthis oil preparation may be a promising alternative natural dermatological application with enhanced antibacterial activity.
The antibacterial effect of oil extracts used in our study was performed in vivo only. Thus the limitation of our study was not investigating the in vitro effect. We relied on histopathological examination, the pathological appearance and healing of the wound lesions of the mice, levels of biomarkers related to wound healing and the assessment of wound area.
ACKNOWLEDGMENTS
The authors would to thank the Faculty of Medicine and the Medical Research Institute, Alexandria University for their support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
GAE, SAA, and IAA contributed to the conception and design of the study, and in vivo part of the study. DMY contributed to the design of the animal model. EF contributed to the histopathological preparation and examination of the skin tissue lesions. SAA and GAE contributed to the results and interpretation of the data. GAE, SAA, IAA, and EF wrote the manuscript. All authors read, revised and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Ethics Committee, Faculty of Medicine, Alexandria University, Alexandria, Egypt, with IRB No.: 00007555-FWA No.:00018699.
- Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28 (3):603-661.
Crossref - Rasigade JP, Vandenesch F. Staphylococcus aureus: a pathogen with still unresolved issues. Infect Genet Evol. 2014;21:510-514.
Crossref - Tarai B, Das P, Kumar D. Recurrent Challenges for Clinicians: Emergence of Methicillin-Resistant Staphylococcus aureus, Vancomycin Resistance, and Current Treatment Options. J Lab Physicians. 2013;5(2):71-78.
Crossref - Al-Obaidi JR, Halabi MF, AlKhalifah NS, Asanar S, Al-Soqeer AA, Attia MF. A review on plant importance, biotechnological aspects, and cultivation challenges of jojoba plant. Biol Res. 2017;50(1):25.
Crossref - Sanchez M, Avhad M, Marchetti J, Martinez M, Aracil J. Jojoba oil: A state of the art review and future prospects. Energy Convers Manag. 2016;129:293-304.
Crossref - Hussain AI, Rathore HA, Sattar MZ , Chatha SA, Sarker SD, Gilani AH. Citrullus colocynthis (L.) Schrad (Bitter Apple Fruit): A Review of its Phytochemistry, Pharmacology, Traditional Uses and Nutritional Potential. J Ethnopharmacol. 2014;155(1):54-66.
Crossref - Youn C, Archer NK, Miller LS. Research Techniques Made Simple: Mouse Bacterial Skin Infection Models for Immunity Research. J Invest Dermatol. 2020;140(8):1488-1497.e1.
Crossref - Kraft WG, Johnson PT, David BC, Morgan DR. Cutaneous infection in normal and immunocompromised mice. Infect Immun. 1986;52(3):707-713.
Crossref - Ellis S, Lin EJ, Tartar D. Immunology of wound healing. Curr Dermatol Rep. 2018;7(4):350-358.
Crossref - Rodrigues M, Kosaric N, Bonham CA, Gurtner GC. Wound healing: A cellular perspective. Physiol Rev. 2019;99(1):665-706.
Crossref - van Kessel Kok PM, Bestebroer J, van Strijp Jos AG. Neutrophil-Mediated Phagocytosis of Staphylococcus aureus. Front Immunol. 2014;(5):467.
Crossref - Fine N, Tasevski N, McCulloch CA, Tenenbaum HC, Glogauer M.The Neutrophil: Constant Defender and First Responder. Front Immunol. 2020;11:571085.
Crossref - Caley MP, Martins VL, O’Toole EA. Metalloproteinases and Wound Healing. Adv Wound Care (New Rochelle). 2015;4(4):225-234.
Crossref - Kaiser P, Wachter J, Windbergs M. Therapy of infected wounds: overcoming clinical challenges by advanced drug delivery systems. Drug Deliv and Transl Res. 2021;11(4):1545-1567.
Crossref - Roy S, Santra S, Das A, et al. Staphylococcus aureus Biofilm Infection Compromises Wound Healing by Causing Deficiencies in Granulation Tissue Collagen. Ann Surg. 2020;1(6):1174-1185.
Crossref - Williamson DA, Carter GP, Howden BP. Current and emerging topical antibacterials and antiseptics: agents, action, and resistance patterns. Clin Microbiol Rev. 2017;30(3):827-860.
Crossref - Punjataewakupt A, Napavichayanun S, Aramwit P. The downside of antimicrobial agents for wound healing. Eur J Clin Microbiol Infect Dis. 2019;38(1):39-54.
Crossref - Kampf G. Acquired resistance to chlorhexidine-is it time to establish an ‘antiseptic stewardship’ initiative? J Hosp Infect. 2016;94(3):213-227.
Crossref - McNamara PJ, Levy SB. Triclosan: an instructive tale. Antimicrob Agents Chemother. 2016;60(12):7015-7016.
Crossref - Hoang TPN, Ghori MU, Conway BR. Topical Antiseptic Formulations for Skin and Soft Tissue Infections. Pharmaceutics. 2021;13(4):558.
Crossref - Chen S, Song J, Sun C, et al. Herbal genomics: Examining the biology of traditional medicines. Science. 2015; 347(6219), S27-S29.
Crossref - Brown ED, Wright GD. Antibacterial drug discovery in the resistance era. Nature. 2016;529(7586):336-343.
Crossref - Tada AJZ, Sugimoto N, Sato K, Zmayaki T, Tanamoto K. Analysis of the con¬stituents in jojoba wax used as a food additive by LC/MS/MS. J Food Hyg Soc Japan. 2005;46(5):198-204.
Crossref - El-Mallah MH, El-Shami SM. Investigation of liquid wax components of Egyptian jojoba seeds. J Oleo Sci. 2009;58(11):543-548.
Crossref - Habashy RR, Abdel-Naim AB, Khalifa AE, Al-Azizi MM. Antiinflammatory effects of jojoba liquid wax in experimental models. Pharmacol Res. 2005;51(2):95-105.
Crossref - Raziyeva K, Kim Y, Zharkinbekov Z, Kassymbek K, Jimi S, Saparov A. Immunology of Acute and Chronic Wound Healing. Biomolecules. 2021;11(5):700.
Crossref - Jauregui RG, Fleige H, Bubke A, Rohde M, Weiss S, Forster R. IL-1β Promotes Staphylococcus aureus Biofilms on Implants in vivo. Front Immunol. 2019;17:10:1082.
Crossref - Wright HL, Cross AL, Edwards SW, Moots RJ. Effects of IL-6 and IL-6 blockade on neutrophil function in vitro and in vivo. Rheumatology. 2014;53(7):1321-1331.
Crossref - Johnson BZ, Stevenson AW, Prele CM, Fear MW, Wood FM. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines. 2020;30;8(5):101.
Crossref - Larjava H, Haapasalmi K, Salo T, Wiebe C, Uitto VJ. Keratinocyte integrins in wound healing and chronic inflammation of the human periodontium. Oral Dis. 1996;2(1):77-86.
Crossref - Saarialho-Kere UK, Kovacs SO, Pentland AP, Olerud JE, Welgus HG, Parks WC. Cell-matrix interactions modulate interstitial collagenase expression by human keratinocytes actively involved in wound healing. J Clin Invest. 1993;92(6):2858-2866.
Crossref - Fernandes P. Fusidic Acid: A Bacterial Elongation Factor Inhibitor for the Oral Treatment of Acute and Chronic Staphylococcal Infections. Cold Spring Harb Perspect Med. 2016;6(1):a025437.
Crossref - Hajikhani B, Goudarzi M, Kakavandi S, et al. The global prevalence of fusidic acid resistance in clinical isolates of Staphylococcus aureus: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2021;10(1):75.
Crossref - Edslev SM, Clausen ML, Agner T, Stegger M, Andersen PS. Genomic analysis reveals different mechanisms of fusidic acid resistance in Staphylococcus aureus from Danish atopic dermatitis patients. J Antimicrob Chemother. 2018;73(4):856-861.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.