M.F. Elkady1,2, Soha Farag3* and Ahmed M. Haddad3
1Chemical and Petrochemical Engineering Department, Egypt-Japan
University of Science and Technology, New Borg El-Arab City, Alexandria, 21934, Egypt.
2Fabrication Technology Department, Advanced Technology and New Materials Researches, Institute, City of Scientific Researches and technological applications, New Borg El-Arab City, Alexandria, 21934, Egypt.
3Environmental Biotechnology Department, Genetic Engineering and Biotechnology Research Institute, City of Scientific Research and Technology Applications, Alexandria, Egypt.
ABSTRACT
Nano-magnetite was successfully immobilized onto the isolated lead resistant bacteria species using co-precipitation technique to synthesize novel nano-magnetic bacterial bio-composite material. This isolated bacteria was identified as Enterobacter sp. B2 under accession number KT213696. Scanning Electron Microscope micrographs implied that the magnetite nano-particles were dispersed onto the surface of bacteria. The magnetic properties of magnetic bio-composite material were determined using Vibrating Sample Magnetometer (VSM). VSM evident that the bio-composite is characterized by its supermagnetic properties with 50.8 emu/g saturation magnetization that facilitate the material handling and separation. The feasibility of the novel synthesized magnetic bio-composite material for lead decontamination was explored against the variation of the processing parameters during the bioremediation process. The results elucidated that the equilibrium of lead bioremediation process achieved 90.5% within 60 minutes with bio-composite dosage of 0.1 g. The increments of both initial lead concentration and solution temperature have negative impact on the bio-remediation process.
Keywords: Magnetic immobilized bacteria; Enterobacter sp.;Bio-composite; Lead removal; Bioremediation.
INTRODUCTION
Heavy metals are the main toxic substances in most of industrial wastewater and are highly lethal to human beings and other water and soil living things. Heavy metal pollution is a serious environmental problem. Anthropogenic activities and the rapid development of many industries such as mining operations, surface finishing, energy and fuel production, fertilizers, pesticides metallurgy and the discharge of industrial wastes have resulted in the accumulation of metals in the environment and the food chain, bringing about serious environmental pollution, threatening ecosystems and human health1, 2.
The occurrence of heavy metals in water environment is known to cause acute destruction to aquatic life, beside the fact that these metals kill microorganisms during biological treatment of wastewater with a subsequent delay of the bio treatment process. Most of the heavy metal salts are soluble in water and form aqueous solutions and consequently cannot be separated by conventional physical means of separation3.
Lead (Pb), a major heavy metal pollutant that is found in soil, water and air. This metal is considered as a hazardous waste and highly toxic to human, animals, plants and microbes4. The toxicity of Pb(II) is due to conformational changes of nucleic acids and proteins, inhibition of enzyme activity, disruption of membrane functions and oxidative phosphorylation, as well as impairing the cell osmotic balance. Pb(II) also shows a stronger affinity for thiol and oxygen groups than essential metals such as calcium and zinc5.
Lead inhibits some enzymatic processes involved in heme synthesis, so the elevated blood lead concentrations have been associated with anemia due to the reduced hemoglobin synthesis6-8. Moreover, increased blood lead levels are linked to renal failure6,9,10. Exposure to lead contamination during early childhood has been its association with mental retardation and has been proven to affect intelligence of children even in low concentration 11-12.
Several methods have been conventionally applied for removal of heavy metals from wastewater such as precipitation, flocculation, adsorption to suspended solids during primary sedimentation or adsorption to extracellular polymers, ion-exchange and filtration13.
Many microorganisms, such as algae, yeast and bacteria can absorb dissolved metals from their environments onto their bodies and can be used for removing heavy metal ions effectively. Microorganisms uptake metals either actively (bioaccumulation) and/or passively (biosorption)14.
During the last few decades, biological methods such as biosorption and bioaccumulation for the removal of the toxic heavy metal ions have been extensively studied15-17. Biosorption of heavy metals by microbial cells (inactivated algal, fungal and bacterial biomass) has been known as a prospective alternate to existing technologies for recovery of heavy metals from industrial waste streams. Most studies of biosorption for metal removal have involved the use of either laboratory grown microorganism or biomass produced by the pharmacology and food processing industries or wastewater treatment units 3,18. Moreover, the ability of microbial stains to grow in the presence of heavy metals would be helpful in the waste water treatment where microorganisms contribute in the degradation of organic matter in biological processes for waste water treatment19,20.
Heavy metal resistant Microbes can detoxify the metals by reducing its bioavailability through different mechanisms such as; precipitation of metals as phosphates, carbonates and/or sulfides; volatilization via methylation or ethylation; physical exclusion of electronegative components in membranes and extra cellular polymeric substances (EPS); energy-dependent metal efflux systems; and intra cellular sequestration with low molecular weight, cysteine-rich proteins21,22.
In recent decades, many literatures have reported the correlative advancement on the heavy metal treatment by immobilized microorganisms. Some ideal results have been acquired in wastewater treatment with immobilized methods23. However, as far as separation is concerned, this method needs to be upgraded to satisfy the commercial application. Magnetic particles have been applied as a new sorbent to adsorb metal ions, in which the difficulty of separation was resolved in an external magnetic field24.
In this study, a lead resistant bacterium was isolated and identified. The bacterial isolate was able to remove up to 100 ppm lead from contaminated waste water. The isolated lead resistance bacteria species was immobilized with magnetic nano-particles to synthesize novel nano-magnetic bacterial bio-composite material. This bio-composite material characterized by its facile handling at the water purification processes regarding to its magnetic properties. The lead bioremediation efficiency of this novel magnetic bio-composite was compared with that of the free bacterial species from the polluted synthetic wastewater. Moreover, the different parameters affecting on the lead bioremediation process using nano-magnetic bacterial bio-composite material was examined.
MATERIALS AND METHODS
Isolation of Lead (Pb2+) resistant bacteria
Soil samples were collected from polluted soil near the tannery effluent of tanning company. Ten gram soil polluted was inoculated into Fifty mL LB medium [25] contains in g/L : tryptone,10; NaCl, 5; and yeast extract, 5; supplemented with 50 ppm lead and incubated for 48 h at 30°C and 150rpm in a shaking incubator. One mL from each culture was transferred into LB medium amended with 100 ppm lead and incubated for 48 h at 30°C in a shaking incubator. Several sequential subcultures in LB medium amended with increasing lead concentration concentrations 20, 40, 60, 80, 100, 120, 140 ppm were done with incubation for 48 h at 30°C 150 rpm in a shaking incubator to select the highest resistant bacteria, morphologically different colonies were streaked on LB agar plates (1.5% agar) for purification before being inoculated into the liquid medium to check their decolorizing ability. The bacterial isolates with the strongest resistance ability given a prefix of “B1, B2, and B3”. Then the purified isolates were preserved in mixture of LB medium and glycerol at -20°C.
Identification of the bacterial isolate
The most resistant bacterium B2 was selected for identification on molecular level using 16SrRNA gene sequencing. Genomic DNA was isolated from an overnight 5 mL LB culture according to standard method of Sambrook25. 16SrRNA was amplified using the universal primers 5‘-AGAGTTTGATCMTGGCTCAG-3‘ and 5‘-TACGGYACCTTGTTACGACTT-3‘ based on the 16SrRNA gene of E. coli. The PCR mixture consisted of 25 pmol of each primer, 10 ng of bacterial genomic DNA, 200 mM dNTPs and 2.5 U of Taq polymerase in 50 µl of polymerase buffer. The PCR was carried out for 30 cycles of 94°C for 1 min, 53°C for 1 min and 72°C for 2 min. After completion of the PCR, a fraction of the PCR product was examined using agarose gel and the remnant was purified using GenElute™ PCR Clean-Up Kit. The 16S rRNA gene fragment (1500 bp length) was sequenced and DNA similarities were assessed using Blast program (www.ncbi.nlm.nih.gov/blast). The molecular phylogeny was performed using Blast Tree View (www.ncbi.nlm.nih.gov/projects/treeview).
Biomass production
Overnight preculture was prepared by inoculation of a single colony of bacterial isolate B2 into 30 mL sterile LB medium. The grown culture was used entirely to inoculate the main culture (3L of LB medium) in order to obtain enough biomass. The culture was incubated at 30°C and 160 rpm for 24 h. The biomass was collected by centrifugation of the culture at 3500 rpm for 30 min at 4°C. The supernatant was decanted and the cells were collected and lyophilized.
Immobilization of isolated biomass to synthesize the nano-magnetic bacterial bio-composite material
Five grams from the lyophilized biomass was dispersed in 150 ml solution composed from 5 mmol ferrous sulphate and 12 mmol ferric chloride. Thirty mL ammonia solution (5 mole/L) was added drop wise into the previous solution mixture with continuous stirring at 60°C. After completeness of the ammonia solution addition, a black precipitate from magnetite particles was appear at the reaction solution onto the suspended lyophilized biomass. Subsequently, the stirring and heating were turned off and the black magnetic precipitate was separated using external magnetic field. The separated powder slurry was washed several times with deionized-distilled water and dried at 30°C using vacuum oven. The dried magnetic bacteria powder material was stored at dissector to be utilized at the water treatment process.
Characterization of nano-magnetic bacterial bio-composite material
In order to confirm the immobilization of the isolated bacteria biomass with magnetite nano-particles, XRD and SEM of the prepared nano-magnetic bacterial bio-composite were examined. The crystalline structural of both the isolated biomass and the magnetic bio-composite were determined using X-ray diffractometry (Schimadzu-7000 diffractometer) that contains a copper target. Data were collected between 10° and 80° in 2¸. The binding characteristic was determined with the aid of transform infrared spectrophotometer (Shimadzu FTIR-8400 S). The sample/KBr mass ratio used for the preparation of the disks was 1:100. They were grinded together in mortar and encapsulated for prepared translucent sample disks, then detected using FTIR. The scanning electron microscope (JEOL JSM-6360LV) was used to take pictures of the magnetic biomass at different magnifications. One drop from the free biomass suspension was added at glass substrate and other drop from the magnetic bio-composite matrix was added at another glass substrate. The two glasses substrates contains the biomaterials were dried for 24 h at 25°C using vacuum oven. After complete the drying process the two glasses were sputtered by gold target and supported on the copper holder for examination. Finally, the magnetization properties of the prepared magnetic bio-composite were determined at room temperature using Vibrating Sample Magnetometer (VSM, Dexing, Model:250) in the range of -5000 to 5000 Oe.
Bioremediation monitoring process for lead decontamination using batch mode
The lead bioremediation behaviors of the free biomass before and after magnetite immobilization were compared using batch technique to determine the effect of magnetite immobilization process. The most proper matrix either free biomass or magnetic bio-composite that recorded the highest lead bioremediation will be selected as an efficient matrix for monitoring the influence of bioremediation processing parameters onto the matrix lead sorption efficiency. Various materials dosages (0.01g-1g) from either free biomass or magnetic bio-composite were mixed separately with 25 ml synthetic waste lead solution with initial concentration of 100 ppm at 100ml stopper conical flasks using shaking water bath. The lead solution was maintained at pH 7 and 22°C under continuous stirring at 100 rpm for 2 h. After finishing the mixing period, the solid adsorbent material matrices were separated and the remaining concentrations of lead solutions after the treatment process were determined using atomic absorption spectrophotometer (Shimadzu, AA-7000). In order to optimize the treatment processing parameters for lead bioremediation process using the novel nano-magnetic bacteria B2 bio-composite material, the experiments were done at the same previously described batch mode manner at 100 ml stopper conical flasks using shaking water bath 25 ml of lead solution at constant initial concentration was mixed with specific weights from the magnetic bio-composite material and shaking at various intervals under determined solution pH and temperature. The initial pH of the lead solution was adjusted to the initial desired values of 1, 4, 6, 8 and 10 using either HCl or NaOH solution to evaluate the effect of pH on the lead bio-remediation process. The shaking interval period studied over the range 5- 150 min. The initial lead ion concentration was changed at studied range10- 500 ppm. The various utilized bio-composite material dosages were from 0.05- 1.5g. Finally the lead solution temperature was adjusted using shaking waster batch at the range 15- 85°C. After finishing the mixing time, the magnetic bio-composite powder material was separated from the treatment media using external magnetic field and the remaining lead ion concentration was determined using atomic absorption spectrophotometer (Shimadzu, AA-7000). The percentage of lead ion bio-remediation was calculated using the following equation:
% lead bio-remediation = (Ci-Cf)/ Ci
Where, Ci: is the initial lead ion concentration at the solution and Cf: is the final and remaining lead ion concentration at the solution after treatment process.
RESULTS AND DISCUSSION
Isolation and identification of lead resistant bacteria
Several lead resistant bacterial isolates were isolated from the enrichment and sequential subcultures with different lead concentrations. The best lead resistant isolate, B2, which was able to grow in the presence of up to 100 ppm Pb(II), was selected for further work and identification. The genomic DNA was purified and the PCR product of the 16srRNA gene (1500bp) was sequenced. Subsequently, the sequence has been deposited in the GenBank with the accession number (KT213696) and aligned against the GenBank database. The closest known sequence in the database was Enterobacter sp. with 99% similarity to the bacterial strains. The Phylogenetic relationship among the tested isolate and the closely related species was analyzed using Blast Tree View as shown in Fig. 1.
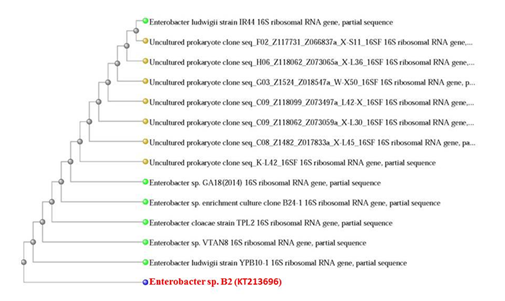
Fig. 1. Phylogenetic relation 16S rRNA sequence of the Pb2+ bacterial strain (Enterobactersp. B2) with 16S rRNA of closely related bacteria in the GenBank database. The dendogram was generated by the neighbor-joining method using Blast Tree View.
Characterization of nano-magnetic bacterial bio-composite material
The best lead resistant strain (B2) was lyophilized and immobilized with magnetite nano-particles to synthesize nano-magnetic bacteria bio-composite material that characterized by its magnetic properties. This magnetic property facilitates its handling at the water purification process through utilization of external magnetic field as indicated in Fig. 2. The physico-chemical properties of the magnetic bio-composite were determined using various characterization techniques.
Fig. 2. Facile separation of nano-magnetic bacteria bio-composite using external magnetic field.
SEM micro-graphs of both free biomasses B2 before and after magnetite immobilization were compared at Fig. 3. It was evident from Fig. 3(A) that the isolated Enterobacter sp. Species have rod-like shape and this shape was maintained after magnetic immobilization without any distortion. However, small dark spherical spots were observed at the magnetite immobilized biomass as shown in Fig. 3(B). These spherical dark spots represent the magnetite nano-particles that were immobilized onto the bacteria surface with average diameters of 40nm. These SEM images confirm the immobilization of magnetite nano-particles onto bacteria surface to attain the novel nano-magnetic bacteria bio-composite material.
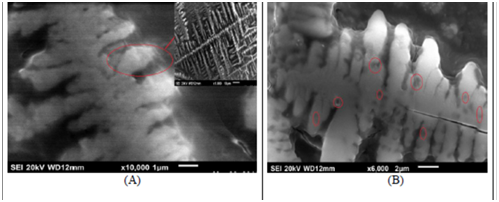
Fig. 3. SEM mocrographs of (A) free biomasses B2, (B) nano-magnetic B2 bio-composite.
In order to compare the surface nature of the free biomasses B2 before and after magnetite immobilization, the FTIR spectroscopy method was used to characterize the surface functional groups to identify the magnetite binding characteristic of the bio-composite. As seen in Fig. 4, the FTIR spectrum of free biomass displays a number of absorption peaks, reflecting the complex nature of the biomass. The spectrum pattern of the free biomass showed changes of certain bands in the region of 1575–527 cm”1 as compared magnetite immobilized B2 at the bio-composite26. It was observed at the two FTIR spectrums characteristics band around 1546 cm”1 that is indicative of C–N stretching and N–H deformation. The broad band at 3297.30 cm”1 was assigned for the existence of OH at both free bacteria and magnetic composite. The peak at 1640.20 cm”1 is mainly attributed to C-O stretching that can be regarded to amide I and amide II bands of protein peptide bonds at bacteria strain27. There was also clear that aromatic –CH stretching peak at 902 cm”1 was assigned for free biomass B2 that was disappeared after magnetite immobilization at the bio-composite28. Two intense peaks were detected at nano-magnetite bacteria bio-composite at 845 and 615 cm-1 band, which are due to the stretching vibration mode associated to the metal-oxygen absorption band (Fe – O bonds in the crystalline lattice of Fe3O4) that was immobilized at the magnetic bio-composite 29. So, the FTIR spectrums confirm the formation of the nano-magnetic bacteria bio-composite.
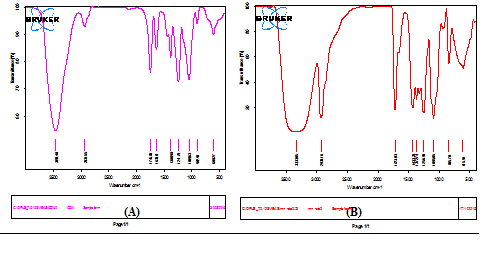
Fig. 4. FTIR spectrums of (A) free biomasses B2, (B) nano-magnetic B2 bio-composite.
After confirming the immobilization and binding of magnetite nano-particles onto the bacteria to prepare novel nano-magnetic bacteria bio-composite, the physico-chemical properties of this bio-composite were examined using XRD and VSM analysis. In order to assign the crystalline structure of the prepared nano-magnetic bacteria bio-composite, XRD of the material was investigated at Fig. 5. It can be seen from the Figure that the bio-composite has almost semi-crystalline structure and there are some characteristic peaks with some degree of intensity were observed at XRD spectrum. In order to identify these peaks, the XRD pattern was compared with the standard pattern of magnetite JCPDS Card No. (79 – 0417). The analysis of the diffraction pattern showed the formation of a cubic spinel structure of Fe3O4, due to the strongest reflection that proceeds from the (311) plane at 2¸ = 35.4°, which is characteristic of such phase30. The other peaks indexed as planes of cubic crystal of iron oxide Fe3O4 (220), (400) and (511) are assigned for peaks present at 2¸ = 30.5°, 43° and 48° respectively. Therefore, it was confirmed about the presence of the crystalline structure of immobilized magnetite nano-particles onto the magnetic bio-composite.
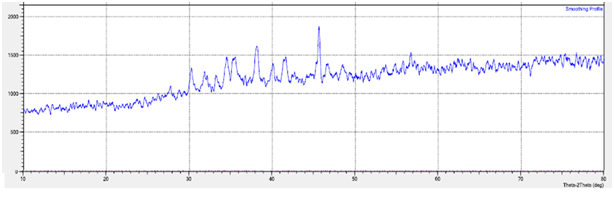
Fig. 5. XRD pattern of prepared nano-magnetic B2 bio-composite.
The magnetic properties of the novel prepared of nano-magnetic B2 bio-composite were explored using VSM analysis at room temperature. Fig. 6 evident that the magnetization curve showed any hysteresis behavior and it exhibits immeasurable values of coercivity field and remnant magnetization. The Figure showed that the magnetization is completely saturated at value of 6.8 emu/g and the ratio of saturation remanence to saturation magnetization (Mr/Ms) is 0.049. Therefore, the prepared magnetic B2 bio-composite material was characterized by its super-magnetic that facilitates its handling during the water purification process using magnetic field31.
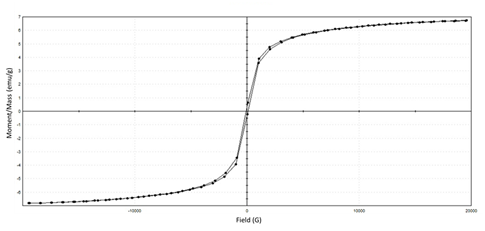
Fig. 6. Magnetic properties of prepared nano-magnetic B2 bio-composite.
Adsorption behavior of free Enterobacter sp. B2 and nano-magnetic B2 bio-composite
To ensure that loading of the bacteria with nano-magnetic particles did not inhibit its Pb2+ adsorption capacity, the removal of lead ions (100 ppm) by the bacterial strain Enterobacter sp. B2 was tested before and after immobilization with nano-magnetic particles. Similar adsorption behavior of both free bacteria and nano-magnetic B2 bio-composite was found using different doses for 2 h as a contact time at pH 7 and 22°C as shown in Fig. 7. The results showed that the free and immobilized B2 have the same behavior in lead removal. Similarly Li et al. (2009) [32] shows that the production of 2-HBP by free cells and Fe3O4-coated cells were capable of biodesulfuriztion of dibenzothiophene (DBT) to 2-hydroxybiphenyl (2-HBP) demonstrated similar time courses and desulfurizing activities during the reaction. The immobilized cells were successfully reused for BDS over seven batch cycles but the uncoated free cells could be used only once.
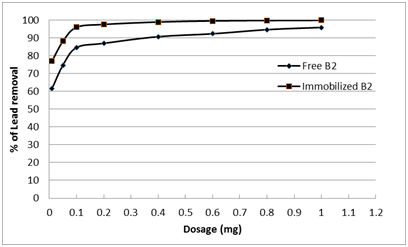
Fig. 7. Adsorption behavior of freeEnterobacter sp. B2 and nano-magnetic B2 bio-composite (pH=7, initial lead concentration=100 ppm and solution temp.=22˚C).
Investigation of adsorption parameters
The adsorption process of lead on nano-magnetic B2 bio-composite has been discussed considering the effect of different factors.
Effect of contact time
The effect of different contact times from 5 to 150 min on the sorption of Pb2+ by nano-magnetic B2 bio-composite was showed that the removal of Pb2+ increased exponentially by increasing the contact time (Fig. 8). During the first 60 min, there was a sharp increase of the adsorption capacity where 90% of the Pb2+ was removed. After 60 min the Pb2+ removal capacity increased slowly by increasing the contact time up to 150 min, after 120 min the removal capacity was 95% while it was 98% after 150 min. Therefore, 60 min was selected as the optimum time for sorption of Pb2+ under the selected experimental conditions. In consistent with our findings, Elkady et al., 2011; Abou-Mesalam, 200433,34 found that the adsorption of metal ions such as Cd2+ and Pb2+ from aqueous waste solutions on inorganic ion exchange material increased exponentially by increasing the contact time. Moreover, almost 90% removal percentage of Pb2+ was achieved after 60 min.
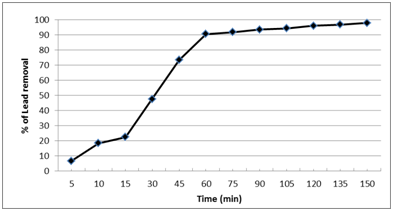
Fig. 8.Effect of contact time on the lead removal by nano-magnetic B2 bio-composite. (pH=7, magnetic B2 dosage=0.1 gm, initial lead concentration=100 ppm and solution temp.=22˚C).
Effect of initial lead ion concentration
The effect of different Pb2+ initial concentrations (from 10 to 500 ppm) on the removal percentage and the removed amount of lead ions was illustrated in Fig. 9. It was observed that the Pb2+ removal percentage is inversely proportional with the initial Pb2+ solution concentrations which reach to 76.5% at maximum concentration 500 ppm. Accordingly initial lead ion concentration of 100 ppm was selected as the optimum concentration according to the experimental conditions where 90.5% of Pb2+ was removed by the nano-magnetic Enterobacter sp. B2 bio-composite. This may be attributed to the increase of adsorbed Pb2+ ions onto the external surface of the bacteria which increases significantly its local concentration. This leads to the formation of Pb2+ clouds on the bacterial surface which hinders the diffusion of further lead ions and decreases the rate of ion exchanges through the bacterial surface. This explanation is compatible with the findings of Pons et al. (1993)35 about the ‘screening effect’ of the outer layer in the microbial cells resulting in a lower metal uptake at higher concentrations.
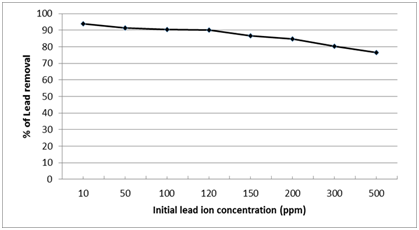
Fig. 9.Effect of initial lead ion concentration on the efficiency of nano-magnetic B2 bio-composite for lead removal. (pH=7, magnetic B2 dosage=0.1 gm, contact time=60 min and solution temp.=22˚C).
Effect of nano-magnetic B2 bio-composite dosage
Adsorbent dosage is one of the important parameters of adsorption. The effect of different magnetic B2 dosage (from 0.05 to 1.5 g) indicated that, the removal percentage of Pb2+ by using 0.1g of B2 bio-composite was about 90% however, by increasing the dose of the bio-composite up to 1.5 g, the removal of Pb2+ slightly improved by about 10% only as illustrated in Fig. 10. So the 0.1g of the bio-composite was selected for further work to economize the bacterial biomass and its result (90% of lead removal) is suitable to know the effect of other parameter. It was found that increasing the adsorbent dosage with fixed metal ion concentration provided more available area and the number of exchangeable sites for sorption and exchanging metal ions increased. This leads consequently to increase the extent of metal ion removal36. The studies done by many references were in agreement or had the same trend with our own findings33,37,38.
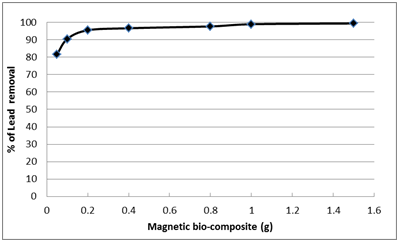
Fig. 10. Effect of nano-magnetic B2 bio-composite dosage on lead removal (pH=7, initial lead concentration=100 ppm, contact time=60 min and solution temp.=22˚C).
Thermal effect
The effect of solution temperature variation (15- 85°C) on the removal percentage of Pb2+ was presented in Fig. 11, which revealed that the percentage of Pb2+ removal was inversely proportional with the solution temperature. The optimum temperature for Pb2+ removal was the ambient temperature (25°C) where Pb2+ removal percentage was about 90%. However, equilibrium sorption studies for temperature effect revealed that temperature elevation improves ion sorption for several studied sorption processes using chemically prepared ion exchangers39. But by using immobilized bacteria (nano-magnetic B2 bio-composite) may effect on the activity of it to absorb the metal, nevertheless, using the ambient temperature is an advantage, as it avoids energy input for heating which make the treatment process more practical and economical.
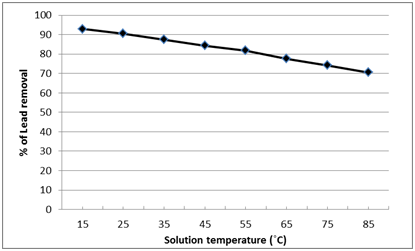
Fig. 11. Effect of solution temperature on the lead removal by nano-magnetic B2 bio-composite (pH=7, magnetic B2 dosage=0.1 gm, initial lead concentration=100 ppm and contact time=60 min).
Effect of initial solution pH
It is well known that metal sorption increased with increasing pH because of the competition between protons and heavy metals at low pH. At low pH, the cell surface sites are strictly linked to the protons, in that way they are unobtainable for other cations. However, with an increase in pH the surface sites are available to the cations37,40. Therefore, selecting the different pH values (1, 3, 5, 7, 9, 11) of the solution for achieving maximum productivity in the removal of pb+2 by nano-magnetic B2 bio-composite is important. The revealed data was shown in Fig. 12. It was observed that the sorption of pb+2 onto nano-magnetic B2 bio-composite increased with the increasing of the initial pH up to 5 and approaches a plateau at pH range 5–11 and its removal percentage was about 90%. Similarly Etemadifar et al. (2014)41 found that the pH 6.84 is the optimal pH for the bioconversion of dibenzothiophene (DBT) to 2-hydroxybiphenyl (2-HBP) in oil model by nanomagnet immobilized Rhodococcus erythropolis R1. In additionally Wang and Chen (2006) and Blackwell et al. (1995)42,43 proved in their result that the highest adsorption occurs at pH ranges from 4 – 8 which is widely known as being optimal for metal sorption of almost all types of biomass.
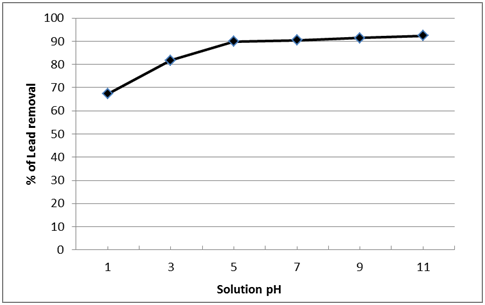
Fig. 12. Effect of initial solution pH on the lead removal by nano-magnetic B2 bio-composite (Magnetic B2 dosage= 0.1 gm, initial lead concentration=100 ppm, and contact time=60 min, solution temp.=22˚C).
CONCLUSION
The bacterial cell competent to absorb lead was isolated and identified as Enterobacter sp. B2. The isolated bacteria biomass was immobilized by nano-particles and set by using nano iron to form nano-magnetic bacterial bio-composite material. This biocomposite material was characterized using X-ray Diffraction (XRD), Fourier Transform Infrared (FTIR) and Scanning Electron Microscope (SEM) to emphasis the crystalline, chemical and morphological structures of it. The optimum conditions for lead removal by magnetic B2 bio-composite was monitored to achieve the superlative absorbed material for lead bioremediation to reach 90% removal at 100 ppm of lead using 0.1 g nano-magnetic B2 bio-composite at pH 7 and 22°C within 60 min. The using of magnetic bio-composite material represents an innovative process for metal removal from industrial effluent with great adeptness, separation and reprocess.
REFERENCES
- Volesky, B. (ed): Biosorption and biosorbent. In Biosorption of heavy metals, Boca Raton, Florida, USA, 1990; pp 3-5.
- Chisti, Y. Environmental impact of toxic pollutants. Biotech. Adv., 2004; 6: 431-432.
- Hussein, H., Ibrahim, S.F., Kandeel, K., Moawad, H. Biosorption of heavy metals from waste water using Pseudomonas sp. Electronic J. Biotechnol., 2004; 7: 38-42.
- Low, K.S., Lee, C.K., Liew, S.C. Sorption of cadmium and lead from aqueous solution by spent grain. Process Biochem., 2000; 36: 59-64.
- Bruins, M.R., Kapil, S., Oehme, F.W. Microbial resistance to metals in the environment. Ecotoxicol. Environ. Saf., 2000; 45: 198–207.
- Factor-Litvak, P., Wasserman, G., Kline, J.K., Graziano, J. The Yugoslavia prospective study of environmental lead exposure. Environ. Health Persp., 1999; 107: 9-15.
- Tripathi, R.M., Raghunath, R., Mahapatra, S., Sadasivan, S. Blood lead and its effect on Cd, Cu, Zn, Fe and hemoglobin levels of children. Sci. Total. Environ., 2001; 277: 161-168.
- Barbosa Jr, F., Tanus-Santos, J.E., Gerlach, R.F., & Parsons, P.J. A critical review of biomarkers used for monitoring human exposure to lead: advantages, limitations, and future needs. Environ. Health Persp., 2005;113: 1669-1674.
- Ehrlich, R., Robins, T., Jordaan, E., Miller, S., Mbuli, S., Selby, P., Wynchank, S., Cantrell, A., De Broe, M., D’Haese, P., Todd, A., Landrigan, P. Lead absorption and renal dysfunction in a South African battery factory. Occup. Environ. Med., 1998; 55: 453-460
- Weaver, V.M., Jaar, B.G., Schwartz, B.S., Todd, A.C., Ahn, K.D. , Lee, S.S., Wen, J., Parsons, P.J., & Lee, B.K. Associations among lead dose biomarkers, uric acid, and renal function in Korean lead workers. Environ. Health Persp., 2005; 113: 36-42.
- Canfield, R.L., Henderson, C.R., Cory-Slechta, D.A., Cox, C., Jusko, T., Lanphear, B.P. Intellectual impairment in children with blood lead concentrations below 10 ¼g per deciliter. N. Engl. J. Med., 2003; 34: 1517-1526.
- Pocock, S.J., Smith, M., Baghurst, P. Environmental lead and children’s intelligence: A systematic review of the epidemiological evidence. Br. J. Med., 1994; 309: 1189-1197.
- Qodah, Z. A. Biosorption of heavy metal ions from aqueous solutions by activated sludge. Desalination, 2006; 196: 164–176.
- Hussein, H., Farag, S., Moawad, H. Isolation and characterisation of Pseudomonas resistant to heavy metals contaminants. Arab J. Biotechnol., 2003; 7: 13-22.
- Davis, T.A., Volesky, B., Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res., 2003; 37: 4311- 4330
- Mehta, S.K., Gaur, J.P. Use of algae for removing heavy metal ions from wastewater: Progress and prospects. Crit. Rev. Biotechnol., 2005; 25: 113-152.
- Romera, E., Gonzalez, F., Ballester, A., Blazquez, M.L., Munoz, J.A. Biosorption with algae: A statistical review. Crit. Rev. Biotechnol., 2006; 26: 223-235.
- Costa, A.C.A., Leite, S.G.C. Metal biosorption by sodium alginate immobilized Chlorerella homosphaera. Biotechnol. Lett., 1991; 13: 559-562.
- Munoz, R.A., Munoz, M.T., Terrazas, E., Guieysse, B., Mattisasson, B. Sequential removal of heavy metals ions and organic pollutants using an algal-bacterial consortium. Chemosphere, 2006; 63: 903-991.
- Prasenjit, B., Sumathi, S. Uptake of chromium by Aspergillus foetidus. J. Mater. Cycl. Waste Manag., 2005; 7: 88-92.
- Chatterjee, S, Mukherjee, A., Sarkar, A., Roy, P. Bioremediation of lead by lead-resistant microorganisms, isolated from industrial sample. Adv. Biosci. Biotechnol., 2012; 3: 290-295.
- Silver, S. Bacterial resistances to toxic metals -A review. Gene., 1996; 179: 9-19.
- Zhang, Y., Banks, C. Factors affecting the removal of selected heavy metals using a polymer immobilised Sphagnum moss as a biosorbent. Environ. Technol., 2005; 26: 733-743.
- Hu, J., Chen, G., Lo, I.M. Removal and recovery of Cr(VI) from wastewater by maghemite nanoparticles. Water Res., 2005; 39: 4528-4536.
- Sambrook, J., Fritsch, E.F., Maniatis, T. Molecular Cloning: a Laboratory Manual., Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press., 1989.
- Kuyucak, N., Volesky, B. Biosorbents for recovery of metals from industrial solutions. Biotechnol. Lett., 1988; 10: 137–142.
- Karande, V.A, Patil, R.N., Tiwari, A.P., Satvekar, R.K., Raut, A.V., Ghosh, S.J., Pawar, S.H. The isolation and characterization of magnetotactic bacteria from iron ore soil for synthesis of magnetic nanoparticles as potential use in magnetic hyperthermia. IJPES., 2014; 4: 321-327.
- Tunali, S., abuk, A., Akar, T. Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chem. Engine. J., 2006; 115: 203–211.
- Lopez, A., González, F., Bonilla, A., Zambrano, G., Gómez, E. Synthesis and characterization of Fe3O4 magnetic nanofluid, Revista Latinoamericana de Metalurgiay. Materiales., 2010; 30: 60-66.
- Vaidyanathan, G., Sendhilnathan, S., Arulmurugan, R. Structural and magnetic properties of Co1″xZnxFe2O4nanoparticles by co-precipitation method. J. Magnetism and Magnetic Mater., 2007; 313: 293 – 299.
- Simmons, S., Sievert, S., Frankel, R., Bazylinski, D., Edwards, K. Marine Magnetotactic Bacteria in a Seasonally Stratified Coastal Salt Pond. Appl. Environ. Microbiol., 2004; 70: 62-30.
- Li, Y.G., Gao, H. S., Li, W.L., Xing, J.M., Liu, H.Z. In situ magnetic separation and immobilization of dibenzothiophene-desulfurizing bacteria. Biores. Technol., 2009; 100: 5092–5096.
- Elkady, M.F., Abu-Saied, M.A., Abdel Rahman, A.M., Soliman, E.A., Elzatahry, A.A., Elsayed Yossef, M., Mohy Eldin, M.S. Nano-sulphonated poly (glycidyl methacrylate) cations exchanger for cadmium ions removal: Effects of operating parameters. Desalination., 2011; 279: 152-162.
- Abou-Mesalam, M.M. Applications of Inorganic Ion Exchangers: II—Adsorption of Some Heavy Metal Ions from Their Aqueous Waste Solution Using Synthetic Iron(III) Titanate. Adsorption., 2004; 10: 87–92.
- Pons, M.P., Fusté, M.C. Uranium uptake by immobilized cells of Pseudomonas strain EPS 5028. Appl. Microbiol. Biotechnol., 1993; 39: 661– 665.
- Saeed, A., Akhter, M.W., Iqbal, M. Removal and recovery of heavy metals from aqueous solution using papaya wood as a new biosorbent. Sep. Purif. Technol., 2005; 45: 25–31.
- Abdel-Ghani, N.T., Elchaghaby, G.A. Influence of operating conditions on the removal of Cu, Zn, Cd and Pb ions from wastewater by adsorption. Int. J. Environ. Sci. Technol., 2007; 4: 451–456.
- Mehrasbi, M.R., Farahmandkia, Z., Taghibeigloo, B., Taromi, A. Experimental determination of Cd2+adsorption mechanism on lowcost biological waste. Water Air Soil Pollut., 2009; 199: 343–351.
- Abd El-Latif, M.M., Elkady, M.F. Kinetics study and thermodynamic behavior for removing cesium, cobalt and nickel ions from aqueous solution using nano-zirconium vanadate ion exchanger. Desalination., 2011; 271: 41–54.
- Ahuja, P., Gupta, R., Saxena, R.K. Sorption and desorption of cobalt by Oscillatoria anguistissima. Curr. Microbiol., 1999; 39: 49-52.
- Etemadifar, Z., Derikvand, P., Emtiazi, G., Habibi, M.H. Response Surface Methodology Optimization of Dibenzothiophene Biodesulfurization in Model Oil by Nanomagnet Immobilized Rhodococcus Erythropolis R1. J. Materials Sci. Engineering B., 2014; 4: 322-330. doi: 10.17265/2161-6221/2014.10.008
- Wang, J., Chen, C. Biosorption of heavy metals by Saccharomyces cerevisiae: Rev. Biotechnol. Adv., 2006; 24: 427-451.
- Blackwell, J.K., Singleton, I., Tobin, M.J. Metal cation uptake by yeast: a review. Appl. Microbiol. Biotechnol., 1995; 43: 579-584.

