ISSN: 0973-7510
E-ISSN: 2581-690X
The precise relationship between interleukins-33 and IL-5, as well as some trace elements and asthma, is unknown. The target of research was to compare and link the above-mentioned serological parameters in asthmatic patients and healthy controls. In 69 asthmatic patients and 35 healthy controls, serum levels of IL-33, IL-5, zinc, copper, iron, total IgE, Forced expiratory volume (FEV) and Forced expiratory volume (FEV) were compared. Spirometry was used to assess the (FEV) and (FVC) in asthmatic patients, as well as their age and body mass index (BMI). When asthmatic patients were matched to controls, mean levels of IL-33, IL-5, and total IgE appeared highly significant difference (p < 0.001). There was a substantial decline in zinc levels in the asthmatic group, but no significant drop in Copper levels. There was also a statistically significant difference in high Iron mean levels among asthmatic patients. In addition, the findings revealed a significant positive correlation between Iron and IgE levels in patients and the levels of (IL-33 and IL-5), plus a significant negative correlation with Zinc levels. Only Copper had no relationship with the interleukins studied. IL-33, also known as IL-5, is a novel inflammatory marker implicated in asthma progression by interacting with IgE, Zinc, Iron, but not Copper levels. As a result, it could be a one-of-a-kind therapeutic target in these patients.
Asthma, Copper, IL-5, IL-33, Iron, Zinc
Asthma is a chronic inflammatory illness of the lungs characterized by fluctuating airflow closure and associated symptoms such as airway inflammation and damage.1 Asthma is thought to be an immunological illness mediated by T helper 2 (Th2) cells. Interleukin (IL)-33 is a cytokine produced by Th2 cells that actions as a chemoattractant for human Th2 cells. Mast cells produce IL-33 after being activated by immunoglobulin IgE.2 Interleukin-5 (IL-5) is important in the pathophysiology of asthmatic airway inflammation,3 while activated CD3+ T cells are predominant origin for IL-5 in atopy with asthma, also CD8 lymphocytes are the chief source in non-atopy asthma.4 CD8 cells operate as a moderator of eosinophil initiation, regulating adhesion, membrane receptor expression, and chemotaxis.5 The development of asthma is influenced by a variety of genetic and environmental variables.6 Many researchers have suggested that dietary changes may have had a role in increased asthma susceptibility.7 It is thought that the decrease in anti-oxidant intake, as indicated in pregnant women’s diets, may enhance the newborn baby’s vulnerability to allergens.8 Nutrition has also been identified as a significant influence in the development of several chronic respiratory, cardiovascular, and gastrointestinal disorders.9 Trace elements are vital micronutrients which present in extremely low levels in the body, accounting for less than 0.01 percent of overall body weight.10 They are essential for the normal functioning of the immune system and play a significant role in a variety of physiological functions. These elements and minerals must be present in adequate proportions in the body, and they must be capable of interacting with other elements to produce key molecules and participating in a variety of important chemical reactions.11 Trace element deficiency has been linked to the formation of free radicals, which causes tissue damage. Infectious disorders and trace element deficiency are frequently detected together, resulting in complex interactions.12 Immunomodulatory actions of important trace metals like zinc and copper alter susceptibility and the course of a range of illnesses. This is because these elements are found in the structure of antioxidant enzymes such as superoxide dismutase. Antioxidant is a property of enzymes, can modulate the host immune system, and can change the viral DNA.10 Variations in zinc and copper levels reduce the antioxidant system’s efficacy, causing hyperreactivity and inflammation in the respiratory tract.13 The major goal of this study was to compare the outcomes of patients with asthma to healthy controls in terms of serum levels of IL33, IL-5, and trace metals (zinc, iron, and copper). In addition, the link between the parameters studied was investigated, as well as the idea that small trace element levels are a risk factor for asthmatic symptoms. We wanted to see if there was a link between bronchial asthma and trace element and interleukin levels in the blood.
The participants in this study were 69 asthmatic patients (39 males and 30 females), ranging in age from 12 to 40 years (Mean SD: 25.15±17.87). Patients with allergic asthma who have visited the Zahraa allergic center of Al-Karkh hospital with asthma depend on medical diagnosis of wheeze with history of recurrent, dyspnea and cough before 12 months at least, and anthropometric measurements (height, weight) complete system examination to rule out other diseases. As control subjects, 35 healthy people of similar ages (19, 16 of males and females respectively) were recruited, after rejection criteria: family history of asthma, history of childhood asthma, a febrile illness or chest infection within the previous four weeks, or episodes of cough and wheezing in the past 12 months, and sera total IgE value of > 100 IU/ml. Physical Examination Asthmatic patients were diagnosed established on clinical examination, history and by inclusion of alterable airway obstruction, distinct as rise in volume of forced expiratory in 1 s (FEV1) by 12%, 15 min after salbutamol inhalation (400 μg/spacer). 14 Patients who suffered from any chronic disease were excluded from the study.
Measurement of total serum IgE, IL-5, IL-33 levels and trace elements (zinc, copper, iron) concentrations
Upon recruitment, all subjects had a forearm venous blood sample (5 ml) collected for a complete blood count, cytokine, and IgE measurement. After allowing the blood to clot, it was centrifuged, and the sera was collected and separated into many Eppendorf tubes, which were then frozen at -20°C and thawed immediately before analysis. According to manufacturer manuals:
- The total IgE concentration in the blood was determined using the ELISA technique (Human, Germany).
- IL-5 and IL-33 serum levels were also analyzed using an ELISA method (BioSource, Belgium, and R&D Systems, respectively). The detection limits for cytokines were 2.0 pg/ml for IL-5 and 1.5 pg/ml for IL-33. Cytokine levels below the revelation limits were considered zero.
- The contents of zinc and copper in the blood were determined using an assay kit (Bussero (Millan) ITALY).
- Photometric Colorimetric Test to estimate iron levels using a kit from the Human Gesellschaft für Biochemica und Diagnostica mbH (Germany).
- Spirometry was used to evaluate the Forced Expiratory Volume (FEV) and Forced Vital Capacity (FVC) in asthmatic patients using a portable spirometer.
Statistics analysis
The current study’s obtained data was analyzed using IBM’s SPSS statistics software 25.0 version. Descriptive data analysis: Frequency analysis, Pearson Correlation: to analyze the relationship between variables and Scatter diagram displayed the correlation, and Independent t-Test: for means comparison were employed.15
Asthma appears to be caused by a variety of aspects, including immunological, genetic and environmental influences.16 Cytokines show a vital role in organizing the chronic inflammation of asthma by inducing, stimulating, and supporting the persistence of different inflammatory cells in the respiratory tract.17 This study looked at 69 asthmatics and 35 non-asthmatics. The characteristics of asthmatic patients and the control group are shown in (Table 1), and mean ages, BMI (Kg/m2), total IgE (IU/ml), Zinc, Copper, Iron, IL-33, IL-5, FEV1 and FVC were compared between the groups. Present results of study appear mean serum IgE level was significantly higher in asthmatic patients, which supported by results published by Sandstrom.18 IgE plays an essential function in the start and expansion of the inflammatory flow and so allergic response happens.19 Mean serum level of zinc was 60.92±5.63 (range of 49-75μg/dl) in the asthmatic, which was significantly lower than 94.84±9.94 (range of 74-114μg/dl) in the control group (p < 0.001) while Copper mean levels 100.28±25.89 (range of 44-145μg/dl) lower than control (103.88±17.83 (range of 80-150μg/dl) with non-significant. The mean iron levels in patients were 1.940.24 (range 89-310g/dl) higher than the control (1.020.14 g/dl) with significant differences at (p-value 0.04), different studies have been suggested that trace elements might be involved in inflammatory process such as asthma. Variations in trace elements such as zinc, lead and copper happen during of acute and chronic inflammatory like asthma, zinc and copper are required for peak activity of the immune system.20 The results demonstrate that serum levels of IL-33 (553.90188.25pg/ml) and IL-5 (36.048.19pg/ml) are significantly different from healthy controls (105.4421.01 pg/ml and 15.245.71 pg/ml) respectively. IL-33, a fellow the family of IL-1 cytokine, is considered to be vital for the stimulation of T helper-2 cell dominant immune responses such as host defense against allergic diseases, nematodes and interfere with viral infection.21,22 Several stimuli comprising innate immune initiates like viral infections and parasite and or in backgrounds of allergic responses lead to produce IL-5 chiefly by T helper type 2 cells.23 This study assessed the serum levels of iron, copper, zinc, IgE, levels in asthma compared with healthy group. The link between IL-33 and asthma and allergies is yet unknown. Several animal research using recombinant protein in leukocyte cultures suggest the involvement of IL-33 in Th2-type immune response enhancement; however, only a few human studies have been undertaken.8 Some allergic illnesses, such as anaphylaxis, allergic rhinitis, atopic dermatitis and allergic conjunctivitis, have been shown to be affected by IL-33.24
Table (1):
Laboratory results of study subjects Independent samples t-test.
| Variables | Study Groups (Mean±SD) | t-test | p-value | |
|---|---|---|---|---|
| Case | Control | |||
| Age ( years) | 22.06±16.36 | 28.24±18.92 | -1.74 | 0.11 NS |
| BMI (Kg/m2 ) | 23.24±6.25 | 25.76±5.81 | -1.56 | 0.40 NS |
| IgE( IU/ml) | 196.68±54.21 | 14.18±4.75 | 23.71 | <0.001 HS** |
| Zinc Levels μg/dl | 60.92±5.63 | 94.84±9.94 | -20.98 | <0.001 HS** |
| Copper Levels μg/dl | 100.28±25.89 | 103.88±17.83 | -0.81 | 0.42 NS |
| Iron Levels μg/dl | 1.94±0.24 | 1.02±0.14 | 23.36 | 0.04 S* |
| IL-33 (pg/ml) | 553.90±188.25 | 105.44±21.01 | 16.74 | <0.001 HS** |
| IL-5 (pg/ml) | 36.04±8.39 | 15.24±5.71 | 14.48 | 0.01 S* |
| FEV1 | 2.28±0.23 | 3.93±0.08 | -54.45 | <0.001 HS** |
| FVC | 3.57±0.36 | 4.97±0.04 | -26.70 | <0.001 HS** |
*Independent samples t-test, NS: Non significant* at p > 0.05 HS: High significant** at p ≤ 0.001.
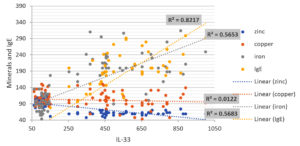
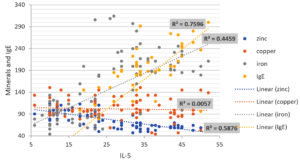
The serum levels of IL-33 in asthmatic patients were found to be significantly higher than in healthy participants in the current investigation. In addition, there was a substantial positive association between Iron and IgE levels in patients and the concentrations of IL-33 and IL-5, in addition to a significant negative correlation with Zinc levels. Only Copper had no relationship with the interleukins examined. In addition, the current investigation discovered a link between IL-33 serum levels and total IgE levels. Many studies have found a link between total IgE and asthma severity. Innate cells secrete IL-4 after being stimulated by IL-33, which causes B-cell growth and IgE production. Simultaneously, IL4 promotes the interaction of CD40 on B-cells and CD40 ligand on T-cells, resulting in the generation of total serum IgE. As a result, IL-33 could play a part in all IgE-mediated allergy disorders.22 Some studies have found higher levels of IL-33 in asthmatic patients and linked it to disease severity, such as one study that found elevated expression of IL-33 in epithelial cells and bronchoalveolar lavage fluid of asthma patients compared to healthy controls.19,23 In contrast to other studies, Hussein et al found that serum zinc levels in asthmatic patients were elevated.10 As a result, there is some disagreement over serum copper and zinc levels in asthmatic patients. The correlation between minerals and IgE levels with IL-33 and IL-5 levels in patients was depicted in a scatter diagram in (Fig. 1, 2) and ( Table 2). There was a significant positive correlation between total IgE and Iron Levels with IL-33 and IL-5, whereas FEV1, FVC, and Zinc Levels results showed high significantly negative correlations with IL-33 and IL-5. Only Copper had no association with the interleukins that were tested. As a result, it appears that a drop in the zinc-to-copper ratio is more relevant than variations in zinc or copper levels. The levels of IgE and iron in patients’ serum were substantially greater than in control groups, according to our findings.25,26 IgE and blood iron levels have a favorable relationship. Our findings revealed high significant levels of blood zinc, iron, and IgE in asthma patients, implying that there is a link between serum mineral levels, IgE levels, and allergic illnesses such asthma.27 It is effective to develop novel therapeutic techniques against IgE-mediated allergies by identifying a target as a primary trigger of inflammation in asthma. IL-33 could be one of these molecules, operating early in the allergy cascade after epithelium injury caused by various environmental stressors or cellular damage. It attracts and activates the disease-causing cells, indicating that it plays a crucial role in asthma pathogenesis.28 Growing research suggests that IL-33 could be a new therapeutic target for allergic illnesses including asthma. Recent research has found that IL-33 antagonist has positive effects in murine models of allergic rhinitis, lower airway inflammation, and allergic contact dermatitis, suggesting that IL-33 could be a therapeutic target for allergies.29 Finally, an increase in blood iron, zinc, and copper levels in asthma patients appears to play an important role in asthma induction, but serum zinc levels in asthmatics and healthy people appear to be similar.
Table (2):
Correlation of IL-5 and IL-33 with Minerals and IgE Levels.
| Factors | IL-5 | IL-33 | ||
|---|---|---|---|---|
| (r) | p-value | (r) | p-value | |
| Age | -0.08 | 0.40 NS | -0.04 | 0.64 NS |
| BMI | -0.18 | 0.06 NS | -0.08 | 0.40 NS |
| IgE | 0.87 | <0.001 HS | 0.90 | <0.001 HS** |
| Zinc Levels | -0.79 | <0.001 HS | -0.77 | <0.001 HS** |
| Copper Levels | -0.07 | 0.45 NS | -0.11 | 0.27 NS |
| Iron Levels | 0.72 | <0.001 HS | 0.77 | <0.001 HS** |
| FEV1 | -0.81 | <0.001 HS | -0.84 | <0.001 HS** |
| FVC | -0.73 | <0.001 HS | -0.84 | <0.001 HS** |
*Pearson correlation coefficient, NS: Non significant* at p > 0.05 HS: High significant** at p ≤ 0.001.
Fig. 1. Scatter diagram represented the correlation between Minerals and IgE Levels with IL-33 levels.
(IgE = positive weak correlation, Iron = positive high correlation, Zinc = negative strong correlation, Copper = No correlation).
It’s thought that elevated IL-33 is responsible for the maintenance of airway inflammation and hypersensitivity, particularly in severe asthmatic patients, and the findings confirmed that IL-33 and IL-5 play a vital role in asthmatic patients with mineral toxicity. To prove the role of these cytokines as a potential therapeutic target in asthma and other allergic illnesses, more research is needed.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
ETHICS STATEMENT
Not applicable.
AVAILABILITY OF DATA
All data sets generated or analyzed during this study are included in the manuscript.
- Sly PD, Flack F. Susceptibility of children to environmental pollutants. Ann N Y Academy of Sciences. 2008;1140(1):163-183.
Crossref - Ali M, Zhang G, Thomas WR, et al. Investigations into the role of ST2 in acute asthma in children. Tissue Antigens. 2009;73(3):206-212.
Crossref - Ghassemian A, Park JJ, Tsoulis MW, Kim H. Targeting the IL-5 pathway in eosinophilic asthma: a comparison of mepolizumab to benralizumab in the reduction of peripheral eosinophil counts. Allergy, Asthma Clin Immunol. 2021;17(1):1-7.
Crossref - Pelaia C, Paoletti G, Puggioni F, et al. Interleukin-5 in the pathophysiology of severe asthma. Front Physiol. 2019;10:1514.
Crossref - Tomasiak-Lozowska MM, Bodzenta-Lukaszyk A, Tomasiak M, Skiepko R, Zietkowski Z. Rola interleukin 13 i 5 w astmie The role of interleukin 13 and interleukin 5 in asthma. Postepy Hig Med Dosw (Online). 2010;64:146-155.
- Quirt J, Hildebrand KJ, Mazza J, Noya F, Kim H. Practical guide for allergy and immunology in Canada 2018. Allergy Asthma Clin Immunol. 2018;14:50.
Crossref - Kim SH, Lee J, Oh I, et al. Allergic rhinitis is associated with atmospheric SO2: Follow-up study of children from elementary schools in Ulsan, Korea. PloS One. 2021;16(3):e0248624.
Crossref - Molnar D, Galffy G, Horvath A, et al. Prevalence of Asthma and Its Associating Environmental Factors among 6-12-Year-Old Schoolchildren in a Metropolitan Environment-A Cross-Sectional, Questionnaire-Based Study. Int J Environ Res Public Health. 2021;18(24):13403.
Crossref - Riccioni G, D’Orazio N. The role of selenium, zinc and antioxidant vitamin supplementation in the treatment of bronchial asthma: adjuvant therapy or not? Expert Opin Investig Drugs. 2005;14(9):1145-1155.
Crossref - Hussein MM, Yousif AA, Saeed AM. Serum Levels of Selenium, Zinc, Copper and Magnesium in Asthmatic Patients: a Case Control Study. Sudan Journal of Medical Sciences. 2008;3(1):45-48
Crossref - Shazia Q, Mohammad ZH, Rahman T, Shekhar HU. Correlation of oxidative stress with serum trace element levels and antioxidant enzyme status in Beta thalassemia major patients: a review of the literature. Anemia. 2012;2012:270923.
Crossref - Lukac N, Massanyi P. Effects of trace elements on the immune system. Epidemiol Mikrobiol Imunol. 2007;56(1):3-9.
- Mao S, Wu L, Shi W. Association between trace elements levels and asthma susceptibility. Respir Med. 2018;145:110-119.
Crossref - National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Bethesda: National Heart, Lung, and Blood Institute. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007.
- Louten J, Rankin AL, Li Y, et al. Endogenous IL-33 enhances Th2 cytokine production and T-cell responses during allergic airway inflammation. Int Immunol. 2011;23(5):307-315.
Crossref - Saluja R, Ketelaar ME, Hawro T, Church MK, Maurer M, Nawijn MC. The role of the IL-33/IL-1RL1 axis in mast cell and basophil activation in allergic disorders. Mol Immunol. 2015;63(1):80-85.
Crossref - Sandstrom T. Omalizumab in the management of patients with allergic IgE-mediated asthma. J Asthma Allergy. 2009;2:49-62.
Crossref - Komai-Koma M, Brombacher F, Pushparaj PN, et al. Interleukin-33 amplifies IgE synthesis and triggers mast cell degranulation via interleukin-4 in naive mice. Allergy. 2012;67(9):1118-1126.
Crossref - Khanam UA, Rennie DC, Davis K, Lawson JA. Are Dietary Factors Associated with Lung Function in Canadian Adults? Can J Diet Pract Res. 2019;81(1):28-36.
Crossref - Muhsin JM, Rawdhan SO. Determination of the Cytomegalovirus (CMV) infection Role with the Disturbances of Immunoglobulin E (IgE) and Interleukin-33 (IL-33) Concentrations in the Pathogenesis of Asthma and Atherosclerosis in a Sample of Iraqi Patients. J Pure Appl Microbiol. 2019;13(2):1003-1010.
Crossref - Hamzaoui A, Berraies A, Kaabachi W, Haifa M, Ammar J, Kamel H. Induced sputum levels of IL-33 and soluble ST2 in young asthmatic children. J Asthma. 2013;50(8):803-809.
Crossref - Azazi EA, Elshora AE, Tantawy EA, Elsayd MA. Serum levels of Interleukin-33 and its soluble receptor ST2 in asthmatic patients. Egypt J Chest Dis Tuberc. 2014;63(2):279-284.
Crossref - Rogala B, Gluck J. The role of interleukin-33 in rhinitis. Curr Allergy Asthma Rep. 2013;13(2):196-202.
Crossref - Pouramjad SM, Egtesadi SH, Moosavi SA, Nour Mohammadi I, Yazdani RO. Study of zinc serum concentration and effect of zinc supplementation on lung function in asthmatic patients. Razi Journal of Medical Sciences. 2009;15:55-61.
- Gusmira YH, Irsa L, Lubis B, Evalina R, Lubis M. Correlation between Lead Serum Level and Total Immunoglobulin E (Ige) Level in School-Aged Children. Eur J Med. 2018;(6):13-19.
Crossref - Joseph CL, Havstad S, Ownby DR, et al. Blood lead level and risk of asthma. Environ Health Perspect. 2005;113(7):900-904.
Crossref - Walsh GM. An update on biologic-based therapy in asthma. Immunotherapy. 2013;5(11):1255-1264.
Crossref - Taniguchi K, Yamamoto S, Hitomi E, Inada Y, Sugioka T, Hamasaki Y. Blockade of interleukin-33 attenuates allergic contact dermatitis in model mice: Possible mechanism via eosinophil infiltration. J Clin Exp Dermatol Res. 2013;4(3):1-4.
Crossref - Schober P, MMedStat, Boer C, Lothar A. Schwarte. Correlation Coefficients: Appropriate Use and Interpretation. Anesth Analg. 2018;126(5):1763-1768.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.