ISSN: 0973-7510
E-ISSN: 2581-690X
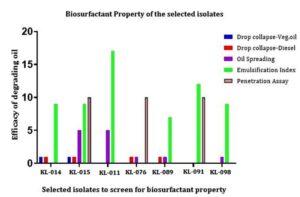
The present study focusses on proving the biosurfactant activity of the 7 isolated bacterial strains. Plants could not absorb enough nutrients as a result of contamination in the soil. This contaminants can be chemical pollutants or oil spillage. By considering the earlier potential applications of biosurfactants, this research was aimed to prove the role of a plant probiotic bacteria as oil degrading bacteria. In order to prove this approach, many screening methods were performed such as drop collapse, oil spreading (mm), emulsification stability testing by calculating emulsification index (E24%), penetration assay etc. Among the 7 selected isolates, isolate KL-015 have shown positive results with all the biosurfactant screening analysis. A unique role was proven by KL-011 isolate as it has shown a high oil degrading zone, i.e., 0.6 mm in diameter as well as the formation of more foam layer or emulsified layer with the emulsification index of 17.39% which indicates its role in having a biosurfactant molecule within it.
Biosurfactant, Oil Spillage, Drop Collapse, Oil Spreading, Emulsification Index, Penetration Assay
Oil contamination is a global hazard in today’s world. In order to overcome the effect of oil, a strategy called Bioremediation is necessary to the world. Bioremediation is an effective phenomenon to clean up the contaminated environment.1 One of the most recent criteria that has emerged as a part of bioremediation is bioaugmentation. The use of biosurfactant is being a vital example of bioaugmentation in breaking down the chemicals present in the soil. If the soil is contaminated with pollutants such as hydrocarbons, the plant may not grow healthy. Biosurfactants are totally different from the synthetic surfactants. The common feature among the both surfactant molecules is the chemical composition which with it is made. They hold the amphipathic molecules with both hydrophilic and hydrophobic subunits that separate at physical interaction. The polar moieties might be cationic, anionic, non-ionic, or amphoteric molecules, whereas the non-polar moieties are frequently chains of hydrocarbons. Surfactants can create microemulsions, in which hydrocarbons are soluble in water or vice versa, and lower surface and interfacial tensions attributable to the mix of hydrophobic and hydrophilic moieties.2 Such chemicals produced by living entities are termed as biosurfactants.
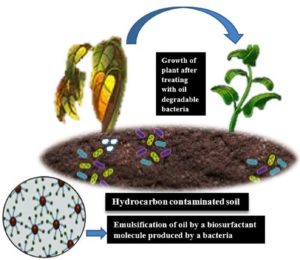
Biosurfactants produced by microbes can exist to remove metal contaminants and hydrocarbons from the environment.3 To improve the wettability and supply an invariant distribution of fertilizers to the soil, biosurfactants can be introduced into the agricultural fields. Apart from aiding in developing soil fertility, they also assist in the disbandment and lower the absorption of the toxicants and hinder the caking while being stored.4 However, there are certain microorganisms which absorbs the hydrocarbons and utilize them for their growth, nourishment, and metabolic pathways are mainly present in the environment.5 Such microbes can be categorized as oil degrading bacteria or biosurfactant producing bacteria as shown in Figure 1.
Rhamnolipids
Biosurfactants derived from the lipid classes – mannosyl-erythritol, rhamnolipids, and lipo-peptides can be administered as biopesticides to combat a broad range of insects and disease-causing microbes because due of their antimicrobial activity.6-8 The most prevalent kind of biosurfactant is glycolipid. One of the most significant glycolipids, rhamnolipids are renowned for having exceptional physicochemical traits.9 Sophorolipids, trehalolipids, and mannosylerythritol lipids, among other popular glycolipid biosurfactants, comprise mono- and disaccharides together with hydroxy-aliphatic acids.10 Different types of rhamnolipids can be produced as a result of microbial fermentation. Rhamnolipids vary depending on the growth and environmental variables in terms of the degree of polymerization, number of side chains, and level of unsaturation in the chains of fatty acids.11 By making the molecules more hydrophobic, rhamnolipids can facilitate the emulsification of hydrophobic components. Apart from the rhamnolipid classification, a new classification of biosurfactant compounds has emerged which are extracted from the plants and animal based raw material as “first generation biosurfactants”, saponins, sugar-esters, alkanolamines etc., as “second generation biosurfactants”. These second generation compounds are prepared from biological processes and are termed as microbial surfactants too. Based on the molecular weight, they are classified as low molecular weight surfactants (LMWS) and high low molecular weight surfactants (HMWS). These HMWS are commonly made of exo- polysaccharides and thus referred to polymeric surfactants or bioemuslifers.12,13
Microbiota & their Biosurfactant property
Many bacterial isolates were identified which can degrade the hydrocarbons and utilize them as a source of carbon and energy.14 Utilizing microorganisms and their metabolic activities to boost oil production from the minimum available resources is known as “microbial enhanced oil recovery.” Acidification of the stationary surface (solids) is the phenomenon underlying hydrocarbon release. It has been reported that several bacteria, including Bacillus subtilis, Torulopsis bombicola, and Pseudomonas aeruginosa, consume crude oil and hydrocarbons as their only carbon sources and can be used to remove oil pollutants.15 Not only have these bacterial species, a numerous rhizosphere-associated bacteria make biosurfactants that can be essential for motility, signaling, and the development of biofilms. Biosurfactant controls plant-microbe interactions in killing plant pathogens and boosts nutritional bioavailability for beneficent plant-associated microbiota. Biosurfactants are used in soil stabilization to improve the properties of agricultural soil.
Microscopic organisms can develop autonomously in various settings and create vast quantities of valuable goods from cost-effective, regenerative sources that are abundantly available. Microbial sources are usually biodegradable, resulting in reduced concentrations of pollutants and toxic effects.16,17
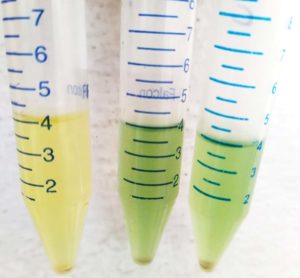
The current study emphasizes the application of 7 isolates that were screened from the preliminary studies of a plant probiotics bacteria for the biosurfactant production analysis. Here are the different methodologies employed for the isolation of a bacteria which has the capacity to behave as a biosurfactant producer or an oil degrading bacterial strain. Few among the important methods used to screen are here below explained. The supernatant is prepared with the prior inoculation of the isolates in a liquid media and maintained under standard conditions. The culture is centrifuged at 3000 rpm for about 30 minutes. This is the freshly extracted supernatant used for the biosurfactant screening as shown in the Figure 2.
Method: 1
Drop Collapse assay
This assay was developed by Jain et al. and later modified periodically using different parameters.18-22 A 10 μl of broth is taken and placed in one drop of oil on a glass slide. After one minute absorb the changes within the drop. The drop activity is observed immediately using a magnifying glass as shown in Figure 3. If the drop spreads it indicates positive. If the drop remains stable it is negative. The activity of the collected supernatant was compared with the control (water).
Method: 2
Oil spreading/displacement technique
The bacterial isolates were inoculated into liquid media and incubated for 24 hrs under standard conditions. The supernatant is prepared by the centrifugation of the bacterial culture at 3000 rpm for 30 min. The cell-free extract was used as the sample for the oil-spreading method. 20 μl of freshly extracted supernatant of culture is placed at the centre of the crude oil drop. Take 20 ml of distilled water on to a Petri plate. Add 1 ml of crude oil at the centre of the water placed on a Petri plate. The crude oil considered for the experiment is diesel. The ring formation due to the displacement of crude oil was measured as shown in Figure 4. If biosurfactant is present in the supernatant, then a clear zone is formed at the center of the oil layer.23
Method: 3
Emulsification of stability test
Emulsification stability test was measured according to the method described by Balogun and Fagade.24 Take 2 ml of diesel and 2 ml of supernatant solution, vortex them for about 2 min. check the height of the different layers formed. Now incubate the tubes for 24 hrs under a normal standard temperature. Measure the height of the emulsified layer and the total height of the liquid. Calculate the emulsification index using a standard formula – dividing the height of the emulsified layer (mm) with the total height of the liquid (mm) and multiplied by 100.25-29 The sample that shows the maximum height of the emulsified layer indicates the more foam activity and has a high detergent property.
Method: 4
Penetration Assay
Take a 96 well plate in order to perform the assay. Fill the well with 150 mu of hydrophobic paste (It is a combination of oil and silica gel), and cover the paste with 10 μl of oil. Prepare a mixture of coloured supernatant by mixing 10 μl of red staining solution and 90 μl of supernatant. Now add this coloured supernatant on to the surface of the paste. Keep the setup undisturbed for 15 min. Observe for the specific changes such as the upper layer turns cloudy white from red color and the supernatant moves downwards from the top to bottom crossing the oil layer.30
Initially, 7 bacterial strains were screened out from the preliminary studies. They are KL-014, KL-015, KL-011, KL-076, KL-089, KL-091, and KL-098. Upon performing the tests to check for the oil degrading bacteria and identify the bacteria which has emulsifying capacity, the isolate KL-015 have shown more ability of degrading oil which was mentioned in Table 1 and also with the formation of emulsifying activity. Surprisingly among all the isolates, KL-011 have shown the formation of more foam layer or emulsified layer which have proven its ability of high production of a biosurfactant component as shown in the Figure 5. According to Table 2, the foaming capacity is analysed by emulsification stability test method where in it is estimated as a value of percentage of the foam layer formed from the total sample after incubation time.
Table (1):
Results of Biosurfactant properties.
| Isolates | Drop Collapse Assay | Oil Spreading (mm) | Penetration Assay | |
|---|---|---|---|---|
| Veg. oil | Diesel | |||
| KL-014 | + | + | 0 | – |
| KL-015 | + | + | 0.5 | + |
| KL-011 | – | – | 0.6 | – |
| KL-076 | – | + | 0.3 | + |
| KL-089 | – | + | 0.3 | – |
| KL-091 | – | – | 0 | + |
| KL-098 | – | – | 0.2 | – |
Table (2):
Emulsification Stability Testing Results.
Isolates |
Sample height (mm) |
Foam Layer (mm) |
Aqueous Layer (mm) |
Total Height (mm) |
Emulsification Index (%) |
|---|---|---|---|---|---|
KL-014 |
1.8 |
0.3 |
1.3 |
3.4 |
8.823529 |
KL-015 |
1.9 |
0.3 |
1.2 |
3.4 |
8.823529 |
KL-011 |
0.7 |
0.4 |
1.2 |
2.3 |
17.3913 |
KL-076 |
2.3 |
0 |
1.3 |
3.6 |
0 |
KL-089 |
2.6 |
0.3 |
1.1 |
4 |
7.5 |
KL-091 |
2 |
0.4 |
1.1 |
3.5 |
11.42857 |
KL-098 |
2.2 |
0.3 |
1.1 |
3.6 |
8.33333 |
The sample is exposed to drop collapse assay with two different oils – vegetable oil and crude oil. The action of the isolate is different for a different variety of oil. Upon the analysis for drop collapse assay, KL -014, KL-015 have shown the ability to cleave the oil molecules while KL-076 and KL-089 were partially active. KL-011, KL-091 and KL-098 have shown negative results. Oil displacement test resulted in specific activity of KL-011 in the formation of bigger zone when compared with the other isolates.
Figure 6 depicts the efficiency of a plant probiotic bacteria in behaving as an oil degrading bacteria. Such bacteria which can be supplied to the plant as a probiotic bacteria which helps the plant roots to uptake the minerals without any hydrocarbon obstacles.
Biosurfactants’ dynamic characteristics allowed them to be used in agriculture, primarily to substitute synthetic surfactants in contaminant killers and agribusiness compositions, endorsing the advancement of “Agri-Science” in this field in reaction to the requirement to minimize negative consequences for the environment and human health compounds.31,32 Biosurfactants function as surface-active molecules that lower the interfacial tension (IFT) between various fluids. They also optimize the oil in water by reducing the size of the oil droplets.33 The potential benefits of biosurfactants encompass their high biodegradability characteristic, risk aversion of toxicity, extraction from renewable resources, ability to operate in circumstances of severe pH and temperature, and long-term excellent biocompatibility.34 There are various characteristics that synthetic and biological surfactants have in common, including the ability to reduce surface tension, foam, emulsify, stabilise, solubilize, and detergent property.35-39 As drug-delivery systems, they can potentially have a substantial influence on the biopharmaceuticals.40,41 Oil, pharmaceutical, medical, farming, environmental remediation, and other sectors produce synthetic surfactants, which are noxious to the environment.42,43Biosurfactants also play a vital role in the food industry especially during the cooking of fats in controlling the consistency, increasing the shelf- life of the product and well mix-up of aromatic flavours. The biosurfactants have the ability to avoid biofilm formation by controlling microbial interactions.44 In addition to serving as emulsifiers in food, they behave as a crucial lubricants in the petrochemical industry, and as immuno- modulators in vaccines, and participants in gene therapy.45 Moreover, because most microbial strains generate a variety of biosurfactant chemicals, their use may be constrained by the level of purity needed for particular applications, such as those in the pharmaceutical and medical industries.46 The best method to inculcate the biosurfactant molecules as per the applications are using them as a nano-materials. These molecules have a variety of functions in nanotechnology. They serve as capping and reducing agents in the synthesis of nanoparticles (NP), as well as emulsifiers in nanoemulsions and self-assembly structures for encapsulation, functionalization, or templates.47 Finally, they can also be prepared using various agriculture based substrates along with microbial fermentations which can make its cost more cheeper.48
Biosurfactants have emerged as appealing microbial products in the burgeoning biotechnology sector due to their benefits over synthetic compounds concerning environmental sustainability and industrial concerns about manufacturing eco-friendly products. This study proves that the oil-degrading bacteria which were isolated from the rhizosphere soil play a dual role as a plant probiotic bacteria and biosurfactant producer too. It also explains the use of these isolates in the treatment of bioremediation, i.e., in helping the plant to develop its healthy morphology by absorbing sufficient minerals. The decomposition of hydrocarbons in polluted water and soil is amongst the most interesting uses for biosurfactants. One of the major application of biosurfactant producing bacteria is the development of Microbial Enhanced Oil Recovery (MEOR), a biosparging process. Apart from MOER, they can be used as a pathogen eliminator within the plant and increase the availability of plant associated bacteria to the plant. When compared their role with chemical surfactant molecules, the biosurfactants are less toxic, easily biodegradable, have good commercial value, and eco-friendly too. A plan to continued study is yet to be developed on acquiring thorough articulations of the molecular structures, which allows the quantitative assessment of yield and efficiency of the isolates’ growth conditions.
ACKNOWLEDGMENTS
The authors would like to thank KL University, Vaddeswaram, Andhra Pradesh, for their support in the analysis part of this work.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
The article does not contain any study with animals and human subjects performed by any of the authors.
- Hamzah A, Tavakoli A, Rabu A. Detection of toulene degradation in bacteria isolated from oil contaminated soils. Sains Malays. 2011;40(11):1231-1235.
- Farias CBB, Almeida FCG, Silva IA, et al. Production of green surfactants: market prospects. Electron J Biotechnol. 2021;51:28-39.
Crossref - Sun X, Wu L, Luo Y. Application of organic agents in remediation of heavy metals – contaminated soil. Ying Yong Sheng Tai Xue Bao. 2006;17:1123-1128.
- Makkar RS, Rockne KJ. Comparison of synthetic surfactants and biosurfactants in enhancing biodegration of polycyclic aromatic hydrocarbon. Environ Toxicol Chem. 2003;22(10):2280-2292.
Crossref - Eeonu CS, Tagbo R, Anike EN, Oje OA, Onwurah IN. Biotechnological tools for environment sustainability: prospects and challenges for environment in Nigeria- a standard review. Biotechnol Res Int. 2012;450802.
Crossref - Rodrigues L, Banat IM, Teixeira J, Oliveira R. Biosurfactants: Potential applications in medicin. J Antimicrob Chemother. 2006;57(4):609-618.
Crossref - D’aes J, De Maeyer K, Pauwelyn E, Hofte M. Biosurfactants in plant-Pseudomonas interaactions and their importance to biocontrol. Environ Microbiol Rep. 2010;2(3):359-372.
Crossref - Ochoa-Loza FJ, Artiola JF, Maier RM. Stability constants for the complexation of various metals with a rhamnolipid biosurfactant. J Environ Qual. 2001;30(2):479-485.
Crossref - Arima K, A Kakinuma, Tamura G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: Isolation, Characterization and its inhibition of fibrin clot formation. Biochem Biophysics Res Commun. 1968;31(3):488-494.
Crossref - Drakontis CE, Amin S. Biosurfactants: Formulations, properties, and applications. Curr Opin Colloid Interface Sci. 2020;48:77-90.
Crossref - Lawniczak L, R Marecik, Chrzanowski L. Contributions of biosurfactants to natural or induced bioremediation. Appl Microbiol Biotechnol. 2013;97(6):2327-2339.
Crossref - Jayashree R, Murugaragavan R. Biosurfactants and its applications. Sabujeema Int Multi e-Magaz. 2021;4(1):61-64.
- Nikolova C, Gutierrez T. Biosurfactants and their applications in the oil and gas industry: current state of knowledge and future perspectives. Front Bioeng Biotechnol. 2021;9(1):1-46.
Crossref - Vnosa AD, Zhu X. Biodegradation of crude oil contaminating marine shorelines and freshwater wetlands spill. Sci Texhnol Bull. 2003;8(2):163-178.
Crossref - Das K, Mukherjee AK. Crude petroleum-oil biodegradation efficiency of Bacillus subtilis and Pseudomonas aeruginosa strains isolated from petroleum oil contaminated from North-East India. Bioresour Technol. 2007;98(7):1339-1345.
Crossref - Youssef N, Elshahed MS, McInerney MJ. Microbial processes in oil fields: culprits, problems, and opportunities. Adv Appl Microbiol. 2009;66:141-251.
Crossref - Khire JM. Bacterial Biosurfactants, and Their Role in Microbial Enhanced Oil Recovery (MEOR). In: Sen, R. (eds) Biosurfactants. Advances in Experimental Medicine and Biology, 2010; 672.
Crossref - Bodour A, Drees K, Maier R. Distribution of biosurfactant-producing bacteria in undisturbed and contaminated arid southwestern soils. Appl Environ Microbiol. 2003;69(6):3280-3287.
Crossref - Jain D, Collins-Thompson D, Lee H, Trevors JT. A drop- collapsing test for screening surfactant-producing microorganisms. J Microbiol Methods. 1991;13(4):271- 279.
Crossref - Batista S, Mounteer A, Amorim F., Totola MR. Isolation and characterization of biosurfactant/bioemulsifier producing bacteria from petroleum contaminated sites. Bioresour Technol. 2006;97(6):868-875.
Crossref - Plaza G, Zjawiony I, Banat I. Use of different methods for detection of thermophilic biosurfactant- producing bacteria from hydrocarbon-contaminated bioremediated soils. J Petro Science Eng. 2006;50(1):71- 77.
Crossref - Youssef N, Duncan K, Nagle D, Savage KN, Knapp RM, McInerney MJ. Comparison of methods to detect biosurfactant production by diverse microorganisms. J Microbiol Methods. 2004;56(3):339-347.
Crossref - Deepika KV, Kalam S, Sridhar PR, Podile AR, Bramhachari PV. Optimization of rhamnolipidbiosurfactant production by mangrove sediment bacterium Pseudomonas aeruginosa KVDHR42 using response surface methodology. Biocatal Agric Biotechnol. 2016;5:38-47.
Crossref - Balogun SA, Fagade OE. Emulsifying bacteria in produce water from Niger-Delta, Nigeria. African J Microbiol Res. 2010;4(9):730-734.
- Willumsen P, Karlson U. Screening of bacteria, isolated from PAH-contaminated soils, for production of biosurfactants and bioemulsifiers. Biodegradation. 1996;7(5):415-423.
Crossref - Denger K, Schink B. New halo and thermotolerant fermenting bacteria producing surface-active compounds. Appl Microbiol Biotechnol. 1995;44(1- 2):161-166.
Crossref - Bento FM, Camargo FA, Okeke BC, Frankenberger Jr WT. Diversity of biosurfactant producing microorganisms isolated from soils contaminated with diesel oil. Microbiol Res. 2005;160(3):249-255.
Crossref - Bosch M, Robert M, Mercade M, Espuny M, Parra J, Guinea J. Surface-active compounds on microbial cultures. Tenside Surfactants Detergents. 1988;25:208- 211.
Crossref - Makkar R, Cameotra S. Biosurfactant production by a thermophilic Bacillus subtilis strain. J Ind Microbiol Biotechnol. 1997;18(1):37-42.
Crossref - Walter, V., Syldatk, C., Hausmann, R. Screening Concepts for the Isolation of Biosurfactant Producing Microorganisms. In: Sen, R. (eds) Biosurfactants. Advances in Experimental Medicine and Biology, 2010, vol 672. Springer, New York, NY.
Crossref - Sachdev DP, Cameotra SS. Biosurfactants in agriculture. Appl Microbiol Biotechnol. 2013;97:1005-1016.
Crossref - Kohl J, Kolnaar R, Ravensberg WJ. Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front Plant Sci. 2019;10:845.
Crossref - Khire JM. Bacterial biosurfactants, and their role in microbial enhanced oil recovery (MEOR). Adv Exp Med Biol. 2010;672:146-57.
Crossref - Noorjahan M, Reddy GD, Khayyum M, et al. Significant of biosurfactants in the lubrification, mineral flotation, and petroleum recovery. Green Sustainable Proc Chem Environ Eng Sci. 2021:329-346.
Crossref - Santos DKF, Rufino RD, Luna JM, Santos VA, Sarubbo LA. Biosurfactants: multifunctional biomolecules of the 21st century. Int J Mol Sci. 2016:17(3):1- 31.
Crossref - Shakeri F, Babavalian H, Amoozegar MA, Ahmadzadeh Z, Zuhuriyanizadi S, Afsharian MP. Production and application of biosurfactant in biotechnology. Biointerface Res Appl Chem. 2020;11:10446-10460.
Crossref - Shao B, Liu Z, Zhong H, et al. Effects of rhamnolipids on microorganism characteristics and applications in composting: A review. Microbiol Res. 2017;200:33-44.
Crossref - Jahan R, Bodratti AM, Tsianou M, Alexandridis P. Biosurfactants, natural alternatives to synthetic surfactants: physicochemical properties and applications. Adv Coll Interface Sci. 2020;275(1):102061.
Crossref - Prakash AA, Prabhu NS, Rajasekar A, et al. Bio- electrokinetic remediation of crude oil contaminated soil enhanced by bacterial biosurfactant. J Hazard Mater. 2021;405:124061.
Crossref - Jimoh AA, Lin J. Biosurfactants: A new frontier for greener technology and environmental sustainability. Ecotoxicol Environ Saf. 2019;184:109607.
Crossref - Vecino X, Cruz JM, Moldes AB, Rodrigues LR. Biosurfactants in cosmetic formulations: Trends and Challenges. Crit Rev Biotechnol. 2017;37(7):911-923.
Crossref - Liu L. Penetration of Surfactants into Skin. Journal of Cosmetic Science. 2020;71(2):91-109.
- Johnson P, Trybala A, Starov V, Pinfield VJ. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adavnces in Colloid Interface Science. 2021;288:102340.
Crossref - Ahamed MI, Prasad R. Microbial biosurfactants: preparation, properties and applications. Nature. 2021;1(1):1-61.
- Zahoor, F., Jamil, N., & Batool, R. Microbial Biosurfactants: Characterization, Properties, and Environmental Applications. In Sustainable Management of Environmental Contaminants: Eco- friendly Remediation Approaches, 2022, pp. 371-389. Cham: Springer International Publishing.
Crossref - Sarubbo LA, Rocha RB, Luna JM, Rufino RD, Santos VA, Banat IM. Some aspects of heavy metals contamination remediation and role of biosurfactants. Chemical Ecology. 2015;31(8):707 -723.
Crossref - Nitschke M, Marangon CA. Microbial surfactants in nanotechnology: recent trends and applications. Crit Rev Biotechnol. 2021;42(2):294-310.
Crossref - Meena, Khem Raj, et al. Benchmarking of different microbes for their biosurfactants antifungal action against plant pathogens. Indian J Exp Biol. 2022;60(12):931-938.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.