ISSN: 0973-7510
E-ISSN: 2581-690X
Dental caries are one of the leading infectious microbial diseases globally. Streptococcus species are the predominant causative agents for the formation of dental caries. Various antibiotics have been reported for the treatment of dental caries in humans. However, owing to the increasing evidence of microbial resistance, there is a need to develop safe and effective alternative treatments for infections. Traditional medicinal plants and their bioactive products have been explored worldwide for the treatment of various diseases and infections. These plants have great potential for creating novel medications without any side effects. The present study aimed to elucidate the antibacterial potential of medicinal plants against biofilm-forming bacteria from dental caries. Bacteria from dental caries were isolated and identified using 16S rRNA sequencing technique and the predominant bacterial isolates were Streptococcus mutans (MH889143), Enterococcus faecalis (MH793461), Rothia dentocariosa (MH824681), and Streptococcus anginosus (MH889145). The antibacterial potential of seventeen medicinal plants was determined against these bacterial isolates using the agar well diffusion method. The aqueous extracts of Moringa oleifera, Ficus benghalensis, Ficus racemosa, Ficus religiosa, Senegalia catechu, Pistacia integerrima, and Quercus infectoria showed significant inhibition against all bacterial isolates. Pistacia integerrima and Quercus infectoria showed the maximum inhibition. The present study confirmed that traditional medicinal plants could be helpful for the treatment of oral and dental ailments.
Dental caries, Medicinal plants, Antibacterial activity
Dental caries are a prevalent dental disease worldwide. The World Health Organization, reported that 60%–90% of school-going children and more than 90% of adults suffer from dental cavities leading to the formation of caries. Therefore, dental caries is a major public health problem globally1
In the oral cavity, different microbial species known as oral microbiota, are found. Over 750 species of microorganisms have been reported in the oral cavity2. The formation of dental caries is due to acidogenic and aciduric gram-positive bacteria that metabolize sucrose to organic acid, which dissolves the calcium phosphate in teeth resulting in tooth decay3.
Dental caries is a biofilm-mediated infection caused by predominant bacteria such as Streptococcus spp., Lactobacillus, Rothia dentocariosa, and Actinomyces spp. These cariogenic oral bacteria attach themselves to the tooth surface resulting in the formation of a biofilm, which restricts the penetration of drugs4.
Various antibiotics have been reported to prevent the formation of dental plaque and caries. However, antibiotic resistance is a major health issue worldwide. Therefore, there is a need to explore safe and alternative therapies for combating infection. Medicinal plants are considered a more suitable, safe, and cost-effective alternative to synthetic medicines5.
Medicinal plants are capable of producing a large number of secondary metabolites such as tannins, terpenoids, alkaloids, and flavonoids that possess antimicrobial properties6. Recently, there has been increasing interest in the extraction of biologically active compounds from plant species for various therapeutic applications. Plant extracts are an important source of biologically active compounds, making them effective alternatives to routinely used drugs7. Many plants have been reported in Ayurveda, whose potential is still unexplored. Therefore, the present investigation aimed to evaluate the antibacterial potential of the aqueous extract of traditional medicinal plants against bacteria isolated from dental caries.
Preparation of plant aqueous extracts
The different plant materials used in Ayurveda and traditional system of medicine were collected and authenticated in collaboration with the College of Ayurved, Bharati Vidyapeeth, Pune, and Agharkar Research Institute, Pune, respectively. Selected plants were washed with sterile distilled water and dried in shade. Each dried plant material was ground into a fine powder. Plant extracts were prepared by dissolving 1 g of dried plant in 25 mL sterile distilled water, boiled on slow heat for 5 min and then filtered through Whatman filter paper. Plant extracts were stored in a small bottle at 5°C until further use.
Antibacterial activity of the plant extracts
Isolation and characterization of bacterial isolates
Oral samples collected from dental caries using sterile swabs were spread onto Mitis Salivaris Agar (MSA) medium and incubated at 37°C for 48 h. Random colonies were selected and transferred to fresh Mitis Salivaris Agar medium. Purified bacterial colonies were identified using 16S rRNA sequencing. The sequences were searched for homology using BLAST. The sequences were submitted to GenBank for assigning accession numbers.
Antibacterial assay
Bacterial isolates were inoculated into brain heart infusion broth and incubated overnight at 37°C. The antibacterial activity of the aqueous plant extract was determined using a modified agar well diffusion assay. Bacterial culture (100 μL, approximately 106 CFU/mL) was spread uniformly onto Mueller–Hinton agar medium. Aqueous extracts of plants (100 μL) were added to the wells. Chlorhexidine was used as a control. Plates were maintained at 37°C for 24 h. Antibacterial activity was checked by the presence of a zone of inhibition8.
Determination of minimum inhibitory concentration (MIC)
The MIC was determined using the broth dilution method using 96-well microtiter plates. In each well, 100 µL of BHI broth, 50 µL of overnight bacterial culture, and 50 µL of the stock solution of each extract (concentration 1 mg/mL) was added. Serial dilution was performed using a micropipette up to the 10th well. Control wells were prepared with the culture medium, broth suspension, and the extract of each plant. The plates were incubated at 37°C for 24 h. The MIC value of the plant extract was evaluated as the lowest concentration that completely inhibited bacterial growth after 24 h of incubation9.
Statistical analysis
The means and standard deviations were calculated for each sample. Analysis of variance (ANOVA) was used to assess the statistical significance of the differences between the control and the samples treated with each extract. Differences were considered significant at p < 0.05.
Identification of isolated bacteria
The random bacteria colonies isolated on Mitis Salivaris Agar were identified using 16S rRNA sequencing and were found to be Streptococcus mutans (MH889143), Enterococcus faecalis (MH793461), Rothia dentocariosa (MH824681), and Streptococcus anginosus (MH889145). The accession numbers mentioned in brackets were assigned after submitting the sequences to GenBank, NCBI.
Table (1):
Medicinal plants used for the study.
Sr. No. |
Medicinal Plant |
Part of plant used for the study |
|---|---|---|
1 |
Moringa oleifera |
Seed |
2 |
Achyranthes aspera |
Bark |
3 |
Ficus benghalensis |
Bark |
4 |
Ficus racemosa |
Bark |
5 |
Ficus religiosa |
Bark |
6 |
Senegalia catechu |
Bark |
7 |
Bauhinia variegata |
Bark |
8 |
Pistacia integerrima |
Galls |
9 |
Butea monosperma |
Seed |
10 |
Pterocarpus marsupium |
Bark |
11 |
Aegle marmelos |
Bark |
12 |
Solanum xanthocarpum |
Bark |
13 |
Terminali aarjuna |
Bark |
14 |
Momordi cacharantia |
Bark |
15 |
Nigella Sativa |
Seed |
16 |
Quercus infectoria |
Galls |
17 |
Citrullus colocynthis |
Bark |
Antibacterial assay
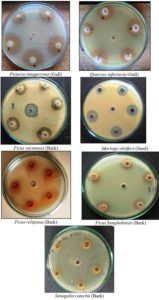
The antibacterial potential of medicinal plants was evaluated according to their zone of inhibition and compared to that of 0.2% chlorhexidine used as a standard. Seventeen plants were screened for their antibacterial potential. Seven plants, namely, Pistacia integerrima, Quercus infectoria, Moringa oleifera, Ficus religiosa, Ficus benghalensis, Ficus racemosa, and Senegalia catechu exhibited significant antibacterial activity. The maximum zone of inhibition against the dental bacterial isolates was observed in P. integerrima and Q. infectoria.
Four plants, namely Achyranthes aspera, Bauhinia variegata, Pterocarpus marsupium, and Terminalia arjuna showed moderate antibacterial activity. Six plants, Butea monosperma, Aegle marmelos, Solanum xanthocarpum, Momordica charantia, Nigella sativa, and Citrulluscolocynthis did not show any inhibitory effect on oral bacteria.
The antibacterial activity potential of all the aqueous extracts of medicinal plants evaluated against the four isolated bacteria is summarized in Table 2.
Table (2):
Antimicrobial activity (zone of inhibition, mm) of various plant extracts against isolated oral pathogens.
| Zone of inhibition in mm against the bacteria | |||||
|---|---|---|---|---|---|
| Sr. No | Plant extracts used | S. mutans | E. faecalis | R. dentocariosa | S. anginosus |
| Mean ± S.D. | Mean ± S.D | Mean ± S.D | Mean ± S.D | ||
| 1 | Moringa oleifera (seeds) | 17.00 ± 1.41 | 15± 1.41 | 16.50 ± 0.70 | 17.00 ± 2.82 |
| 2 | Achyranthes aspera (bark) | 8.00 ± 1.41 | 6.00 ± 8.48 | No zone | No zone |
| 3 | Ficus benghalensis (bark) | 17.50 ± 2.12 | 13.50 ± 2.12 | 13.50 ± 2.12 | 14.00 ± 1.41 |
| 4 | Ficus racemosa (bark) | 14.00 ± 1.41 | 14± 1.41 | 15.00 ± 1.41 | 15.50 ± 2.12 |
| 5 | Ficus religiosa (bark) | 17.50 ± 2.12 | 13.00 ± 2.82 | 12.50 ± 0.70 | 8.50 ± 0.70 |
| 6 | Senegalia catechu (bark) | 17.00 ± 1.41 | 13.50 ± 0.70 | 6.50 ± 9.19 | 12.00 ± 1.41 |
| 7 | Bauhinia variegata (bark) | 15.00 ± 1.41 | 10.50 ± 2.12 | 12.00 ± 1.41 | 13.00 ± 1.41 |
| 8 | Pistacia integerrima (gall) | 26.00 ± 1.41 | 25.50 ± 0.70 | 23.00 ± 1.41 | 19.50 ± 3.53 |
| 9 | Butea monosperma (seed) | No zone | No zone | No zone | No zone |
| 10 | Pterocarpus marsupium(bark) | 11.50 ± 0.70 | 6.00 ± 8.48 | 7.00 ± 9.89 | 12± 1.41 |
| 11 | Aegle marmelos (fruit) | No zone | No zone | No zone | No zone |
| 12 | Solanum xanthocarpum (bark) | No zone | No zone | No zone | No zone |
| 13 | Terminalia arjuna (bark) | 13.00 ± 1.41 | 11.00 ± 2.82 | 10.50 ± 0.70 | 5.00 ± 7.07 |
| 14 | Momordica charantia (seed) | No zone | No zone | No zone | No zone |
| 15 | Nigella sativa (seed) | No zone | No zone | No zone | No zone |
| 16 | Quercus infectoria (gall) | 23.00 ± 1.41 | 19.00 ± 7.07 | 17± 1.41 | 18.00 ± 2.82 |
| 17 | Citrullus colocynthis (bark) | No Zone | No zone | No zone | No zone |
| 0.2% CHX (control) | 18.60±0.65 | 19.00±0.91 | 21.83±0.57 | 24.33±0.27 | |
| F-Value | 38.2 | 7.31 | 13.71 | 13.91 | |
| P-Value* | <0.001† | <0.001† | <0.001† | <0.001† | |
P-value derived from ANOVA Test; †significant at p < 0.05, zone of inhibition in mm. Values are mean of duplicate readings (mean ± S.D)
Determination of minimum inhibitory concentration (MIC)
The MIC was determined for the seven plant extracts that showed significant antibacterial activity. The MIC values of the different plant extracts against the isolated bacteria are presented in Table 3. The inhibitory effects, expressed as the MIC values, of the seven plant extracts on bacterial growth were in the range of < 0.73 to > 2.53 μg/mL.
Table (3):
Minimum inhibitory concentration (MIC values of potential plant extracts against isolated bacteria.
| Minimum inhibitory concentration (µg/ml) | ||||
|---|---|---|---|---|
| Plants extract | S. mutans | E. faecalis | R. dentocariosa | S. anginosus |
| Pistacia integerrima | 1.33 | 1.27 | 1.89 | 1.58 |
| Quercus infectoria | 1.57 | 2.53 | 2.50 | 2.36 |
| Moringa oleifera | 0.83 | 1.07 | 1.27 | 1.25 |
| Ficus religiosa | 0.73 | 0.81 | 0.93 | 1.16 |
| Ficus benghalensis | 0.81 | 0.86 | 1.02 | 1.14 |
| Ficus racemosa | 0.96 | 1.09 | 0.88 | 1.19 |
| Senegalia catechu | 0.90 | 0.80 | 0.77 | 1.04 |
| 0.2% CHX | 0.40 | 0.57 | 0.78 | 1.25 |
The present study aimed to screen seventeen traditional medicinal plants against bacterial isolates that are commonly associated with dental caries. Streptococcus mutans, Enterococcus faecalis, Rothia dentocariosa and Streptococcus anginosus were isolated from dental caries samples. These bacteria are reported to play an important role in dental caries because of their ability to form biofilms10.
Traditional medicinal plants and plant-derived bioactive compounds have been a source of novel drug discovery for various ailments. These could be a safer and cost-effective alternative for the treatment of various bacterial infections and to combat the increasing drug resistance of these bacteria. Microbial growth in biofilms has significantly increased the possibility of survival in the presence of antibiotics. Ayurvedic medicinal plants were explored in the present study. The plants were selected based on their beneficial capability in oral health benefits, as reported in the Charak Samhita11. The seventeen aqueous extracts were prepared and checked for antibacterial activity using the agar well diffusion method against S. mutans (MH889143), E. faecalis (MH793461), S. anginosus (MH889145), and R. dentocariosa (MH824681). Results showed that seven plants, namely, P. integerrima, Q. infectoria, M. oleifera, F. religiosa, F. benghalensis, F. racemosa, and S. catechu, exhibited significant antibacterial activity.
The aqueous extracts evaluated in our study showed excellent results with P. integerrima galls exhibiting a zone of inhibition of 26. 00 ± 1.41 mm, 25.50 ± 0.70 mm, 23.00 ± 1.41 mm, and 19.50 ± 3.53 mm against S. mutans, E. faecalis, R. dentocariosa, and S. anginosus, respectively. The maximum antibacterial activity was observed in P. integerrima against all the isolated oral bacteria. The excellent antimicrobial activities observed in our study are very significant when compared to those obtained with hot water extracts of P. integerrima gall which showed non-significant (p > 0.05) zones of inhibition of 9 mm against Bacillus subtilis and Salmonella typhi and of 8 mm against Enterococcus faecalis, Staphylococcus aureus, and Pseudomonas aeruginosa12.
A previous report suggested the antibacterial potential of P. integerrima gall against various bacteria such as Bacillus cereus (zone of inhibition, 20.03 ± 0.15 mm), S. aureus (16.60 ± 0.31 mm), P. aeruginosa (14.60 ± 0.31 mm), Escherichia coli (11.60 ± 0.23 mm), and Klebsiella pneumoniae (6.60±0.31 mm)12.
The presence of secondary metabolites in the gall of Q. infectoria could be responsible for its antibacterial activity against various pathogens. The high tannin content in the galls of Q. infectoria has been reported as responsible for its antibacterial activity13 studied the antibacterial activity of methanol and acetone extracts of Q. infectoria galls against S. mutans and observed a zone of inhibition of 22.67 ± 0.33 mm and 21.33 ± 0.33 mm, respectively. Significant antibacterial activity by aqueous extracts of Q. infectoria was observed against the bacteria isolated in our study.
In addition to these two prominent plants, five other plants, i.e., M. oleifera, F. religiosa, F. benghalensis, F. racemosa, and S. catechu exhibited antibacterial potential against the isolated bacteria. The significant inhibition of Streptococcus species and similar antibacterial activities of these plants has been reported previously14,15. P. integerrima and Q. infectoria showed the most promising results against oral bacterial isolates. It was observed that these plants may contain broad-spectrum antibacterial compounds that could be used for preventive treatments in dental caries.
Traditional Indian medicinal plants have immense antimicrobial potential. The aqueous extracts of M. oleifera, F. benghalensis, F. racemosa, F. religiosa, S. catechu, P. integerrima, and Q. infectoria showed significant antibacterial activity against isolated bacteria which are majorly associated with dental caries. The highest inhibition was shown by P. integerrima and Q. infectoria. These medicinal plants should be further explored for their potential application in the treatment of oral diseases.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Dixit LP, Shakya A, Shrestha M, Shrestha A. Dental caries prevalence, oral health knowledge and practice among indigenous Chepang school children of Nepal. BMC Oral Health.2013;13:20.
Crossref - Jenkinson HF, Lamont RJ. Oral microbial communities in sickness and in health. Trends Microbiol. 2005;13(12):589-95.
Crossref - Adyanthaya A, Ismail S, Sreelakshmi N. Indian traditional medicinal herbs against dental caries – an unsung past to a bright future. Saudi Journal of Oral and Dental Research. 2016;1(1):1-6.
- Aas JA, Griffen AL, Dardis SR, et al. Bacteria of dental caries in primary and permanent teeth in children and young adults. J Clin Microbiol. 2008;46(4):1407-1417.
Crossref - Balunas MJ, Kinghorn A. Drug discovery from medicinal plants. Life Sciences. 2005;78(5):431-441.
Crossref - Mujeeb F, Bajpai P, Pathak N. Phytochemical Evaluation, Antimicrobial Activity, and Determination of Bioactive Components from Leaves of Aegle marmelos. BioMed Res Int. 2014;497606.
Crossref - Katiyar C, Gupta A, Kanjilal S, Katiyar S. Drug discovery from plant sources: An integrated approach. Ayu. 2012;33(1):10-19.
Crossref - Yim N-H, Jung YP, Cho W-K, et al. Screening of aqueous extracts of medicinal herbs for antimicrobial activity against oral bacteria. Integrative Medicine Research. 2013;2(1):18-24.
Crossref - Klancnik A, Piskernik S, Jersek B, Mozina SS. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J Microbiol Methods. 2010;81(2):121-126.
Crossref - MacFarlane TW, Samaranayake LP. Microbiology of dental caries. Clinical Oral Microbiology.2014;3(1):35-50.
- Shastri K, Chaturvedi G. Charak Samhita, Vidyotini Hindi commentary Varanasi: Chaukhamba Bharati Academy. 2018;1-2.
- Khalid A, Waseem A, Saadullah M, et al. Antibacterial activity analysis of extracts of various plants against gram -positive and -negative bacteria. Afr J Pharm Pharmacol. 2011;5(7):887-893.
- Basri DF, Tan LS, Shafiei Z, Zin NM. In vitro antibacterial activity of galls of Quercus infectoria Olivier against oral pathogens. Evid Based Complement Alternat Med. 2012; 632796.
Crossref - Gayathri M, Kannabiran K. Antimicrobial activity of Hemidesmus indicus, Ficus bengalensis and Pterocarpus marsupium roxb. Indian J Pharm Sci. 2009;71(5):578-581.
Crossref - Hossain MS, Sayeed MA, Uddin MN. In-vitro antimicrobial activity of methanolic extract of Ficus racemosa Linn. fruits. Journal of Scientific and Innovative Research.2014;3(34):446-449.
© The Author(s) 2021. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.