ISSN: 0973-7510
E-ISSN: 2581-690X
Bioremediation is an important technology to remediate the chromium (Cr) contaminated soil and water. In this study, Shewanella putrefaciens (MTTC8410) was used to investigate the influence of carbon concentration, pH, and temperature on reduction of hexavalent chromium [Cr(VI)] into trivalent chromium [Cr(III)]. The increased bacterial growth rate was significantly reduced the Cr(VI) concentration. In batch mode experiments, 1% starch recorded the highest reduction of Cr(VI) (90%) followed by 1% glucose (88% reduction) and a reduction of 77% was by 1% cellulose. By using various pH conditions the maximum Cr(VI) reduction was achieved at pH 7.0. In this experiment the maximum Cr(VI) reduction (75%) was observed at 35°C, followed by 30°C with 62% of Cr(VI) reduction. Bioreactor analysis revealed the highest reduction of Cr(VI) (88%) in unsterile tannery effluent. The significant levels of physico- chemical parameters were reduced in unsterile tannery effluent, as compared to the sterile tannery effluent. The experimental results revealed that the S. putrefaciens (MTTC8410) could be used as a potential bacterial strain for reduction of Cr(VI) from contaminated groundwater.
Shewanella putrefaciens, Bioremediation, Cr(VI) reduction, Tannary effluent, Black gram, Phytotoxicity assay
Chromium (Cr) is the seventh most abundant element on earth, the twenty-first metal in the earth’s crust and is mined as chromites1,2. The tannery industry is among the most contaminating industrial sectors and in the process of leather manufacture each tannery industry uses vast amounts of chemicals. The leather industries which widely use compounds containing Cr(III) in the tanning process generally release Cr(VI) effluents into natural water supplies, often without adequate effluent treatment, leading to anthropogenic contamination in water sources3. Chromium is one of the main contaminants in various engineering processes including tannery, electroplating, mining, fibre, metal processing, fertilizer, coloring and manufacturing of pigments4. Globally, Cr contamination estimates that the sector is accountable for the annual disposal into water of 300-400 million tons of heavy metals, solvents, toxic sludge and other wastes5. Among tannery effluent pollutants, Cr(VI) is one of the most toxic and causes various human health problems symptoms including nasal and skin irritation and lung carcinoma. While Cr(III) is harmless and insoluble, this is vital for human diet6,7. Cr can be found in various oxidation forms; Cr (VI) is the most toxic and soluble and Cr (III) is the least toxic form to human beings.
The traditional Cr management method included physical techniques (reverse osmosis, soil washing, membrane separation, exchange of ions, and adsorption) to remove Cr from the environment. Similarly, various chemical methods have also been used to extract Cr from aqueous media, including graphene-coated iron oxide (GCIO) nanoparticles8, maghemite nanoparticles9, iron oxide/carbon10 and mackinawite (FeS)-coated sand11. Such strategies pose further drawbacks such as high costs, energy and secondary hazardous waste generation12-15. Biological removal of Cr is too good a process and option for the physical and chemical methods, as it is environmentally safe, less costly and lacks secondary emissions. Microbial bioremediation is a cost-effective and beneficial biosource for removing Cr from tannery and other industrial waste along with other harmful pollutants16,17. There are many microorganisms that play a crucial role in water, soil and air. A number of researchers previously successfully isolated a variety of possible microorganisms, minimizing effectiveness with Cr(VI). Some bacterial and fungal species such as Escherichia coli, Shewanella oneidensis, Bacillus firmus, Aspergillus niger and Pleurotus ostreatus were used to transform the Cr(VI) into Cr(III)18-22. The novelty of this study is to evaluate the ability of live cells of a bacterium namely S.putrefaciens (MTTC8410) to reduce the Cr(VI) into Cr(III). The batch process and bioreactor study was carried out for Cr reduction from chromium contaminated ground water.
The present study was aimed at evaluating S.putrefaciens’ Cr(VI) reduction capability. Studies of the batch mode were performed by living bacterial cells. Under optimized conditions, the bacterial strain on Cr contaminated tannery effluent is checked via bioreactor analysis. The toxicity of treated wastewater samples from the bioreactor was examined using seed germination of on black gram.
Bacterial strain
The S. putrefaciens (MTTC8410) utilized in this study was obtained from MTCC Chandigarh in India. It was sub cultured in the laboratory using Luria broth medium. The bacterial strain was maintained by nutrient agar slant. The bacterial strain was subcultured every month and preserved using a glycerol solution 20% and stored at 4°C for further studies.
Media and chemicals
The optimization of potential bacterial strain was performed by using mineral salt medium. All analytical grade chemicals and reagents were purchased from Loba Chemie Laboratories (Mumbai, India) and Hi-media Laboratories (Mumbai, India).
Influence of Cr(VI) on bacterial growth
An influence of different concentrations of Cr(VI) on the bacteria’s growth rate was investigated. Shewanella putrefaciens strain (MTTC8410) was grown at 120 rpm and pH 7.0 in a rotary shaker (model: OrbiTech LETT, India), whereas the temperature in LB broth medium was 37°C supplemented by Cr(VI) range 10 to 30 mg/ L23. Every 4 hrs time interval 5 ml samples were obtained from the conical flasks. Followed by the samples were collected and centrifuged for 10 min at 3000 rpm. The pellet was then dissolved with sterile distilled water and calculated growth rate was measured at 600 nm using UV-spectrophotometer (model: Cyber lab-UV-100, USA)24.
Biochemical tests of S.putrefaciens (MTTC8410)
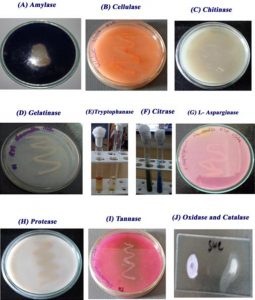
The S.putrefaciens (MTTC8410) was qualitatively investigated for its ability to produce various enzymes viz, amylase25, cellulose26, chitinase27, gelatinase28, tryptophanase29, citrase, L-asparginase30, protease31, tannase32, oxidase and catalase by appropriate standard method.
Effect of carbon sources on reduction of Cr(VI)
Shewanella putrefaciens (MTTC-8410) was used to evaluate the influence of various carbons sources on the reduction of Cr(VI). So far, 100 ml mineral salt medium (MSM) was prepared in 250 ml conical flasks with various concentrations of carbon sources (0.5, 1 and 1.5 per cent) and amended with 10 mg Cr(VI) and all flasks were sterilized. After the sterilization individually, 1% of S. putrefaciens (MTTC-8410) was transferred by aseptically33. Flasks were then kept for 10 days in shaker at 37°C at125 rpm. Then 5 ml of sample were taken from all conical flasks at every 24 hrs time interval. After this the collected samples were spun at 3000 rpm for 10 min. Then aqueous phase was withdrawn from the centrifuge tube and chromium was estimated by DPC (diphenylcarbazide) method using in UV-spectrophotometer at 540 nm (model: Cyber lab-UV-100, USA). The culture growth rate was measured simultaneously, at 600 nm.
Effect of different pH conditions on Cr(VI) reduction
S. putrefaciens was used to assess the effectiveness of different pH conditions on the Cr(VI) reduction. In which various pH of (5 to 9) 100 ml (MSM) medium were prepared with optimized carbon source 0.5 per cent starch in 250 ml conical flasks and adjusted with 10 mg Cr(VI) and all flasks were sterilized. After the sterilization 1% of S. putrefaciens (MTTC-8410) was transferred to individual conical flask by aseptically34. Flasks were then kept for 10 days in shaker at 37°C with 125 rpm. Then 5 ml of sample were taken from all conical flasks at every 24 hrs time interval. After this the collected samples were spun at 3000 rpm for 10 min. Then aqueous phase was withdrawn from the centrifuge tube and Cr(VI) was estimated by DPC method in UV-spectrophotometer at 540 nm (model: Cyber lab-UV-100, USA). Simultaneously the culture growth rate was measured at 600 nm.
Influence of temperature on Cr(VI) reduction
Shewanella putrefaciens (MTTC-8410) was used to assess the influence of different temperatures on reduction of Cr(VI). Hence, in 250 ml conical flasks 100 ml (MSM) medium was prepared with optimized carbon source 0.5% starch and amended with 10 mg Cr(VI) and all flasks were sterilized. After the sterilization 1% of S. putrefaciens was transferred to individual conical flask by aseptically. Then conical flasks were kept in shaker under different temperatures (25 to 45°C) at 125 rpm for 10 days35. Followed by 5 ml of sample was taken from all conical flasks at every 24 hrs time interval. After this the collected samples were spun at 3000 rpm for 10 min. Then aqueous phase was withdrawn from the centrifuge tube and Cr(VI) was estimated by DPC method in UV-spectrophotometer at 540 nm (model: Cyber lab-UV-100, USA). Simultaneously the culture growth rate was measured at 600 nm.
Analytical method
The [Cr(VI)] was determined using the (DPC) procedure using a UV-spectrophotometer (model: cyber lab-UV-100, USA)36. Around 0.025 g of DPC was mixed with 9.67 ml of acetone and 330 ml of 3 M H2SO4. The absorbance was calculated at 540 nm and all assays were performed in triplicates, and mean values were recorded.
Bioreduction of chromium from tannery effluent using lab scale bioreactors
The tannery effluent treatment method was planned and the setup was made as shown in (Fig. 1). This set up was prepared based on the pilot scale water treatment plant37. The tannery effluent was obtained from tannery industry in Periyakulam, district of Erode, Tamil Nadu. The sample was collected in sterile container and was taken to the laboratory and analyzed for various physicochemical parameters38. The established lab scale bioreactors have a capacity of 10 liters and are made of tarson. Mechanical stirrers were installed in the reactor tank and the settling tanks. Artificial aerator was connected to both bioreactors for aeration purpose. About 10 liters of tannery effluent containing 20 mg of Cr(VI) was subjected to primary (bioprocess) treatment and inoculated with S.putrefaciens. Then 5 ml of sample was taken from all conical flasks at every 24 hrs time interval. After this the collected samples were spun at 3000 rpm for 10 min. Then aqueous phase was withdrawn from the centrifuge tube and Cr(VI) was estimated by DPC method using UV-spectrophotometer at 540 nm (model: Cyber lab-UV-100, USA). Simultaneously the culture growth rate was measured at 600 nm. Further, all physic-chemical parameters were analyzed before and after the treatment of tannery effluent.
Reactor 1 (R1)
Unsterile tannery effluent + 0.5% starch + 1% inoculum
Reactor 2 (R2)
Sterile tannery effluent + 0.5% starch + 1% inoculum
Phytotoxicity assay
Pot culture experiments were performed to evaluate the toxicity of the treated and untreated tannery effluent. The fertile soil was collected without any contamination near Omalur Taluk of Salem district. The Cr(VI) amended soil was filled in sterile plastic cups with the dimensions of 5 x 15 cm. The black gram seeds obtained from the licensed agency and surface sterilized by mercury chloride (HgCl2) (0.1%, w/v). Four seeds were sowed in each cup and the set up was kept for phytotoxicity assay. In each pot containing black gram (Vigna mungo) the treated and untreated tannery effluents at varying percentages viz 25, 50, 75 and 100% were irrigated and allowed to germinate the plants. Control pot was irrigated with tap water to compare the efficiency of the treatment. Germination of the seeds was noted after 3rd day of irrigation and calculated39.
Statistical Analysis
All tests were performed in triplicates and reduction of Cr(VI) was evaluated using error bars40. Data were statistically analyzed using the Microsoft ® Excel (Version 2010) statistical package.
Effect of Cr(VI) on bacterial growth rate
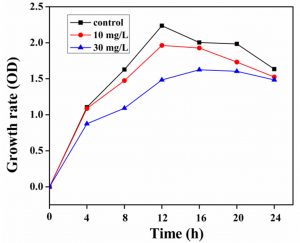
The influence of different Cr(VI) (10 and 30 mg/L) concentrations on the growth of S.putrefaciens has been performed and presented in (Fig.2). The interaction between cell growth and varying Cr(VI) concentration was studied at 24 hrs time of incubation. The rate of bacterial growth in LB media without Cr(VI) was very significant as compared to the LB medium containing Cr(VI) 10 and 30 mg / L containing. In which 10 mg/L of Cr(VI) has a minor effect on the development of bacterial cells. Cr(VI) at a concentration of 10 to 30 mg/L have negatively effect on the growth of the bacteria. The previous study reported that concentration of Cr(VI) in the range of 10 and 20 mg/L would have a small impact on Bacillus sp. growth, while 100 mg/L of Cr(VI) was greatly inhibit the bacterial growth41. The present study reported a decrease in Escherichia coli ATCC 33456 growth rate during lag phase due to the increased concentrations of Cr(VI)42.
Biochemical tests of S. putrefaciens
The enzymatic characteristic of S. putrefaciens was presented in Fig. 3. The strain developed a clear zone around the culture in starch agar medium, but in the case of cellulose, chitinase and pectinase enzyme assay, the culture could not develop the clear zone around the colonies due to the lack of enzyme producing gene. The S. putrefaciens (MTTC-8410) can produce enzymes like gelatinase, tryptophanase, citrase, L- Asparginase, protease, oxidase and catalase and it was confirmed by the strain developed a clear zone in respective media. Similar results were reported by Cr reducing Pseudomonas species43.
Effect of different concentrations of glucose on Cr(VI) reduction
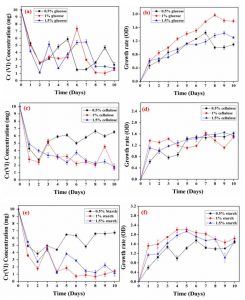
The effect of different doses of glucose (0.5, 1 and 1.5 percent) on reduction of Cr(VI) by S. putrefaciens was presented in Fig. 4a. In which, by using 0.5% of glucose, no significant Cr(VI) reduction was observed from 1st to 5th day of experiment. After the 5th day of experiment, slight significant Cr(VI) reduction (82%) was observed. By using 1% of glucose, the maximum Cr(VI) reduction (88%) was observed in 8th day to 10th of day of experiment. The previous study reported that glucose was donor of electrons, which could easily be oxidized glucose44. Some previous research reported that glucose can play a good carbon source while act as a efficient electron donor to reduce Cr(VI) by bacteria45-47. By using 1.5% of glucose, 80% of Cr(VI) reduction was observed. Overall in this different concentration of glucose optimization, 1% of glucose achieved very significant Cr(VI) reduction. Previously many researchers reported that glucose is a known electron donor to several bacterial strains for Cr(VI) reduction48. Fig. 4b showed the growth rate of S. putrefaciens by various doses of glucose in MSM medium containing 10 mg of Cr(VI). In this study by using 0.5% of glucose, there was no significant growth observed from 1st day to 10th day of experiment and it does not influence the Cr(VI) reduction. But by using 1 and 1.5% of glucose the significant Cr(VI) reduction was observed. In which both 1 and 1.5% of glucose can influence the Cr(VI) reduction as well as growth rate of S. putrefaciens.
Different concentrations of cellulose on Cr(VI) reduction
The effect of different doses of cellulose (0.5, 1 and 1.5 percent) on reduction of Cr(VI) by S. putrefaciens was presented in (Fig.4c). In this experiment, by using 0.5% of cellulose, there was very poor Cr(VI) reduction was observed. By using 1% of cellulose, 77% of Cr(VI) reduction was observed in 6th to 8th day of experiment. Followed by using 1.5% of cellulose, there was 75% of Cr(VI) reduction from 5th to 10th day of experiment. Fig. 4d showed the growth rate of S. putrefaciens under various concentrations of cellulose (0.5, 1 and 1.5%) in MSM medium containing 10 mg of Cr(VI). In this study by using 0.5% of glucose, better significant growth was observed from 1st day to 10th day of experiment and it also contribute poor influence on Cr(VI) reduction. But by using 1 and 1.5% of cellulose, significant chromium reduction was observed. In which both 1 and 1.5% of glucose can influence the Cr(VI) reduction as well as growth rate of S. putrefaciens. Previous study reported that a number of electron donors play an important role for the reduction of Cr(VI) by Ganoderm lucidum49.
Effect of different concentrations of starch on Cr(VI) reduction
The effect of different doses of starch (0.5, 1 and 1.5 percent) on reduction of Cr(VI) by S. putrefaciens was presented in (Fig.4e). In this study, by using 0.5% starch, there was considerable Cr(VI) reduction was observed in first two days, after that on the 3rd day of experiment it loss the Cr(VI) capacity. Using 1% of starch, 90% of Cr(VI) reduction was observed in 6th to 9th day of experiment. Followed by using 1.5% of starch, there was 79% of Cr(VI) reduction was achieved from 7th to 8th day of experiment. The electron donors of carbon sources influenced the Cr(VI) reduction by using Brevibacterium casei50. Fig.4f was showed the growth rate of S.putrefaciens under various concentrations of starch (0.5, 1 and 1.5%) in MSM medium containing 10 mg of hexavalent chromium. In this study, by using 1% of starch was can influenced the maximum level of Cr(VI) reduction and growth rate of S.putrefaciens when compared to 0.5 and 1.5% of starch.
Influence of different concentrations of pH (5,6,7,8 and 9)
The reduction of Cr (VI) under various pH conditions by using S.putrefaciens was shown in Figure 5a. The pH has an influence on Cr(VI) bioreduction. The effects of incubation period on reduction of Cr(VI) at various pH (5, 6, 7, 8 and 9) was analyzed (The operation conditions; 10 mg of Cr (VI)/100 ml medium, temperature 37°C and 1ml of one OD inoculums). In this study, all pH from 5 to 9 was very significantly reduced the Cr(VI) from the aqueous solution. In this experiment the maximum Cr(VI) reduction was observed at pH7. The similar result was observed that the maximum removal of Cr(VI) (88%) by PCP3 strain at pH 7.051. So the further studies were carried out at pH7. The reduction of Cr(VI) during low pH may be due to the relationship of bioaccumulation and functional group dissociation52. The (Fig. 5b) was showed the growth rate of S. putrefaciens under various pH conditions. All pH levels significantly influenced the growth rate.
Influence of different temperature of pH (25, 30, 35, 40 and 45°C)
Temperature is one of the important key for microbial growth Cr(VI) reduction. The effectiveness of various temperatures on reduction of Cr(VI) by S. putrefaciens were presented in (Fig. 5c). In this study, the maximum Cr(VI) reduction (75%) was observed at 35°C. Followed by 30°C was showed the 62% of Cr(VI) reduction. The previous study was reported that hat 30°C was significantly encourage the growth of Bacillus KCH3 and growth was inhibited by below15°C and above 40°C53. The significant bacterial growth achieved by 30°C and 35°C when compared to other temperatures (Fig. 5d). The similar result was observed that the maximum removal of Cr(VI) (85.87%) by Brevibacillus sp at 35°C54.
Bioreduction of chromium contaminated tannery effluent through lab scale bioreactor
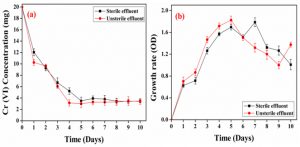
Bioreduction of chromium contaminated tannery effluent through lab scale bioreactor results were presented in (Fig. 6a). In sterile effluent, the maximum Cr(VI) reduction (84%) was observed from 4th day to throughout the study. In unsterile effluent, the maximum Cr(VI) reduction (88%) was observed from 5th day to throughout the study. The significant Cr(VI) reduction was obtained in bioreactor study, due to more biochemical reactions to convert raw materials to products through the action of biocatalyst and enzyme of microorganisms. The growth rate of S.putrefaciens was presented in (Fig. 6b). In unsterile effluent, growth rate was very significantly high because of unsterile effluent have native microbes, hence those native microbes also influence the Cr(VI) reduction and growth rate.
Physicochemical parameters of untreated and treated tannery effluent
The physicochemical parameters of the raw tannery effluent, treated sterile tannery effluent and treated unsterile tannery effluent were described in Table 1. The tannery effluent contained high concentrations of salts and other parameters above the permissible limits. The high concentration of Cr, organic material and salinity in the effluents released from tanneries are considered to be the major hazard55,56. The collected tannery effluent contains higher concentrations of BOD (Biochemical oxygen demand) and COD (Chemical oxygen demand) along with other parameters. The similar results were reported that, tannery effluent was having high BOD and COD concentrations57. After the treatment of sterile tannery effluent by using S.putrefaciens, most of the salts and other parameters were reduced up to permissible limits fixed by the BIS (Bureau of Indian Standards). In this study, because of the use of dissolved organic substances by bacterial strain58, S. putrefaciens significantly decreased the BOD and COD of tannery effluent. Furthermore, after the treatment of unsterile tannery effluent by using S.putrefaciens, most of the salts and other parameters were reduced permissible limits fixed the BIS and other organizations. The physic-chemical parameters of unsterile treated effluent were significantly reduced as compared to the treated sterile tannery effluent.
Table (1):
Physico-chemical parameters of untreated tannery effluent, treated sterile tannery effluent and treated unsterile tannery effluent.
S.No |
Parameters |
Untreated tannery effluent |
Treated sterile tannery effluent |
Treated unsterile tannery effluent |
|---|---|---|---|---|
1 |
Colour |
Dark Black |
Light Black |
Black |
2 |
pH |
7.86 |
7.54 |
7.24 |
3 |
Turbitidy |
Above 200 |
Above 400 |
Above 400 |
4 |
Electrical/Specific conductivity |
7890 Micro mho/cm |
4368 Micro mho/cm |
4308 Micro mho/cm |
5 |
Total dissolved solid |
4385 |
2401 |
2101 |
6 |
Total suspended solid |
660 |
330 |
290 |
7 |
Total Hardness |
2842 |
1042 |
981 |
8 |
Chromium |
20 |
5 |
2.3 |
9 |
Chloride |
1124 |
1216 |
1016 |
10 |
Sulphate |
390 |
234 |
211 |
11 |
Calcium |
656 |
136 |
98 |
12 |
Magnesium |
220 |
170 |
112 |
13 |
Sodium |
118 |
98 |
83 |
14 |
Pottassium |
98 |
73 |
71 |
15 |
Biological oxygen demand |
223 |
78 |
74 |
16 |
Chemical oxygen demand |
458 |
94 |
88 |
17 |
Nitrate |
6.4 |
6.4 |
5.3 |
18 |
Phosphate |
1.0 |
1.0 |
1.0 |
19 |
Manganese |
1.6 |
1.6 |
1.6 |
20 |
Phenolic compound |
2.1 |
2.1 |
2.1 |
21 |
Fluorides |
1.8 |
1.8 |
1.8 |
Phytotoxicity assay by pot culture method
The data regarding phytotoxicity assay of treated and untreated tannery effluent studies has been presented in (Table 2). In treated sterile tannery effluent (25 to 100%), significant black gram seed germination was observed every day. In treated unsterile tannery effluent (25 to 100%), very significant growth was observed when compared to treated sterile effluent. The similar results were reported by using bacterial strain pv2659. But in the case of raw untreated tannery effluent very poor growth rate was observed by using 25 and 50% of raw untreated effluent and there was no growth observed when using 75 and 100% raw untreated tannery effluent. At higher concentrations (80 and 100%) tannery effluent inhibited germination of black gram due to toxicity and stress by using untreated effluent60. The previous study reported that the higher tannery effluent concentration reduces the dehydrogenase activity of the enzyme, which may be changing to disrupt germination and seedling growth61. This study was revealed that after the treatment of tannery effluent by using S.putrefaciens the treated effluent was did not showed toxic effects on black gram plants.
Table (2):
Effectiveness of various concentrations of treated and untreated tannery effluent on germination and seedling growth of black gram (Vigna mungo).
| Reactors | Effluent con. | Shoot length (cm)/days | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Reactor 1 Sterile effluent 0.5% starch |
25% | + | + | + | 2.2 | 3.6 | 7.8 | 10.2 |
| 50% | + | + | + | 1.9 | 3.1 | 6.5 | 8.9 | |
| 75% | – | + | + | 1.6 | 2.8 | 6.1 | 7.7 | |
| 100% | – | + | + | 1.2 | 1.9 | 5.6 | 6.9 | |
| control | – | + | + | 3.4 | 4.1 | 8 | 10.8 | |
| Reactor 1 Unsterile effluent 0.5% starch |
25% | + | + | + | 2.6 | 4.1 | 8 | 11 |
| 50% | – | + | + | 1.9 | 2.8 | 6.2 | 9.3 | |
| 75% | – | + | + | 1.3 | 2.1 | 5.6 | 8.4 | |
| 100% | – | – | + | + | 1.6 | 3.2 | 7.4 | |
| control | + | + | + | 3.1 | 4.6 | 8.8 | 11.5 | |
| Untreated raw effluent | 25% | – | – | + | + | 0.8 | 1.3 | 2 |
| 50% | – | – | – | + | 0.5 | 0.9 | 1.2 | |
| 75% | – | – | – | – | NG | NG | NG | |
| 100% | – | – | – | – | NG | NG | NG | |
| control | + | + | + | 3.2 | 4.4 | 7.2 | 8.2 | |
+ (Germination)
– (No Germination)
Shewanella putrefaciens strain (MTTC8410) was investigated for its an ability to reduce Cr(VI) into Cr(III). In this study the maximum Cr(VI) reduction was achieved at pH 7.0 and temperature 37°C. In the bioreactor test, S.putrefacien maximum Cr(VI) reduction capacity was 97 %. Consecutive experiment for the toxicity of treated tannery effluent on black gram showed no toxicity in plant growth parameters. The bacterially treated tannery effluent has very less amount of Cr, due to this nature its harmless for the agricultural crops. Hence, it was recommended that the S.putrefaciens (MTTC8410) have potential to reduce Cr(VI) from aqueous media.
ACKNOWLEDGMENTS
We would like to express our heartfelt thanks to Vice Chancellor for providing lab facilities. Thanks to Microbial Type Culture Collection (MTCC) and Gene Bank, Chandigarh, India for providing bacterial strain.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors have made a substantial, direct, and intellectual contribution to the work and approve it for publication.
FUNDING
The research was financially supported by the University Grant Commission, New Delhi under the Basic Scientific Research scheme (Sanction No. F-25-1/2013-14(BSR)/11-142/10) and and the FIST grants of Department of Science and Technology (Ref No. SR/FST/LSI-640/2015(C) Dt. 30/5/2016).
ETHICS STATEMENT
This article does not contain any studies with human participants or animals performed by any of the authors.
AVAILABILITY OF DATA
All datasets generated or analyzed during this study are included in the manuscript.
- Cervantes C, Campos-Garcia J. Reduction and Efflux of chromate by Bacteria. Mol Microbiol Heavy Metals. 2007:407-419.
Crossref - Bakshia A, Panigrahib AK. A comprehensive review on chromium induced alterations in fresh water fishes. Toxicol Reports. 2018;5:440-447.
Crossref - Cheung KH, Gu JD. Mechanism of hexavalent chromium detoxification by microorganisms and bioremediation application potential: a review. Int Biodeterior Biodegrad. 2007;59:8-15.
Crossref - Sathvika T, Manasi M, Rajesh V, Rajesh N. Adsorption of chromium supported with various column modelling studies through the synergistic influence of Aspergillus and cellulose. J Environ Chem Eng. 2016;4:3193-3204.
Crossref - UNEP, Clearing the water: A focus on water quality solutions. Nirobi, Kenya. 2010.
- Narayan R, Meena RP, Patel AK, Prajapati AK, Srivastava S, Mondal MK. Characterization and application of biomass gasifier waste material for adsorptive removal of Cr (VI) from aqueous solution. Environ Prog Sustainable Energy. 2016;35:95-102.
Crossref - Kanayochukwu Nduka J, Ijeoma Kelle H. Onyenezi Amuka J. Health risk assessment of cadmium, chromium and nickel from car paint dust from used automobiles at auto-panel workshops in Nigeria. Toxicol Reports.2019;6:449-456.
Crossref - Khare N, Bajpai J, Bajpai AK. Graphene Coated iron oxide (GCIO) nanoparticles as efficient adsorbent for removal of chromium ions: Preparation, characterization and batch adsorption studies. Environ Nanotechnol Monit Manage. 2018;10:148-162.
Crossref - Seraj, Mirzayi S, Nematollahzadeh B, Ali. Engineered maghemite nanoparticles with polyrhodanine for efficient removal of Cr(VI) from water. Environ Nanotechnol Monit Manage. 2018;10:94-103.
Crossref - Neto M, Bellato JO, Silva CR. Iron oxide/carbon nanotubes/chitosan magnetic composite film for chromium species removal. Chemosphere. 2019;218:391-401.
Crossref - Parka M, Parka J, Kangb J, Hanc YS, Jeonga HY. Removal of hexavalent chromium using mackinawite (FeS)-coated sand. J Hazrd Mater. 2018;360:17-23.
Crossref - Chen Z, Huang Z, Cheng Y, et al. Cr(VI) uptake mechanism of Bacillus cereus. Chemosphere. 2012;87:211-216.
Crossref - Jeyasingh J, Philip L. Bioremediation of chromium contaminated soil: optimization of operating parameters under laboratory conditions. J Hazard Mater. 2005;118(1-3):113-120.
Crossref - Rana P, Mohan N, Rajagopal C. Electrochemical removal of chromium from wastewater by using carbon aerogel electrodes. Water Res. 2004;38:2811-2820.
Crossref - Wang L, Wang N, Zhu L, Yu H, Tang H. Photocatalytic reduction of Cr(VI) over different TiO2 photocatalysts and the effects of dissolved organic species. J Hazard Mater. 2008;152:93-99.
Crossref - Imran M, Ahmad I, Barasubiye T, et al. Heavy metal tolerance among free-living fungi isolated from soil receiving long term application of wastewater. J Pure Appl Microbiol. 2020;14:157-170.
Crossref - Mishra A, Saxena A, Surya Pratap Singh. Isolation and characterization of microbial strains from refinery effluent to screen their bioremediation potential. J Pure Appl Microbiol. 2019;13:2325-2332.
Crossref - Ackerley DF, Gonzalez CF, Park CH, Blake R, Keyhan M, Matin A. Chromate-reducing properties of soluble flavoproteins from Pseudomonas putida and Escherichia coli. Appl Environ Microbiol. 2004;70:873-882.
Crossref - Brown SD, Thormpson MR, Verberkmoes NC, et al. Molecular dynamics of the Shewanella oneidensis response to chromate stress. Mol Cell Proteomics. 2006;5:1054-1071.
Crossref - Sau G.B, Chatterjee S, Mukherjee SK. Chromate reduction by cell-free extract of Bacillus firmus KUCr1. Pol J Microbiol. 2010;59:185-190.
Crossref - Srivastava S, Thakur IS. Evaluation of bioremediation and detoxification potentiality of Aspergillus Niger for removal of hexavalent chromium in soil microcosm. J Soil Biol Biochem. 2006;38:1904-1911.
Crossref - Carol D, Kingsley SJ, Vincent S. Hexavalent chromium removal from aqueous solutions by Pleurotus ostreatus spent biomass. Int J Eng Sci Technol. 2012;4:7-22.
- Marzan LM, Hossain M, Mina SA, Akter Y, Chowdhury AMMA. Isolation and biochemical characterization of heavy-metal resistant bacteria from tannery effluent in Chittagong city, Bangladesh: Bioremediation viewpoint. Egypt J Aquat Res. 2017;43:65-74.
Crossref - Thacker U, Parikh R, Shouche Y, Madamwar D. Reduction of chromate by cell-free extract of Brucella sp. isolated from Cr (VI) contaminated sites. Bioresour Technol. 2007;98:1541-1547.
Crossref - Sonia S, Aparna D, Lal Gupta B, Gupta S. Optimization of cellulase production from bacteria isolated from soil. ISRN Biotechnol. 2013:985685.
Crossref - Andro T, Chambost JP, Kotoujansky A, Cattano J, Barras F. Mutants of Erwinia chrysanthemi defective in secretion of pectinase and cellulose. J Bacteriol. 1984;160:1199-1203.
Crossref - Rebecca LJ, Susithra G, Sharmila S, Merina PD. Isolation and screening of chitinase producing Serratia marcescens from soil. J Chem Pharm Res. 2013;5:192-195.
- Aneja KR. Experiments in Microbiology Plant Pathology and Biotechnology, 4th edition, New Age International Publishers, New Delhi, India. 2003.
- Bhattacharya S, Conolly RB, Kaminski NE, Thomas RS, Andersen ME, Zhang Q. A bistable switch underlying B-cell differentiation and its disruption by the environmental contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2010;115:51-65.
Crossref - Gulati R, Saxena RK, Gupta R. A rapid plate assay for screening L-asparaginase producing micro-organisms. Lett Appl Microbiol. 1997;24:23-26.
Crossref - Sneath PHA, Mair NS, Sharpe ME, Holt JG. Bergey’s Manual of Systematic Bacteriology. Williams and Wilkins, USA. 1986;2:1104-1139.
- Osawa R, Walsh TP. Visual reading method for detection of bacterial tannase. Appl Environ Microbiol. 1993;18:74-78.
- Shobana NS. Characterization of chromium bioremediation by Stenotrophomonas maltophilia SRS 05 isolated from tannery effluent. Int Res J Eng Technol. 2007;4:428-403
- Essahale A, Malki M, Marin I, Moumni M. Hexavalent chromium reduction and accumulation by acinetobacter AB1 isolated from Fez tanneries in Morocco. Indian J Microbiol. 2012;52:48-53.
Crossref - Verma T, Singh N. Isolation and process parameter optimization of Brevibacterium casei for simultaneous bioremediation of hexavalent chromium and pentachlorophenol. J Basic Microbiol. 2012;52:1-13.
- Pattanapipitpaisal P, Brown NL, Macaskie LE. Chromate reduction and 16S rRNA identification of bacteria isolated from a Cr(VI)-contaminated site. Appl Microbiol Biotechnol. 2001;57:257-261.
Crossref - Ayyasamy PM, Banuregha ER, Vivekanandhan EG, et al. Bioremediation of sago industry effluent and its impact on seed germination (green gram and maize). World J Microbiol Biotechnol. 2008;24:2677-2684.
Crossref - APHA, American public health association. Standard method for examination of water and wastewater. 21st ed., Washington DC. 2005.
- Saumya S, Yogesh Kumar S. Impact of arsenic toxicity on Black gram and its amelioration using phosphate. ISRN Toxicol. 2013;2013.
Crossref - Steel R, Torrie JH. Principles and procedures of statistics. Mc Graw Hill Book Co. Inc., New York, 1992.
- Liu YG, Xu WH, Zeng GM, Li X, Gao H. Cr(VI) reduction by Bacillus sp. isolated from chromium landfill. Process Biochem. 2006;41:1981-1986.
Crossref - Bae WC, Kang TG, Kang IK, Won YJ, Jeong BC. Reduction of hexavalent chromium by Escherichia coli ATCC 33456 in batch and continuous cultures. J Microbiol. 2000;38:36-39.
- Poornima K, Karthik L, Swadhini SP, Mythili S, Sathiavelu A. Degradation of chromium by using a novel strains of Pseudomonas Species. J Microb Biochem Technol. 2010;2:095-099.
Crossref - Murugavelh S, Mohanty K. Isolation, identification and characterization of Cr(VI) reducing Bacillus cereus from chromium contaminated soil. Chem Eng J. 2013;230:1-9.
Crossref - Pal A, Paul AK. Aerobic chromate reduction by chromiumresistant bacteria isolated from serpentine soil. Microbiol Res. 2004;159:347-354.
Crossref - Das S, Mishra J, Das SK, Pandey S. Investigation on mechanism of Cr(VI) reduction and removal by Bacillus amyloliquefaciens, a novel chromate tolerant bacterium isolated from chromite mine soil. Chemosphere. 2014;96;112-121.
Crossref - Tahri Joutey N, Sayel H, Bahafid W, Ghachtouli NE. Mechanisms of hexavalent chromium resistance and removal by microorganisms. Rev Environ Contam Toxicol. 2015;233:45-69.
Crossref - Zakaria ZA, Zakaria Z, Surif S, Ahmad WA. Hexavalent chromium reduction by Acinetobacter haemolyticus isolated from heavy-metal contaminated wastewater. J Hazard Mater. 2007;146:30-38.
Crossref - RamaKrishna K, Philip L. Bioremediation of Cr(VI) in contaminated soils. J Hazard Mater. 2005;121:109-117.
Crossref - Das AP, Mishra S. Biodegradation of metallic carcinogen hexavalent chromium Cr(VI) by an indigenously isolated bacterial strain. J Carcinog. 2010;9:1-6.
Crossref - Shaili S, Indu Shekhar T. Evaluation of biosorption potency of Acinetobacter sp. For removal of hexavalent chromium from tannery effluent. Biodegradation.2007;18:637-646.
Crossref - Yakup AM, Gulay B, Meltem Y, Sema B, Omer G. Biosorption of Hg2+, Cd2+, and Zn2+ by Ca-alginate and immobilized wood-rotting fungus Funalia trogii. J Hazard Mater. 2004;109:191-199.
Crossref - Sarangi A, Krishnan C. Comparison of in vitro Cr(VI) reduction by CFEs of chromate resistant bacteria isolated from chromate contaminated soil. Bioresour Technol. 2008;99:4130-4137.
Crossref - Chatterjee S, Shekhawat K, Gupta N. Bioreduction of toxic hexavalent chromium by novel indigenous microbe Brevibacillus agri isolated from tannery wastewater. Int J Environ Sci Technol. 2018;16:3549-3556.
Crossref - Dixit S, Yadav A, Dwivedi PD, Das M. Toxic hazards of leather industry and technologies to combat threat: a review. J Cleaner Prod. 2015;87:39-49.
Crossref - Saxena G, Chandra R, Bharagava RN. Environmental pollution, toxicity profile and treatment approaches for tannery wastewater and its chemical pollutants. Rev Environ Contam Toxicol. 2016;240:31-69.
Crossref - Adamu A, Ijah UJ, Riskuwa ML, Ismail HY, Ibrahim UB. Isolation of biosurfactant producing bacteria from tannery effluents in Sokoto metropolis Nigeria. Int J Innovative Sci Eng Technol. 2015;2:366-373.
- Smitha HHS, Raghavendra MP, Shruthi S, Girish K. Bioremediation of rubber processing industry effluent by Arthrobacter sp. Int J Environ Sci Technol. 2012;2:31-34.
- Vijayanand S, Hemapriya J. Biosorption and detoxification of Cr(VI) by tannery effluent acclimatized halotolerant Bacterial Strain pv26. Int J Curr Microbiol App Sci. 2017;3:971-982.
- Mythili K, Karthikeyan B. Bioremediation of tannery effluent and its impact on seed germination (blackgram and sunflower). Curr Bot. 2011;2:40-45.
- Murkumar CV, Chauan PD. Influence of water pollution on germination of gram (Cicer arietinum L.) In: Current pollution Research in India. (Eds: Triveni pk., Goel PK), 1987.
© The Author(s) 2020. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.