ISSN: 0973-7510
E-ISSN: 2581-690X
Among actinobacteria, the genus Streptomyces are found in abundance in specific soil environments. Streptomyces are cultivable using Streptomyces-specific media, including starch casein, yeast extract, or ISP 2 media. Streptomyces isolates can be identified based on their macroscopic culture morphology and microscopic observations, and can be taxonomically placed within the Streptomyces genus. In the present study, mangrove soil samples collected from the coast of Mangalore harboring a multitude of microorganisms were enriched with calcium carbonate and pre-heated to isolate Streptomyces organisms. Cultures were quantified in colony forming units and their diversity was evaluated based on phenotypic features, enzyme hydrolysis, biochemical testing, and antibiotic sensitivity tests. The cross streaking method was used to select Streptomyces isolates, which were then further subjected to intracellular buffer extraction and evaluated against test organisms to determine their antibacterial efficacy. This study highlights the occurrence of prominent Streptomyces species with effective antibacterial activity in a unique environmental habitat of mangrove soil on the Mangalore coast.
Mangrove-soil, Streptomyces, Slide Culturing, Antibacterial Activity, Cross Streak, Intracellular Extraction
Actinomycetes are mainly comprised of the genus Streptomyces under phylum Actinobacteria within the domain bacteria.1 Streptomyces are gram positive bacteria with a mycelial appearance comprised of hyphal structures and spores.2 When cultured on media, Streptomyces proliferate into biomass with distinctive aerial and substratum mycelium. The available nutrients readily penetrate the substratum mycelium for absorption and growth.3,4 To date, several studies have isolated and identified distinct types of Streptomyces species from a range of environments, such as rhizosphere soil, endophytic actinomycetes, marine actinomycetes, and desert actinomycetes, offering natural resources by which to explore the potential of this group of bacteria.5-9 Both physical parameters and nutritional and environmental factors contribute to the genetic capabilities of bacteria, and thus to the organic composition of an organism, and Streptomyces are no exception in terms of their ability to adapt to the different environments that they inhabit.
Streptomyces are known to produce biological molecules in accordance with their environment.10 Therefore, isolating beneficial Streptomyces from specific habitats is an important step for various applications. Streptomyces are a major genus under Actinomycetales that produce approximately 75% of currently available antibiotics, including streptomycin, which was the first antibiotic compound isolated from S. griseus and applied for the treatment of tuberculosis infection.11 Neomycin, an aminoglycoside antibiotic isolated from the soil-welling S. fradiae, inhibits the translation of gram negative organisms such as Escherichia coli, Klebsiella pneumoniae, and Proteus vulgaris.12 Mitomycin C, isolated from S. lavendulae, inhibits DNA synthesis in pathogenic bacteria.13 Vancomycin is a glycopeptide antibiotic produced by the soil-borne S. orientalis, affecting mainly the methicillin-resistant strains of Staphylococcus aureus, S. epidermidus, and Mycobacteria by damaging their cytoplasmic membranes.14 In addition to these antibiotics, studies have also suggested that the extraction of compounds, such as bleomycin—a chemotherapeutic drug derived from S. verticillus—is applicable for the treatment of malignancies.15 Doxorubicin from S. peucetius induces oxidative stress by releasing reactive oxygen species, thereby damaging DNA and triggering apoptosis in cancer cells.16 Maculosin, a promising antioxidant molecule produced by Streptomyces sp. KTM 18, is used to treat deoxygenation in atherosclerosis, as well as neurodegenerative and inflammatory diseases.17 The majority of these species are terrestrial soil and marine inhabiting organisms. In this context, the antimicrobial efficiency of Streptomyces spp. obtained from mangrove soil was evaluated in this study.
Biologically active compounds have been previously extracted from Streptomyces spp. isolated from mangrove forest soil, including S. monashensis, S. mangrovisoli, and S. malaysiense, which showed effective cytotoxicity against colon cancer cell lines.18 Another mangrove-derived Streptomyces strain, S. rochei, has been found to exert significant anti-inflammatory activity and regulate inflammatory mediators, such as tumor necrosis factor-a and interleukins, in macrophages.19 Further studies have found that the secondary metabolites of S. fumigatiscleroticus isolated from mangrove forests acts as a potent antibiotic candidate, cleaving the glycosidic bond of methicillin-resistant S. aureus strains and disrupting their membrane integrity to induce cell death.20
Mangroves are a vegetative system found in coastal regions of marine ecosystems. Due to its unique environmental conditions, including temperature, salinity, pH, humidity, and soil, mangroves are an excellent resource for the study of microorganisms that have adapted to unique environmental conditions.21 The microbes that inhabit this environment no doubt represent a potentially rich resource for the isolation and identification of compounds of pharmacological importance in various therapeutic applications. The mangrove habitat in the coast of Mangalore, Dakshina Kannada district, Karnataka, India has seldom been explored for the occurrence of Actinomyces. In an attempt to identify new compounds of interest, in order to address the problem of antibiotic resistance acquired by infective pathogens, now considered a major threat to public health, the mangrove soil of the Mangalore coast was selected as a study area.
The literature has extensively reported on the importance of the usage of appropriate culture media for the isolation of Streptomyces spp. from different environmental resources.22,23 The colony and culture characteristics of the isolates are also important for the identification of microbes from a given environment. Both primary and secondary metabolites are considered important when speculating on the biological molecules synthesized by Streptomyces, required in turn for the diversified activities and biological applications of the corresponding species. Biological applications, such as antimicrobial, antioxidant, and anti-cancer activities including pro-apoptotic and anti-angiogenic activities in pathological conditions and infectious diseases, are attributable to the mechanism of action of the primary and secondary metabolites.24,25 As mentioned above, environmental factors greatly influence the genetic composition and expression for the synthesis of biologically and physiologically important metabolites. Primary metabolites required for adaptation are known to be synthesized initially during the growth phase, which constitutes enzymes, proteins, and macromolecules. The synthesis and secretion of these bioactive metabolites, either intracellularly or extracellularly, are dictated by both environmental and genetic factors. These metabolites go on to form the building blocks of structural stability during anabolic reactions, as well as being involved in energy generation processes depending on the organism’s requirements.
In various studies, Streptomyces isolated from soil were identified preliminarily based on their morphological appearance on growth media, as well as based on the results of biochemical tests for enzyme production, as illustrated in the Bergey’s manual of determinative bacteriology volume 5 for Actinobacteria.26 Furthermore, the slide culture technique for the microscopic observation of spore arrangements on hyphal structures has also been used to identify species within the Streptomyces genus.27,28 Different types of media and physical conditions can be used for the culture of Streptomyces. Therefore, successful isolation depends on the media used, the use of organic carbon and nitrogen sources, pH, and temperature. According to the International Streptomyces Project (ISP), different media formulations support different culturing purposes, biomass production for primary and secondary metabolites, and pigment production.29
In the present study, Streptomyces spp. were isolated from the mangrove soils of the Mangalore coast, which is considered a unique habitat. Despite the prevalence of Streptomyces spp. in this region, no reports on the various potential biological applications of bacteria isolated from this environment are currently available. Mangrove ecosystems are characterized by marine and fresh water convergence. Therefore, mangrove vegetation has adapted distinctly compared to terrestrial vegetation, including in its microbial load.30-33 To evaluate the occurrence of Streptomyces spp. in this region, the collected soil samples were enhanced and enriched for the effective isolation of Streptomyces spp. using different media. ISP2 media was standardized for the efficient growth of Streptomyces isolates, and their cultural, morphological, and biochemical characteristics were determined. The physical parameters, including pH and temperature, were also analyzed for each of the isolates. Accordingly, most of the isolates performed best at a neutral pH and at 35°C.
In addition, enzyme hydrolysis and antibiotic sensitivity tests were performed to evaluate the antibacterial effects of the isolates. The selected Streptomyces isolates were subjected to intracellular buffer extraction for the identification of isolates with potential to inhibit bacterial growth. This work provides substantiated evidence of the occurrence of Streptomyces spp. isolated from mangroves in the Mangalore coast with potential therapeutic applications to address antibiotic resistance.
Collection of Mangrove Soil Sediments
Mangrove soil sediments were collected from the Mangalore coast at different locations (Thokottu, Tannirbhavi, and Nethravathi river estuaries). The soil samples were collected at a depth of 25 cm and were naturally brown to black in color. The collected sediments were placed in sterile polythene bags for transfer to the molecular research laboratory. Subsequently, each soil sample was air dried at room temperature before further analysis.
Pre-treatment and Physicochemical Analysis of the Sediments
The air-dried soil samples were sieved to obtain fine sediments before examining them for soil texture. The soil sediments were then subjected to chemical pre-treatment using CaCo3 (1%) to suppress bacterial growth. The resulting moistened soils were dried at 50°C for 24 h.
Selective Isolation of Actinomycetes from Mangrove Soil Sediments
Soil dilutions (10-3 and 10-5 dilutions) were inoculated aseptically using the spread plate technique on five separate growth media, namely yeast malt extract agar medium (ISP2), starch casein agar medium, glucose asparagine agar medium, Kenknight and Munaiers agar medium, and starch nitrate agar medium, followed by incubation at 30°C for 7 d. The plates were periodically observed during incubation. Any observed actinomycetes colonies were selected and sub-cultured.
Morphological and Cultural Characteristics
The morphological and cultural characteristics were evaluated according to the Bergey’s manual of determinative bacteriology. After incubating at 30°C for 7 d, the pure cultured mangrove actinomycetes isolates in the starch casein agar media plates were examined for their morphological characteristics, including shape, elevation, texture, soluble pigment production, and color of the aerial and substrate mycelium. Gram staining, acid fast staining, and the cover slip culture method were used to evaluate the microscopic characteristics of the isolates, as well as to study their fragmentation pattern via the formation of spore chains.
Effect of Temperature and pH on Growth
The growth parameters of the selected actinomycetes isolates were investigated according to the studies recommended by ISP. Actinomycetes isolates were streak-inoculated on starch casein agar media slants and incubated at various temperatures (28‒50°C) for 12 d. The culture slants were monitored each day of the incubation period. In addition, all the isolated strains were streak-inoculated on starch casein agar media slants with different pH levels (pH 6.0‒10.0) in media using 0.1 N HCl and NaOH. All of the tested slants were incubated for 12 d and observed for growth at the different pH levels and temperatures.
Antibiotic Sensitivity
Actinomycetes isolates were tested for antibiotic sensitivity using amoxicillin (10 µg), tetracycline (30 µg), polymyxin B (10 µg), and amikacin (10 µg), which were added to the lawn cultures and incubated at 30°C for 7 d. The plates were observed for patterns corresponding to sensitivity or resistance.
Biochemical Analysis and Enzyme Activity
Biochemical tests were performed for each of the isolates to check for indole production, nitrate reaction reduction, urease production, MR-VP, sodium chloride tolerance, and H2S production. In addition, enzyme reactions were also performed to verify starch hydrolysis, catalase activity, casein and gelatin hydrolysis, including citrate utilization, and cellulose degradation. The isolated actinomycetes were screened for the production of extracellular enzymes, such as chitinase, lipase, L asparaginase, tyrosinase, and DNAse. Each actinomycetes isolate was grown on substrate agar plates, including mineral basal medium, Tween-80 agar medium, asparagine agar medium, tyrosine agar medium, and yeast extract medium. All the plates were incubated at 30°C for 7 d and examined for the resulting enzyme activity.
Antimicrobial Activity
The cross streaking of the mangrove actinomycetes against human pathogenic strains, such as Bacillus cereus ATCC 10876, P. vulgaris ATCC 13315, K. pneumoniae ATCC 9621, Salmonella typhimurium ATCC 23564, and E. coli ATCC 8739, was performed to examine their antimicrobial activity. Briefly, isolates were line streaked on sterile nutrient agar plates and incubated at 30°C for 7 d. After observing for growth, the selected bacterial strains were streaked perpendicularly and equidistant from each corner and incubated at 37°C for 24 h.
Intracellular Bioactive Metabolites and Antimicrobial Activity
Actinomycetes were mass cultivated in yeast malt extract broth (ISP2) prepared using malt extract (8.0 g/l), yeast extract (5.0 g/l), and dextrose (2.0 g/l), along with nyastatin (1 µg/ml) and fluconazole (1 µg/ml). The flasks were incubated in a rotary shaker set at 28°C and 120 rpm for 14 d. After incubation, the cultured biomass was separated by centrifuging at 10000 rpm for 15 min. The resulting pellet was homogenized with 0.1 M sodium phosphate buffer and centrifuged at 10000 rpm for 15 min to obtain the cell-free extract.
The antibacterial activity of the intracellular bioactive metabolites was evaluated using the agar well diffusion method. Briefly, sterile molten nutrient agar media was poured into sterile petri dishes. After solidification, the test organisms, including B. cereus ATCC 10876, P. vulgaris ATCC 13315, K. pneumoniae ATCC 9621, S. typhimurium ATCC 23564, and E. coli ATCC 8739 spore suspensions, were swab-inoculated onto the plates. Then, wells were made in the media using a 6-mm cork borer, and 50 µl of each of the intracellular bioactive metabolites of the actinomycetes isolates was loaded into the wells aseptically. Chloramphenicol (1 µg/ml) was used as the positive control and incubated at 37°C for 24 h.
Physicochemical Analysis of Mangrove Soil Samples
Four soil samples collected from mangroves on the Mangalore coast were analyzed for their physicochemical properties, including soil color, texture, and water porosity, as shown in Figure 1. The pH values of the soil samples ranged 6.2–6.9. The gravimetric soil moisture content was determined by oven drying at 110°C. As shown in Table 1, the percentage of water content was found to be in the range of 7.8‒11.36% collected from two different sites at Thokottu, namely from the left and right side of the Nethravathy river bridge. Organic carbon was found to be highly distributed in the Thokottu (L) soil, while sodium was found at high levels in the Jeppinamogaru soil. The highest phosphorus content was found in the Tannirbhavi soil, while potassium was relatively high in the Thokottu (R) soil. The micronutrient availability of copper, zinc, and iron was found to be excessive in the Thokottu mangrove soil.
Table (1):
Physicochemical parameters of the Mangrove soil samples.
| Mangrove Soil | Soil pH | Moisture content | Micronutrients (mg/g) | Macronutrients (mg/g) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Zn | Fe | Cu | C | Na | P | K | |||
| Thokottu (L) | 6.9 | 7.8% | 12.46±0.53 | 13.39±0.3 | 18.33±0.29 | 4.4±0.28 | 34.4±0.24 | 18.46±0.34 | 29.4±0.07 |
| Jeppinamogaru | 6.8 | 7.4% | 11.23±0.28 | 10.3±0.4 | 16.2±0.18 | 3.5±0.38 | 40.25±014 | 12.4±0.35 | 27.64±0.29 |
| Thokottu (R) | 6.2 | 11.36% | 8.36±0.24 | 12.5±0.46 | 23.55±0.48 | 2.3±0.22 | 30.39±0.17 | 15.76±0.29 | 34.29±0.3 |
| Tannirbhavi | 6.7 | 7.14% | 4.8±0.34 | 11.33±0.3 | 21.40±0.38 | 2.5±0.3 | 23.48±0.35 | 19.06 | 26.66±0.20 |
Figure 1. Mangrove soil samples after 1% CaCo3 treatment. (a) Clay soil (Thokottu (L), (b) Loamy soil (Jeppinamogaru), (c) Silt soil (Thokottu(R), (d) Sandy soil (Tannirbhavi)
Isolation and Maintenance of Mangrove Actinomycetes
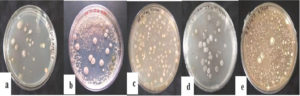
A total of 632 actinomycetes colonies were obtained and quantified using colony forming units (CFU/ml) (Table 2). Among these, 40 morphologically divergent isolates were selected and sub-cultured using various media, as shown in Figure 2. Among the selected media, starch casein agar medium showed good support for mycelial growth and sporulation of the isolates.
Table (2):
Actinomycetes colony count on selective medium from mangrove sediments.
| Mangrove Soil | No of Actinomycetes colonies isolated from mangrove soil | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SCA CFU/ml |
SNA CFU/ml |
ISP2 CFU/ml |
KMA CFU/ml |
GAA CFU/ml |
||||||
| 10-3 | 10-5 | 10-3 | 10-5 | 10-3 | 10-5 | 10-3 | 10-5 | 10-3 | 10-5 | |
| Thokottu (L) | 2.4X10 -5 | 1.5 X10-7 | 2.6X10-5 | 1.1X10-7 | 2.1X10-5 | 1.4X10-7 | 2.0X10-5 | 1.1X10-7 | 2.0X10-5 | 1.0X10-7 |
| Jeppinamogaru | 2.0X10-5 | 1.4 X10-7 | 2.4X10 -5 | 1.0X10-7 | 2.0X10-5 | 1.6 X10-7 | 1.9 X10-5 | 9 X10-7 | 1.9X10-5 | 6X10-7 |
| Thokottu (R) | 2.1X10 -5 | 1.3 X10-7 | 2.0X10-5 | 8X10-7 | 2.2X10-5 | 1.2X10-7 | 1.7X10-5 | 1.0X10-7 | 2.2X10-5 | 1.1X10-7 |
| Tannirbhavi | 1.8X10 -5 | 1.2 X10-7 | 2.2X10-5 | 1.4X10-7 | 1.9X10-5 | 1.0X10-7 | 2.1X10-5 | 8X10-7 | 1.6X10-5 | 7X10-7 |
SCA- Starch casein agar; SNA- Starch nitrate agar; ISP2- Yeast malt extract agar; KMA-Kenknight & munaier’s agar; GAA- Glucose asparagine agar.
Figure 2. Actinomycetes colonies on different media. (a) starch nitrate agar (b) kenknight and munaier’s agar (c) glucose asparagine agar (d) starch casein agar (e) yeast malt extract agar
Culture Characteristics and Microscopic Analysis
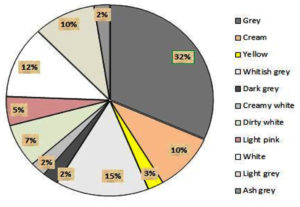
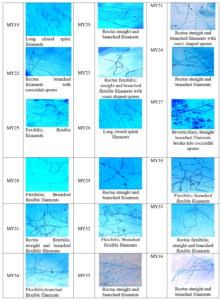
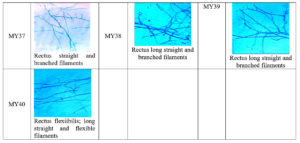
From the actinomycetes cultured on plates, prominent colonies were selected as isolates and sub-cultured for pure culture maintenance. After seven days of growth, the morphological appearance and colony characteristics were evaluated. The isolates showed abundant growth of branched hyphae, as well as aerial and substrate mycelia, on starch casein agar medium. Different colonies were observed, including elevated and flat colonies, with margins that were either circular, regular, or irregular in shape (Table 3). Actinomycetes initially developed as white colonies, later adopting a grey, brown, cream, or pink coloration during sporulation (Figure 3a). Approximately 32% of the actinomycetes mycelia were grey in color, with a varied coloration of cream, white, pink, and yellow colonies observed after isolation. Each of the selected isolates exhibited variation in their coloration. Similarly, a range of colony textures were also observed, from smooth, slimy, and dry to leathery in appearance, along with or without soluble pigment production (Figure 3b).
Table (3):
Culture characteristics of mangrove Streptomyces isolates.
Isolates |
Aerial mycelium |
Substrate mycelium |
Colony shape |
Elevation |
Texture |
Pigmentation |
|---|---|---|---|---|---|---|
MY1 |
Grey |
Yellowish grey |
Circular |
Flat |
Leathery |
– |
MY2 |
Light Grey |
Grey |
Circular |
Flat |
Velvety |
– |
MY3 |
Cream |
White |
Circular |
Flat |
Smooth |
– |
MY4 |
Yellow |
Yellow |
Circular |
Flat |
Smooth |
– |
MY5 |
Grey |
Cream |
Circular |
Flat |
Leathery |
– |
MY6 |
Dark grey |
Grey |
Circular |
Flat |
Velvety |
– |
MY7 |
Creamy white |
White |
Circular |
Flat |
Leathery |
– |
MY8 |
Grey |
Cream |
Irregular |
Concave |
Velvety |
– |
MY9 |
Grey |
Brown |
Circular |
Raised |
Velvety |
– |
MY10 |
Dirty white |
Orange |
Circular |
Flat |
Leathery |
— |
MY11 |
Cream |
Cream |
Circular |
Flat |
Powdery |
– |
MY12 |
Light pink |
Light Brown |
Circular |
Raised |
Powdery |
– |
MY13 |
White |
White |
Circular |
Flat |
Powdery |
– |
MY14 |
Dark grey |
Grey |
Circular |
Flat |
Powdery |
Yellow |
MY15 |
Grey |
Cream |
Circular |
Flat |
Velvety |
– |
MY16 |
Light grey |
Violet |
Circular |
Flat |
Smooth |
Violet |
MY17 |
Pink |
Light Pink |
Circular |
Raised |
Powdery |
Pink |
MY18 |
Grey |
White |
Circular |
Raised |
Powdery |
– |
MY19 |
Grey |
White |
Circular |
Flat |
Powdery |
– |
MY20 |
White |
Light Brown |
Circular |
Flat |
Powdery |
– |
MY21 |
Grey |
Brown |
Circular |
Flat |
Velvety |
– |
MY22 |
Cream |
Cream |
Circular |
Raised |
Smooth |
– |
MY23 |
Whitish grey |
White |
Circular |
Flat |
Powdery |
– |
MY24 |
Light grey |
Brown |
Circular |
Flat |
Powdery |
– |
MY25 |
Cream |
Light brown |
Circular |
Flat |
Smooth |
– |
MY26 |
Dark grey |
Brown |
Circular |
Raised |
Powdery |
– |
MY27 |
White |
Light brown |
Circular |
Flat |
Powdery |
– |
MY28 |
Light grey |
White |
Circular |
Flat |
Powdery |
– |
MY29 |
Grey |
Grey |
Circular |
Flat |
Powdery |
Light Brown |
MY30 |
Light grey |
Brown |
Circular |
Flat |
Velvety |
Brown |
MY31 |
Grey |
White |
Circular |
Flat |
Powdery |
– |
MY32 |
cream |
Light brown |
Circular |
Flat |
Smooth |
– |
MY33 |
Dirty white |
Light brown |
Circular |
Flat |
Leathery |
– |
MY34 |
Light grey |
Brown |
Circular |
Flat |
Powdery |
– |
MY35 |
Ash grey |
White |
Circular |
Raised |
Velvety |
– |
MY36 |
Grey |
White |
Circular |
Flat |
Powdery |
– |
MY37 |
Grey |
Dark grey |
Circular |
Flat |
Velvety |
– |
MY38 |
White |
White |
Circular |
Flat |
Velvety |
– |
MY39 |
White |
White |
Circular |
Flat |
Powdery |
– |
MY40 |
Whitish grey |
White |
Circular |
Flat |
Powdery |
– |
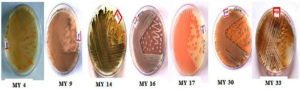
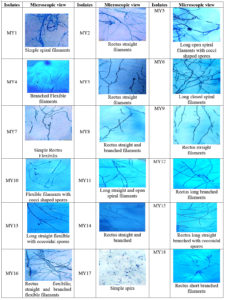
Microscopic examination revealed a long filamentous structure. Gram staining was positive for all the selected isolates, while acid fast staining was negative for all the selected isolates. The spore surface morphology of actinomycetes was observed using the cover slip culture method, and showed different arrays of spore chains. The spore bearing hyphal morphology and their arrangements from different isolates revealed the identity of isolates belonging to the Streptomyces genus. Fragmentation pattern is an important attribute for the identification of the taxonomic characteristics of Streptomyces species. During observation, isolates showed bulging branched mycelium arranged with club shaped microconidal spores at the tip of the hyphae. The spores were 1‒2 µm thick and varied in their shape and size, enclosed by a sporangial envelope containing numerous matured ovoid and cylindrical spores. The polysporous nature of Streptomyces spp. exhibited in different forms was observed for each isolate, and different patterns (e.g. spiral, rectus, biverticillate, flexible, straight, branched, and long hyphal filamentous structures) were observed. These types of spore arrangement patterns have been reported previously in the Streptomyces genus. Accordingly, the selected mangrove actinomycetes were identified, as shown in Figure 4.
Optimum Temperature and pH
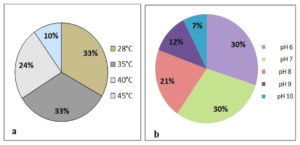
To analyze the sensitivity of actinomycetes isolate growth to temperature, isolates cultured on starch casein agar medium were subjected to growth analysis at temperatures ranging from 28°C to 45°C. All the selected isolates showed efficient growth at 28°C and 30°C. Among 40 selected isolates, 12 isolates also showed good growth at 45°C, while the remaining isolates were unable to withstand temperatures of 45°C. This implies that the majority of actinomycetes isolates have adapted to ambient temperatures of 28-30°C in mangrove soils. Furthermore, a significant number of the selected isolates (25-35%) could withstand temperatures of 40‒45°C (Figure 5a).
Similar to temperature, pH is another key factor in regulating the growth and survival of Streptomyces. Basal media with different pH levels (6.0-10.0) were used to grow Streptomyces strains for 7 d at 30°C. As a result, all the selected actinomycetes isolates were found to withstand pH levels of 6.0 and 7.0. Among the isolates, 28 also showed growth at pH 8.0. However, 12 isolates could not withstand pH 8.0. Sixteen isolates showed growth at pH 9.0, and only ten isolates showed significant growth at pH 10.0. These results indicate that 40% of the Streptomyces isolates were able to sustain high alkaline conditions, and could be considered as facultative alkaliphilic organisms (Figure 5b).
Antibiotic Susceptibility Test
Discs impregnated with antibiotics, including amoxicillin (10 µg), tetracycline (30 µg), polymyxin B (10 µg). and amikacin (30 µg) were placed on growth medium that was pre-inoculated with the Streptomyces isolates to evaluate their sensitivity. The Streptomyces isolates MY1, MY2, MY5, MY8, MY11, MY16, MY18, MY23, MY27, and MY33 showed resistance towards all the antibiotics. Among these isolates, 30 Streptomyces spp. were found to be sensitive to these antibiotics, exhibiting a clear inhibition zone, as shown in Table 4. All the isolates were either sensitive or resistant to the antibiotics tested.
Table (4):
Antibiotic susceptibility of the Mangrove Streptomyces isolates.
| Isolates | Antibiotics sensitive/resistant | |||
|---|---|---|---|---|
| Amoxicillin (10µg) | Tetracycline (30µg) | Polymyxin B (10μg) | Amikacin (30µg) |
|
| MY1 | R | R | R | R |
| MY2 | R | R | R | R |
| MY3 | S | S | S | S |
| MY4 | S | S | S | S |
| MY5 | R | R | R | R |
| MY6 | S | S | S | S |
| MY7 | S | S | S | S |
| MY8 | R | R | R | R |
| MY9 | S | S | S | S |
| MY10 | S | S | S | S |
| MY11 | R | R | R | R |
| MY12 | S | S | S | S |
| MY13 | S | S | S | S |
| MY14 | S | S | S | S |
| MY15 | S | S | S | S |
| MY16 | R | R | R | R |
| MY17 | S | S | S | S |
| MY18 | R | R | R | R |
| MY19 | S | S | S | S |
| MY20 | S | S | S | S |
| MY21 | S | S | S | S |
| MY22 | S | S | S | S |
| MY23 | R | R | R | R |
| MY24 | S | S | S | S |
| MY25 | S | S | S | S |
| MY26 | S | S | S | S |
| MY27 | R | R | R | R |
| MY28 | S | S | S | S |
| MY29 | S | S | S | S |
| MY30 | S | S | S | S |
| MY31 | S | S | S | S |
| MY32 | S | S | S | S |
| MY33 | R | R | R | R |
| MY34 | S | S | S | S |
| MY35 | S | S | S | S |
| MY36 | S | S | S | S |
| MY37 | S | S | S | S |
| MY38 | S | S | S | S |
| MY39 | S | S | S | S |
| MY40 | S | S | S | S |
R: Resistant, S: Sensitive
Biochemical Characteristics
Biochemical tests were carried out for the phenotypically identified Streptomyces isolates. The majority of the Streptomyces strains showed a positive response to the biochemical analysis, including the indole test, starch hydrolysis, catalase test, H2S production test, nitrate reduction test, urease test, methyl red test, Voges‒Proskaeur test, gelatin hydrolysis test, citrate utilization test, and cellulose degradation test. The results of each test are shown in Table 5.
Table (5):
Biochemical characteristics of mangrove Streptomyces isolates.
Streptomyces isolates |
I test |
SH test |
C test |
CH Test |
NR test |
U test |
VP test |
MR test |
GH test |
ST test |
CD test |
CU test |
HP test |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
MY1 |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
– |
+ |
MY2 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
MY3 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
+ |
MY4 |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
– |
+ |
+ |
+ |
MY5 |
+ |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
– |
– |
+ |
MY6 |
+ |
+ |
– |
+ |
– |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
MY7 |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
MY8 |
+ |
+ |
– |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
MY9 |
– |
+ |
– |
+ |
– |
+ |
– |
– |
+ |
– |
+ |
+ |
+ |
MY10 |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
MY11 |
+ |
+ |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
MY12 |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
+ |
MY13 |
+ |
+ |
– |
+ |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
MY14 |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
– |
+ |
MY15 |
+ |
+ |
+ |
+ |
– |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
MY16 |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
+ |
MY17 |
+ |
+ |
– |
+ |
– |
+ |
+ |
+ |
+ |
– |
+ |
+ |
+ |
MY18 |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
MY19 |
+ |
– |
– |
+ |
– |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
MY20 |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
MY21 |
+ |
+ |
– |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
MY22 |
– |
+ |
– |
+ |
– |
+ |
+ |
+ |
+ |
– |
+ |
+ |
+ |
MY23 |
+ |
+ |
– |
– |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
MY24 |
+ |
+ |
– |
– |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
+ |
MY25 |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
– |
+ |
MY26 |
+ |
+ |
– |
+ |
+ |
– |
+ |
– |
+ |
– |
+ |
+ |
+ |
MY27 |
+ |
+ |
– |
+ |
+ |
+ |
– |
+ |
+ |
– |
+ |
+ |
+ |
MY28 |
+ |
+ |
– |
+ |
+ |
– |
+ |
+ |
+ |
– |
+ |
+ |
+ |
MY29 |
+ |
+ |
– |
+ |
+ |
– |
+ |
+ |
+ |
– |
+ |
+ |
+ |
MY30 |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
MY31 |
+ |
+ |
– |
– |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
MY32 |
+ |
– |
– |
– |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
MY33 |
+ |
+ |
– |
+ |
– |
+ |
+ |
+ |
+ |
– |
+ |
+ |
+ |
MY34 |
+ |
+ |
– |
+ |
– |
+ |
– |
– |
+ |
– |
+ |
+ |
+ |
MY35 |
+ |
– |
– |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
+ |
MY36 |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
– |
+ |
+ |
+ |
MY37 |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
MY38 |
+ |
+ |
– |
+ |
+ |
– |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
MY39 |
+ |
+ |
– |
– |
+ |
+ |
– |
– |
+ |
+ |
+ |
+ |
+ |
MY40 |
+ |
+ |
– |
– |
+ |
+ |
+ |
+ |
– |
– |
+ |
+ |
+ |
+; Positive -; Negative.
I- Indole, SH- Starch hydrolysis, C- Catalase, CH- Casein hydrolysis, NR- Nitrate reduction, U- Urease, VP-Voges proskauer, MR- Methyl red, GH- Gelatin hydrolysis, ST- Sodium chloride tolerance, CD- Cellulose degradation, CU- Citrate Utilization, HP- Hydrogen sulphide production
Extracellular Enzyme Production
The Streptomyces isolates were screened for their extracellular enzyme activity for chitinases, lipases, L-asparaginases, tyrosinases, and DNAses by spot inoculation on a suitable agar medium. The different media utilized were mineral basal medium with chitin for chitinase, Tween-80 agar medium for lipase activity, L-asparagine agar medium for L-asparaginase, tyrosine agar medium for tyrosinase, and yeast extract medium containing DNA for DNase activity. All the cultured plates were incubated at 30°C for 5‒7 d.
Antibacterial Activity
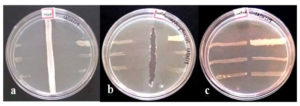
To evaluate the antibacterial potential of the Streptomyces spp., the cross streak method against six test bacteria, namely B. cereus ATCC 10876, P. vulgaris ATCC 13315, K. pneumoniae ATCC 9621, S. typhimurium ATCC 23564, E. coli ATCC 8739, and S. aureus ATCC 23564, was performed. Among 40 isolates, 39 isolates were found to be active against all six pathogens. In contrast, the MY29 isolate did not show any inhibitory effect against any of the test pathogens. As shown in Figure 6, no complete growth of pathogens was observed in the presence of Streptomyces isolates, indicating that the Streptomyces spp. isolated from the mangrove soil have the ability to synthesize a wide spectrum of extracellular bioactive compounds that are effective against virulent bacteria.
Figure 6. Cross streak plate technique to screen the antagonistic activity of Streptomyces isolates against test pathogens such as Bacillus cereus ATCC 10876, Proteus vulgaris ATCC 13315, Klebsiella pneumoniae ATCC 9621, Salmonella typhimurium ATCC 23564, Escherichia coli ATCC 8739 and Staphylococcus aureus ATCC 23565. (a) Vertical streak represents Streptomyces isolates and perpendicular streak are the test pathogens. (b) Vertical lane using standard antibiotic Streptomycin 0.5 mg/ml. (c) Negative control without Streptomyces isolate
Intracellular Extract Preparation and Bioactivity
Based on a preliminary screening of extracellular enzyme activity, the production of bioactive metabolites by the isolates was evaluated by inoculating a loop full culture of isolates in ISP2 media at pH 7.2. The isolates were incubated in a rotary shaker at 28°C and 120 rpm for 14 d. The resulting cell suspensions were centrifuged at 10,000 rpm for 15 min, and the harvested cell biomass was homogenized using 0.1 M sodium phosphate buffer (pH 7). The resulting clear solution containing the bioactive components was separated from the mycelium after centrifugation. The extracts of Streptomyces spp. were verified against the strains B. cereus ATCC 10876, P. vulgaris ATCC 13315, K. pneumoniae ATCC 9621, S. typhimurium ATCC 23564, and E. coli ATCC 8739 using the agar well diffusion method. As summarized in Table 7, the Streptomyces isolates MY1, MY5, MY12, MY13, MY14, MY16, MY23, MY26, MY27, MY33, and MY36 showed potential inhibitory activity against all the six pathogenic strains, while the other isolates did not demonstrate an ability to inhibit the bacterial strains. In agreement with the results of the enzyme activities of the above isolates, the intracellular extracts from the Streptomyces isolates showing antibacterial activity also exhibited significant enzyme activity (Table 6).
Table (6):
Substrate utilization by Mangrove-Streptomyces isolates and enzyme activity.
| Isolates | Enzyme activities | ||||
|---|---|---|---|---|---|
| Chitinase | Lipase | Asparaginase | Tyrosinase | DNAse | |
| MY1 | + | + | + | + | + |
| MY2 | + | + | + | + | – |
| MY3 | + | + | + | + | – |
| MY4 | + | + | + | – | – |
| MY5 | + | + | + | + | + |
| MY6 | – | + | + | + | – |
| MY7 | + | + | + | + | – |
| MY8 | – | + | + | + | – |
| MY9 | – | + | + | + | – |
| MY10 | – | + | + | + | – |
| MY11 | – | + | + | + | |
| MY12 | + | + | – | + | + |
| MY13 | + | + | – | + | + |
| MY14 | + | + | – | + | + |
| MY15 | – | – | – | – | – |
| MY16 | – | + | + | + | + |
| MY17 | + | – | + | – | – |
| MY18 | – | – | – | – | – |
| MY19 | – | – | – | – | – |
| MY20 | – | – | – | – | – |
| MY21 | – | – | – | – | – |
| MY22 | – | – | – | – | – |
| MY23 | – | + | + | + | + |
| MY24 | + | – | – | – | – |
| MY25 | – | – | – | – | – |
| MY26 | – | + | + | + | + |
| MY27 | – | + | + | + | + |
| MY28 | + | – | – | – | – |
| MY29 | – | + | – | + | – |
| MY30 | + | + | – | + | – |
| MY31 | + | – | + | – | |
| MY32 | + | + | – | – | |
| MY33 | – | + | + | + | + |
| MY34 | – | – | – | – | – |
| MY35 | – | – | – | – | – |
| MY36 | – | – | – | – | – |
| MY37 | – | + | – | – | – |
| MY38 | + | + | – | + | – |
| MY39 | – | + | – | + | – |
| MY40 | + | + | – | + | – |
+: Positive -: Negative
Table (7):
Antibacterial activity by well diffusion method.
| Intracellular extract of the Isolates | Diameters of Inhibition zone (mm) | |||||
|---|---|---|---|---|---|---|
| K. pneumoniae ATCC 9621 | S. typhimurium ATCC 23564 | S.aureus ATCC 23565 | B. cereus ATCC10876 | P. vulgaris ATCC 13315 | E .coli ATCC 8739 | |
| MY1 | 8 | 9 | 9 | 10 | 8 | 9 |
| MY5 | 7 | 6 | 7 | 8 | 6 | 7 |
| MY12 | 6 | 8 | 7 | 8 | 6 | 7 |
| MY13 | 7 | 8 | 8 | 7 | 7 | 8 |
| MY14 | 6 | 7 | 7 | 7 | 7 | 7 |
| MY16 | 9 | 9 | 8 | 9 | 9 | 9 |
| MY23 | 6 | 7 | 6 | 8 | 7 | 6 |
| MY26 | 7 | 6 | 7 | 8 | 6 | 8 |
| MY27 | 7 | 7 | 7 | 7 | 7 | 7 |
| MY33 | 6 | 8 | 6 | 7 | 8 | 7 |
| MY36 | 7 | 6 | 7 | 8 | 6 | 6 |
Control- Chloramphenicol (1μg/ml) zone of inhibition 12+2 mm.
The mangroves of the coastal region of Mangalore are among one of the most unexplored sites for the Streptomyces genus, the largest group under the Actinobacteria phylum of Actinomycetes. The diversity and distribution of actinomycetes in the soil of the mangroves of the Mangalore coast are influenced by the physicochemical conditions and nutrients present in this soil environment, representing a unique opportunity to harness a natural resource for the isolation and identification of beneficial Streptomyces spp.
At present, multi-drug resistant organisms are posing an increasing threat to public health on a global scale, becoming causative agents of various infectious diseases.34 Streptomyces are soil microorganisms that play a significant role in producing several beneficial natural products with diverse bioactivities and structural complexities.35 The objective of the present study was to isolate Streptomyces spp. from the soil of mangroves from the Mangalore coast region and analyze their potential antibacterial bioactivity.
The majority of clinical antibiotics currently in use are produced from Streptomyces spp. through industrial processing.36 The emergence of antibiotic-resistant bacteria has driven the search for new molecules of natural origins. In this context, the isolation of Streptomyces spp. from mangrove soils in the relatively unexplored region of the Mangalore coast could enable the identification of novel molecules with antimicrobial activities.
To evaluate the physicochemical nature of the Streptomyces spp. isolated from mangrove soils, each sample was subjected to texture, pH, moisture, and macro/micronutrient composition analysis. As a result, the textures of the soil samples were found to differ, ranging from sandy to silt, loamy, and clay soils. Soil samples collected from the Thokottu region were clay and silt in texture with pH 6.9, while a sandy texture with pH 6.7 was observed in the soil samples from Tannirbhavi. Near the Nethravathi river estuary, the mangrove soil samples were characterized as loamy with pH 6.8. Micronutrient availability, including zinc, iron, and copper, was evaluated using flame photometry and the conductometric method. Potassium, sodium, phosphorus, and organic carbon macronutrients were also analyzed in each soil sample. This was similar to a previous study where non-rhizospheric soil samples collected from Saharan regions in the south of Algeria were evaluated for the isolation and characterization of Actinobacteria—the soil samples were subjected to pH analysis, as well as evaluated for organic carbon and nitrogen availability.37
The Streptomyces isolates from the mangrove soil samples were found to differ greatly in terms of their morphological features, offering a natural source for diversity analysis of Streptomyces spp. The four mangrove sites exhibited varied Streptomyces loads when analyzed based on their biochemical and physiological activities. The isolates showed efficiency in the production of extracellular enzymes in the presence of suitable substrates. Approximately 600 colonies were quantified from the selected mangrove soil sites. The isolation of Streptomyces was achieved upon soil enrichment by pre-treating with CaCO3 (1%) to obtain a significant number of colonies. The successful isolation of morphologically distinctive Streptomyces spp. within specific media provided insights into the diversity and taxonomic features of the Streptomyces isolates found in the soil of mangroves.38
It is well understood that Streptomyces reproduce via spore germination, showing specific spore arrangement patterns during the sporulation stage. This spore arrangement pattern differs significantly among the different species belonging to the Streptomyces genus. By examining the specific types of spore arrangements and structures, the isolates could be preliminarily identified and placed under the genus Streptomyces. To evaluate the corresponding spore patterns, the Streptomyces isolates grown on cover slips using the slide culture technique and stained with lactophenol cotton were microscopically examined, which resulted in the selection of 40 prominent Streptomyces spp. The resulting isolates were verified for positive or negative reactions to biochemical tests according to the Bergey’s manual of determinative bacteriology. As a result, the isolates were confirmed as belonging to Streptomyces. Correspondingly, previous studies on indigenous Streptomyces spp. have shown positive results for the catalase test, nitrate reduction test, and triple sugar iron agar test following the Bergey’s manual of determinative bacteriology for their provisional identification.39
Furthermore, the majority of the isolates were identified as mesophilic and neutrophilic organisms. Although a significant portion of the isolates showed an optimum growth at temperatures of 30-35°C, they were also able to withstand temperatures of 45-50°C, characteristic of facultative thermophiles. Similarly, a significant number of the isolates showed the halophilic characteristic of growth at pH 8-10. In a previous study, Streptomyces sp. M30 was found to exhibit excellent growth stability at 55°C and pH 9, revealing the thermostable and halophilic nature of this isolate.40 Similarly, in a previous study, the majority of the mangrove isolates were found to be mesophilic, growing at an optimum temperature of 30-45°C, with 50% of the isolates able to withstand a temperature up to 50°C. It is worth noting that temperature-sensitive isolates showed varied response to pH levels.
The results of the antibiotic sensitivity of the Streptomyces isolates resulted in the grouping of the isolates into two groups based on their sensitivity to amoxicillin, tetracycline, polymyxin B, and amikacin. The isolates Streptomyces MY1, MY2, MY8, MY11, MY16, MY18, MY23, MY27, and MY33 showed resistance against all four antibiotics. However, among the 40 Streptomyces isolates evaluated, when comparing the preliminary antimicrobial activity using the cross streak method, followed by antibiotic sensitivity, and based on the extracellular enzyme secretion and biochemical reactivity against substrates, 20 isolates were ultimately selected for intracellular extraction from their biomasses.
The isolates that showed the maximum inhibition against the test pathogens were selected for intracellular extraction using 0.1 M sodium phosphate buffer at pH 7.2 from the Streptomyces biomass. The intracellular extract from Streptomyces MY1, MY5, MY12, MY13, MY14, MY16, MY23, MY26, MY27, and MY33 when tested for antibacterial activity against the test organisms showed significant antibacterial activity. These isolates also produced and secreted prominent enzymes, including asparaginases, tyrosinases, chitinases, DNases, and lipases, when tested for enzyme activity. The significant enzyme activity of the selected isolates may have implications for their antibacterial activity. The test pathogens, namely K. pneumoniae ATCC 9621, S. aureus ATCC 23565, S. typhimurium ATCC 23564, B. cereus ATCC 10876, P. vulgaris ATCC 13315, and E. coli ATCC 8739, were significantly inhibited through the In vitro well diffusion of the extract. Previous studies on bioactive compounds obtained from S. mutabilis have shown a wide spectrum of antibacterial activity against S. aureus ATCC 6538, B. subtilis ATCC 6633, P. aeruginosa ATCC 9027, S. typhimurium ATCC 14028, and E. coli 19404, with a rate of growth inhibition ranging from 12.0 ± 0.9 to 20.0 ± 1.9 mm.41
In summary, the isolates Streptomyces MY1, MY16, MY23, MY27, and MY33 exhibited antibiotic resistance against the four selected antibiotics, as well as being efficient producers of prominent enzymes and exerting a significant antibacterial effect. Interestingly, Streptomyces MY33 showed sensitivity to the antibiotics tested, but exhibited significant antibacterial activity without showing efficient extracellular enzyme activity. This implies the existence of an alternate pathway for antibacterial metabolite synthesis or secretion, which may be present in the intracellular extract. Taken together, these findings indicate that the study of the morphological and biochemical enzymatic activities of Streptomyces spp. isolated from mangrove soil, as well as screening for antibacterial activity, offers a significant opportunity for the identification of novel bioactive compounds of microbial origin with therapeutic applications.
The rapid emergence of bacteria resistant to antibiotics has driven the search for new compounds that exhibit specific modes of action. In this context, this work focused on the isolation of Streptomyces spp. from mangrove soil collected from the coastal region of Mangalore—a natural and abundant source of Streptomyces spp. Pre-treatment of the soil samples with heat and chemicals yielded a significant number of Streptomyces spp., which were subsequently evaluated for their biological properties, including antibacterial activity. To this end, the growth of the selected isolates was standardized at optimum temperatures and pH levels, after which the isolates were assayed for their enzyme activity and antibiotic sensitivity. The resulting positive isolates were screened for their antimicrobial activity using the cross streak method, and further confirmed using the well diffusion antimicrobial assay. Subsequently, the antimicrobial efficacy of 11 isolates was analyzed based on the resulting zone of inhibition, which was approximately 10 mm after intracellular buffer extraction. As a result, this work highlights the occurrence of a group of microorganisms, namely Streptomyces spp., in the mangrove soil of the Mangalore coast, which provides an opportunity for the identification of organisms capable of exhibiting effective target-specific activity against antibiotic-resistant bacteria.
ACKNOWLEDGMENTS
The authors would like to thank Mangalore University, India for providing infrastructure for this study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MIK supervised the research work, contributed in graphical analysis and guided in drafting the manuscript. SJA carried out the experiment and drafted the manuscript. Both authors read and approved the final manuscript for publication.
FUNDING
This study was funded by Science & Engineering Research Board (SERB), DST, Govt. of India (EEQ/2016/000418 dated 13-02-2017), and Vision Group of Science &Technology (VGST) Govt. of Karnataka (KSTePS/VGST/CISEE/2018-19/GRD-748/28/2019-20 dated 11-12-2019).
DATA AVAILABILITY
All Data sets generated or analysed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Barka EA, Vatsa P, Sanchez L, et al. Taxonomy, physiology and natural products of actinobacteria. Microbiol Mol Biol Rev. 2016;80(1):1-43.
Crossref - Paradkar A, Trefzer A, Chakraburtty R, Stassi, D Streptomyces genetics: a genomic perspective. Crit Rev Biotechnol. 2003;23(1):1-27.
Crossref - Li Q, Chen X, Jiang Y, Jiang C. Morphological Identification of Actinobacteria. Actinobacteria – Basics and Biotechnological Applications. Intech Open. 2016;3:60-86.
Crossref - Chater KF, Biro S, Lee KJ, Palmer T, Schrempf H. The complex extracellular biology of Streptomyces. FEMS Microbiol Rev. 2010;34(2):171-198.
Crossref - Inahashi Y, Matsumoto A, Omura S, Takahashi Y. Streptosporangium oxazolinicum sp. nov., a novel endophytic Actinomycetes producing new antitrypanosomal antibiotics, spoxazomicins. J Antibiot. 2011;64(4):297-302.
Crossref - Bull AT, Stach JE, Ward AC, Goodfellow M. Marine Actinobacteria: perspectives, challenges, future directions. Antonie Van Leeuwenhoek. 2005;87(1):65-79.
Crossref - Tiwari K, Upadhyay DJ, Mosker E, Susmuth R, Gupta RK. Culturable bioactive actinomycetes from the great Indian thar desert. Ann Microbiol. 2015;65(4):1901-1914.
Crossref - Boubetra D, Bouras N, Zitouni A, et al. Streptosporangium algeriense sp. nov, an actinobacterium isolated from desert soil. Int J Syst Evol Microbiol. 2016;66(2):1034-1038.
Crossref - Berdy J. Bioactive microbial metabolites. J Antibiot (Tokyo). 2005;58(1):1-26.
Crossref - Alferova, IV, Terekhova LP. Use of the method of enriching of soil samples with calcium carbonate for isolation of Actinomyces. Antibiot Khimioter. 1988;33(12):888-890. PMID: 3072928
- Procopio RD, Silva IR, Martins MK, et al. Antibiotics produced by Streptomyces. Braz J Infect Dis. 2012;16(5):466-471.
Crosssref - Dow GT, Thoden JB, Holden HM. The three-dimensional structure of NeoB: An aminotransferase involved in the biosynthesis of neomycin. Protein Sci. 2018;27(5):945-956.
Crossref - Martin TW, Dauter Z, Devedjiew Y, Sheffield P, et al. Molecular Basis of Mitomycin C Resistance in Streptomyces: Structure and Function of the MRD Protein. Structure. 2002;10(7):933-942.
Crossref - Kemung HM, Tan LT, Khan TM, et al. Streptomyces as prominent resource of future anti- MRSA drugs. Front Microbiol. 2018;9:2221.
Crossref - Chen H, Cui J, Wang P, Wang X, Wen J. Enhancement of bleomycin production in Streptomyces verticillus through global metabolic regulation of N-acetylglucosamine and assisted metabolic profling analysis. Microb Cell Fact. 2020;19(32):32.
Crossref - Pham VT, Nguyen HT, Nguyen CT, et al. Identification and enhancing production of a novel macrolide compound in engineered Streptomyces peucetius. RSC Adv. 2021;11:3168-3173.
Crossref - Paudel B, Maharjan R, Rajbhandari P, et al. Maculosin, a non-toxic antioxidant compound isolated from Streptomyces sp. KTM18. Pharm Biol. 2021;59(1):933-936.
Crossref - Law JW, Ser HL, Duangjai A, et al. Streptomyces colonosanans sp.nov., A Novel Antibacterium isolated from Malaysia Mangrove soil exhibiting Antioxidative activity and cytotoxic potential against human colon cancer cell lines. Front Microbiol. 2017;8:877.
Crossref - Gomathi A, Gothandam KM, et al. Investigation of anti-inflammatory and toxicity effects of mangrove-derived Streptomyces rochei strain VITGAP173. J Cell Biochem. 2019;1:1-18.
Crossref - Govindarajan G, Mullick P, Raj BA, et al. Susceptibility pattern of methicillin resistance Staphylococcus aureus (MRSA) by flow cytometry analysis and characterization of novel lead drug molecule from Streptomyces species. J Infect Public Health. 2021;14(12):1831-1841.
Crossref - Priyadarshini A, Singdevsachan SK, Tripathy SK, et al. Isolation and Identification of Actinomycetes from Mangrove soil and extraction of secondary Metabolites for antibacterial activity. Br Biotechnol J. 2016;12(2):1-13.
Crossref - Fang BZ, Salam N, Han MX, et al. Insights on the effects of heat pre-treatment, pH, and calcium salts on isolation of rare Actinobacteria from Karstic Caves. Front Microbiol. 2017;8:1535.
Crossref - Goodfellow M, Fiedler HP. A guide to successful bioprospecting: informed by actinobacterial systematics. Antonie Van Leeuwenhoek. 2010;98(2):119-142.
Crossref - Nachtigall J, Kulik A, Helaly S, et al. Atacamycins A-C, 22-membered antitumor macrolactones produced by Streptomyces sp. C38. J Antibiot. 2011;64(12):775-780.
Crossref - Xie F, Aree WP. Actinobacteria from desert: Diversity and Biotechnological applications. Front Microbiol. 2021;12:765531.
Crossref - Wildermuth H, Wehrli E, Horne RW. The surface structure of spores and aerial mycelium in Streptomyces coelicolor. J Ultrastruct Res. 1971;35(1-2):168-180.
Crossref - Kuster E. Simple Working Key for the Classification and Identification of Named Taxa Included in the International Streptomyces Project. Int J Syst Bacteriol. 1972;22(3):139-148.
Crossref - Arunprasath A, Gomathinayagam M. Mangroves in India: A unique Marine Ecosystem. International Letters of Natural Science. 2015;42:47-49.
Crossref - Kathiresan K. Importance of mangrove forest of India. Journal of Coastal Environment. 2010;1(1):11-26.
Crossref - Kathiresan K. Mangrove forests of India. Current Science. 2018;114(5):976-981.
Crossref - Singh AR, Prabakaran N. Status of Indian Mangroves and a way forward for their Conservation. Jalaplavit. 2020;10(2):1-82.
- Singh V. Antimicrobial resistance in Microbial Pathogens and Strategies for combating them. Science, Technology and Education. 2013;21(2):291-296.
Crossref - Jose PA, Maharshi A, Jha B. Actinobacteria in natural products research: progress and prospects. Microbiol Res. 2021;246:126708.
Crossref - Berdy J. Thoughts and facts about antibiotics: where we are now and where we are heading. J Antibiot. 2012;65(8):385-395.
Crossref - Meklat A, Bouras N, Mokrane S, Zitouni A, et al. Isolation, classification and antagonistic properties of alkalitolerant Actinobacteria from Algerian Saharan Soils. Geomicrobiology Journal Taylor and Francis. 2020;37(9):826-836.
Crossref - Huang H, Lv J, Hu Y, Fang Z, Zang K, Bao S. Micromonospora rifamycinica sp. nov., a novel actinomycete from mangrove sediment. Int J Syst Evol Microbiol. 2008;58(1):17-20.
Crossref - Mohamed H, Miloud, B, Zohra F, Arenzana, JM, Veloso A, Rodriguez-Couto S. Isolation and Characterization of Actinobacteria from Algerian Sahara soils with Antimicrobial activities. Int J Mol Cell Med. 2017;6(2):109-120.
Crossref - Ramazani A, Moradi S, Sorouri R, Javani S, Garshasbi M. Screening of antibacterial activity of Streptomyces species isolated from Zanjan province. Iran. International Journal of Pharmaceutical, Chemical and Biological Sciences. 2013;3(2):342-349.
- Islam MS, Aktar MB, Rahman MM, Uddin KM. Isolation and Characterization of Streptomyces spp collected from Bangladeshi soils on the basis of morphological and biochemical studies. Int J Curr Microbiol App Sci. 2014;3(11):734-742.
- Xin Y, Sun Z, Chen Q, et al. Purification and characterization of a novel extracellular thermostable alkaline protease from Streptomyces sp. M 30. J. Microbiol Biotechnol. 2015;25:1944-1953.
Crossref - Hamed M, Abdrabo MA, Fahmy NM, et al. Distribution and Characterization of Actinomycetes in Mangrove habitats (Red sea, Egypt) with special emphasis on Streptomyces mutabilis M3MT483919. J Pure Appl Microbiol. 2021;15(1):246-261.
Crossref
© The Author(s) 2022. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.