ISSN: 0973-7510
E-ISSN: 2581-690X
Brucellosis caused by various species of the genus Brucella is one of the most important zoonotic diseases of global importance with veterinary, public health, and economic concerns. The study aimed to standardize IgM and IgG-based iELISA to detect anti-Brucella antibodies for serodiagnosis of acute and chronic human brucellosis. The test was standardized using 1:320 dilution of smooth lipopolysaccharide (sLPS) antigen from B. abortus S99 strain, 1:80 serum dilution, 1:4000 anti-human IgM and IgG conjugates, respectively for both IgM and IgG iELISA. The cut-off using 50 each brucellosis positive and negative human sera panel samples was set at ≥ 42 for both IgM and IgG iELISA. A total of 700 human sera samples were evaluated (137 veterinary doctors, 157 artificial inseminators, and 406 veterinary assistants). Overall, the study detected 8.3%, 8.1%, 8%, and 6.1% positivity by in-house IgG iELISA, RBPT, IgM iELISA, and SAT tests, respectively. Considering commercial iELISA kit as a gold standard, the sensitivities of IgM and IgG iELISA were 90% and 97.9%, respectively, whereas, specificities were >99%. The study established >98% specificity and >90% sensitivity for differential detection of immunoglobulin classes in the standardized iELISA. The developed assay outperformed the other evaluated tests with a shorter assay time and can be implemented in both endemic and non-endemic regions for surveillance and diagnosis of human brucellosis.
Brucellosis, Diagnosis, Human, Indirect ELISA
Brucellosis caused by various species of the genus Brucella is one of the most important zoonotic diseases of global importance with veterinary, public health, and economic concerns. Brucella spp. majorly infects a wide range of domestic and wild animals.1 Humans are incidental hosts and often get infected through direct or indirect contact with diseased animals and/ or their products. Brucellosis in animals is generally a disease of the reproductive system causing abortion, retention of placenta, infertility, orchitis, epididymitis, reduction of milk production, and rarely arthritis. Human brucellosis is a multi-system disease with debilitating morbidities.2 Although it is an age-old disease, still possess a great threat to most of the endemic countries in the world.3
Almost all the species of Brucella are involved in causing brucellosis in animals; however, only B. melitensis, B. suis, B. abortus, and B. canis and recently discovered B. inopinata can infect humans.2 B. melitensis is the most common causative agent of human brucellosis and most virulent of them all.4 Brucella spp., being a facultative intracellular pathogen, can survive inside the host macrophages, multiply and cause long-lasting chronic infection. Acute, sub-acute, and chronic brucellosis in humans requires prolonged treatment with a minimum of two to three-drug regimens for complete recovery. Although the rate of mortality is <2%, delayed diagnosis, and inadequate treatment not only cause a severely debilitating and disabling illness but also have major socio-economic ramifications.5 Therefore, an accurate and early diagnosis of human brucellosis is inevitable.
Diagnosis of brucellosis has always been a dilemma due to its non-specific clinical presentations, cross-reacting serological tests, and time-consuming culture methods. Isolation of Brucella spp. from clinical specimens gives the confirmatory diagnosis of brucellosis. However, culturing Brucella spp. requires biosafety containment facilities and trained laboratory personnel. Consequently, less contagious serological and molecular tests are generally preferred for diagnosis and sero-surveillance.6 A major component of the adaptive host immune response to Brucella infection is the generation of protective and long-lasting humoral immunity. Therefore, serological tests are preferred diagnostic tests worldwide. Currently available and widely used serological tests are Rose Bengal Plate Test (RBPT), Standard Agglutination Test (SAT), and 2-Mercaptoethanol (2-ME). However, these tests rely on whole-cell antigens, which reduces their specificity (Sp) and makes them less suitable for definitive diagnosis.7,8 Due to these limitations, there is a growing interest in developing more specific and sensitive diagnostic assays.
Enzyme-linked immunosorbent assay (ELISA) is a widely accepted method for diagnosis and screening of various infectious diseases.9 A previous study reported lower sensitivity (60%) and specificity (84%) for IgM and IgG iELISA, respectively. 10 The other studies have reported a higher Se of 92.3% – 100% for IgG-IgM iELISA but exhibited low Sp of only 55% – 71.3%.11,12 The results of these ELISAs lack good combined Se and Sp for the detection of both IgM and IgG antibodies.13
Hence, the present work aimed to evaluate the diagnostic utility of an indirect ELISA (iELISA) for the detection of both IgM and IgG anti-Brucella antibodies against smooth lipopolysaccharide (sLPS) antigen in human serum samples.
Ethics statement
The study was approved by Institutional Ethics Committee, ICAR- NIVEDI, Bangalore, India (Project ID: IXX10708) and samples were collected after obtaining written consent from all the participants.
Study design, location, and population
This cross-sectional study was conducted at ICAR-NIVEDI in Bangalore from June to December 2021. Seven hundred human sera samples collected from risk personnel (veterinary doctors and artificial inseminators) were included in this study.
Extraction of sLPS antigen
The smooth lipopolysaccharide (sLPS) antigen was extracted from phenol-killed B. abortus S99 cell pellet using the hot phenol water extraction method as described previously.14 Initially, 1 gm wet weight of cells was suspended in 3.4 ml of distilled water and the suspension was heated to 66°C. After reaching the desired temperature, 3.8 ml of pre-heated 90% phenol (v/v) was added to the suspension at 66°C. The mixture was stirred continuously for 15 minutes and subjected to centrifugation at 10,000 rpm for 15 minutes at 4°C. After centrifugation, the brownish layer of phenol at the bottom of the tube was aspirated and the remaining debris was filtered out using Whatman filter paper No. 1. A 10ml suspension of ice-cold methanol containing 0.1ml of methanol saturated with sodium acetate was used to precipitate the sLPS antigen and incubated at 4°C for 2 h. The precipitate was dissolved in 1.6ml of distilled water and incubated for 18 h at 4°C with constant stirring. After centrifugation at 10,000 rpm for 10 min, the supernatant was used for further purification. Protein contaminants were removed by treating the supernatant with 0.16gm of trichloroacetic acid (TCA) and finally, 3.2 ml supernatant was dialyzed against distilled water.
Standardization of in-house anti-human IgM and IgG-based iELISA
Both the assays were carried out following the previously described protocol with few modifications.15 Checkerboard titrations were performed to optimize the concentration of sLPS antigen, dilutions of primary human antibodies, and anti-human IgM and IgG conjugates (Sigma-Aldrich, St. Louis, MO, USA). Using these optimal dilutions of antigen, serum, and conjugates, the analytical sensitivity (ASe) and analytical specificity (Asp) of both IgM and IgG iELISA were determined. Different dilutions of two reference positive serum samples and 8 reference/ hyperimmune sera of cross-reactive Gram-negative bacteria comprising E. coli O157, Salmonella (Poly O), six serovars of Y. enterocolitica (O1 & O2, O3, O5, O8, O9), and V. cholera (Poly Hikojima, Inaba, Ogawa) were used to determine the ASe and Asp, respectively. The ROC curve analysis was performed to derive a cut-off of percentage positivity (PP) values for both IgM and IgG iELISA using panel samples comprising 50 each brucellosis positive and negative human sera samples. The incubation time with both primary and secondary antibodies was reduced to 30 minutes to shorten the assay time.
Sample analysis/evaluation of iELISA
To analyze the diagnostic Se and Sp of iELISA, a total of 700 human sera samples [veterinary doctors-161, artificial inseminators-187 and animal handlers- 406] were included in the study. All these samples were tested by RBPT, SAT, 2-ME, commercial Brucella IgM, and IgG iELISA (NovaLisa, NovaTec, Germany) and compared with in-house IgM and IgG iELISA. RBPT and SAT were performed as described previously by Alton et al., 1988.15 Both RBPT and SAT antigens were obtained from the Institute of Animal Health and Veterinary Biologicals, Bengaluru, India. The commercial IgM and IgG iELISA were performed following the manufacturer’s instructions. The optical density (OD) taken at 450nm was converted into percent positivity (PP) values with reference to the OD of the positive control serum.16 Confirmed human sera positive by RBPT, with 2ME titer of 1:1280 and SAT titer of 1:2560, were used as positive sera controls. To establish diagnostic confirmation, paired sera samples in 20 suspected individuals were collected 3-4 weeks apart and repeated testing by all the serological tests to demonstrate rising antibody titers
Statistical analysis
Statistical analysis was performed using SPSS software, version 22 (IBM, India). The cut-off, Se, Sp, area under the curve (AUC), accuracy, positive predictive value, negative predictive value and kappa statistics were determined using ROC curve analysis and Epitool software (https://epitools.ausvet.com.au/).17
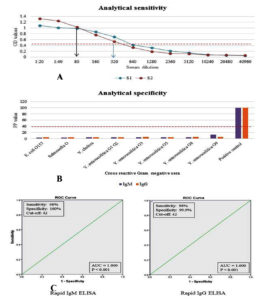
Optimization of test parameters for in-house IgM and IgG iELISA
Checkerboard titration revealed 1:320, 1:80, and 1:4000 as optimal dilutions for sLPS antigen, serum, and conjugates, respectively for both IgM and IgG iELISA. A Se of both IgM and IgG iELISA were up to 1:320 dilutions using two representative positive samples (Figure 1A). Evaluation of Asp for both IgM and IgG iELISA showed non-reactivity with hyperimmune sera against various cross-reactive Gram-negative bacteria (Figure 1B). The ROC curve analysis of 50 each panel samples showed a cut-off value of ≥42 PP with Se of 98% and Sp of 100% for IgM iELISA and a cut-off value of ≥42 PP with Se of 98% and Sp of 99.9% for IgG iELISA (Figure 1C).
Figure 1. Determination of analytical parameters of in-house IgM and IgG iELISA. (A) ASe was estimated using two representative positive samples (S1: IgM positive sample, S2: IgG positive sample) at different dilutions. (B) ASe was estimated with hyperimmune sera against various cross-reactive Gram-negative bacteria. (C) Determination of cut-off using ROC curve analysis of IgM and IgG iELISA drawn using 50 each brucellosis positive and negative human sera
Evaluation of in-house iELISA
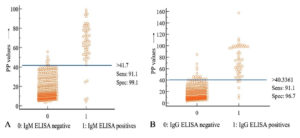
Of 700 samples tested, 8.3%, 8.1%, 8%, and 6.1% were positive by in-house IgG iELISA, RBPT, in-house IgM iELISA, and SAT respectively (Table 1). A total of 57 (8.1%) individuals were reported as positive and suggested brucellosis treatment. Due to insufficient serum samples, we could perform 2-ME only for 311 samples. Of these, 7 (2.3%) showed significant SAT and 2-ME titers among the participants. All 700 samples tested with the commercial IgM and IgG iELISA kit showed 95.8% and 94.4% agreements between in-house and commercial IgG and IgM iELISAs, respectively. Considering RBPT as a gold standard test, the Se and Sp of IgM iELISA were 91.1% and 99.2% and the Se and Sp of IgG iELISA were 92.86% and 99.2%, respectively. Similarly, considering SAT (significant titer ≥ 1:160) as a gold standard test, the Se and Sp of IgM iELISA were 92.8% and 98.9% and the Se and Sp of IgG iELISA were 90.7% and 98.8%, respectively. A very good kappa agreement for IgM and IgG iELISA vs. RBPT and IgM and IgG iELISA vs. SAT (Table 2) was recorded. An interactive dot plot diagram of IgM iELISA showed that at the cut-off value of >41.7 PP, the majority of the positive samples were above the cut-off (Figure 2A). Similarly, for IgG iELISA, most of the positive samples were above the cut-off >40.3 (Figure 2B). From 20 paired sera samples tested, seven samples showed rising titers.
Table (1):
Comparative evaluation of in-house IgM and IgG iELISA with RBPT, SAT, 2-ME, and commercial IgM and IgG ELISA.
| Risk groups | Total | RBPT positives | SAT positives | 2-ME positives | IgM iELISA | IgG iELISA | ||
|---|---|---|---|---|---|---|---|---|
| In-house kit positives | Commercial kit positives | In-house kit positives | Commercial kit positives | |||||
| Veterinary doctors | 137 (19.6) | 11 (8) | 9(6.6) | 5(3.6) | 10 (7.3) | 11(8) | 14(10.2) | 13(9.5) |
| Artificial inseminators | 157 (22.4) | 18 (11.5) | 11(7) | 5(3.6) | 18(11.5) | 18(11.5) | 16(10.2) | 15(9.6) |
| Veterinary assistants | 406 (58) | 28(6.9) | 23(5.7) | NT | 28(6.9) | 28(6.9) | 28(6.9) | 27(6.6) |
| Total | 700 | 57(8.1) | 43(6.1) | 7 (2.3) | 56(8) | 57(8.1) | 58(8.3) | 55(7.8) |
*Due to insufficient sample 2-ME was performed for 311 samples only; NT: Not tested; values in parenthesis represents percentages; ≥1:160 titer in SAT and ≥1:80 titer in 2-ME SAT were considered as significant titers.
Table (2):
Performance characteristics of in-house IgM and IgG iELISA at cut-off 42 (PP value) considering three serological tests as gold standard
| Gold standard 1. RBPT |
Gold standard 2. SAT |
Gold standard 3. Commercial ELISA |
||||
|---|---|---|---|---|---|---|
| IgM iELISA | IgG iELISA | IgM iELISA | IgG iELISA | IgM ELISA | IgG ELISA | |
| Se (95% CI) | 91.1 (80.4-97) | 92.86 (82.7– 98) | 92.8 (80.5-98.5) | 90.7 (77.8–94.7) | 90 (76.3–97.2) | 97.9 (88.7–99.9) |
| Sp (95% CI) |
99.2 (98.2–99.7) | 99.2 (98.2- 99.7) | 98.9 (97.8-99.5) | 98.7 (97.6-99.5) | 99.4 (98.4–99.8) | 99.5 (98.6-99.9) |
| PPV (95% CI) | 86.06 (71.9– 93.7) | 86.3 (72.4 – 93.8) | 81.8 (68.2-90.5) | 79.4 (65.8-88.6) | 88.2 (73.7-95.2) | 91.5 (77.8-97.1) |
| NPV (95% CI) | 99.53 (98.9 – 99.8) | 99.6 (99 – 99.8) | 99.6 (98.8-99.8) | 99.5 (98.7– 99.8) | 99.5 (98.7-99.8) | 99.9 (99.2-99.9) |
| Accuracy | 98.8 (97.7 – 99.5) | 98.9 (97.8 – 99.5) | 98.6 (97.4-99.4) | 98.3 (97.1–99.2) | 98.9 (97.8-99.5) | 99.4 (98.5-99.8) |
| Kappa (95% CI) | 0.9 (0.84 – 0.96) | 0.91 (0.86 – 0.97) | 0.88 (0.81 – 0.95) | 0.86 (0.78 – 0.94) | 0.89 (0.82 – 0.97) | 0.95 (0.91 – 0.99) |
| Positive likelihood ratio | 117 (48.8-281.9) | 120 (49.8-287.2) | 86 (40.8-179.9) | 73 (36.6-147.02) | 142 (53.24-379.7) | 206 (66.5-637.1) |
| Negative likelihood ratio | 0.09 (0.04 – 0.21) | 0.072 (0.03 – 0.19) | 0.072 (0.02 – 0.21) | 0.094 (0.04 – 0.24) | 0.1 (0.04–0.25) | 0.02 (0 – 0.15) |
*CI: confidence interval; PPV: positive predictive value; NPV: negative predictive value.
In India, a significant part of the population involved in livestock-associated activities are always at risk of contracting the disease.18 The diverse clinical symptoms presented by human brucellosis overlaps with various other infectious or non-infectious diseases making brucellosis diagnosis challenging. Hence, a rapid, simple, inexpensive, and consistent diagnostic test is required for brucellosis diagnosis. Isolation of Brucella spp. from human specimens is indisputable proof for brucellosis diagnosis. However, blood cultures have been reported to be positive for 38% to 90% of patients with brucellosis and chances of successfully isolating the organism decrease as the disease progresses.19,20 As a result, detection of anti-Brucella antibodies using serological tests is preferred for routine diagnosis. Since the discovery of the very first test, several serological tests have been developed and improved for the diagnosis of human brucellosis.21 In this study, we have described the diagnostic performance of in-house IgM and IgG iELISA and compared it with conventional serological techniques (RBPT, SAT, and 2-ME) and commercial ELISA kits. As per the results, artificial inseminators (11%) had the highest seropositivity, followed by veterinary doctors (10%) and assistants (7%). Similarly, higher seroprevalence was reported in animal handlers, artificial inseminators, farmers, and veterinary doctors from various parts of the country.22,23
RBPT and SAT are the two inexpensive and valuable laboratory tests for preliminary diagnosis of brucellosis and studies have reported a high level of concordance between SAT and RBPT results.24 In this study, among the screened veterinary health care individuals, 8.1%, 6.1%, and 2.3% were positive by RBPT, SAT, and 2-ME, respectively. The negative or insignificant titers in SAT/2-ME tests in the considerable number of participants (n-13) showed positive serological tests in RBPT, IgM, and IgG iELISA. This noticeable finding of this study implies poor sensitivity of conventional agglutination tests for diagnosis. Largely, the false-negative SAT and 2-ME results could be due to the presence of ‘blocking or incomplete antibodies’ belonging to IgG and IgA classes.25
Several serological techniques have been widely studied for human brucellosis which includes complement fixation tests (warm and cold), Coomb’s test, radio-immunoassay (RIA), and fluorescent polarization assay. These tests have good Se, Sp, and effective for diagnosis of both acute and chronic brucellosis. However, iELISA is preferred over these traditional tests due to its simplified test procedures, time efficiency, and elimination of hazardous materials.13,26
Apart from sLPS, several other immunodominant antigens of Brucella spp. have been used for diagnostic purposes.27 The sLPS is present in all classical species of Brucella except B. ovis and B. canis and therefore, sLPS is well suited for the detection of anti-Brucella antibodies.28 Moreover, because of structural similarity and higher immunogenicity, purified sLPS antigen has the potential to detect a wide range of Brucella biovars.6
The presence of anti-Brucella IgM antibodies are generally indicative of acute brucellosis and IgM antibodies start appearing seven days of the disease onset, reaching highest level within 1 to 3 months after infection. Whereas IgG antibodies appear just about after 3 weeks and reach a peak 6 – 8 weeks after the onset of the disease. Few of the previous studies have reported a Sp of 100% for IgM ELISA and the possibility of over-diagnosis were highlighted in areas with high burden of other infectious diseases with similar clinical manifestations.10,29,30 In this study, 8.3% and 8% of the screened individuals were positive by in-house IgG and IgM iELISA, respectively. However, five participants showed false-positive results in IgM iELISA, whereas other tests were negative which could be due to cross-reacting antibodies. Repeat sampling after 3-4 weeks confirmed false positivity. Therefore, the presence of only IgM antibodies should never be considered as conclusive for acute infections. In such cases, detection of IgG antibodies and/ or rise in antibody titer should be considered for definitive diagnosis. According to our paired serum sample analysis, we observed various conditions which indicated the usefulness of paired serum samples for definitive diagnosis of brucellosis among suspected individuals. Also, anti-Brucella antibodies persist for a long even after successful treatment and recovery.
In comparison, in-house IgG iELISA has detected 8.3% positivity whereas the commercial IgG ELISA kit detected only 7.8% positivity. Overall, comparison between the combined efficiency of IgM and IgG in-house and commercial ELISA kits showed that in-house assays have higher Se and Sp indicating the effectiveness for serodiagnosis of human brucellosis (Table 3). In the present study, we observed that 7% of the screened individuals were positive by RBPT, in-house IgM, and IgG iELISA whereas, SAT and 2-ME detected only 6.1% and 2.3%, respectively. In routine diagnostic laboratories with limited resources, performing SAT and 2-ME for every sample is difficult because of their non-robust and time-consuming. In such cases, robust, specific, and sensitive IgM and IgG iELISA can be used as alternative assays for rapid and reliable disease diagnosis.
Table (3):
Overall comparison of in-house and commercial Brucella IgM and IgG iELISA results considering RBPT as a reference test
In-house (95% CI) |
Commercial (95% CI) |
|
|---|---|---|
Se |
93.3 (83.8 – 98.2) |
91.7 (81.6 – 97.2) |
Sp |
99.4 (98.4 – 99.8) |
99.1 (97.9 – 99.7) |
PPV |
88.7 (74.8 – 95.5) |
83.8 (69.9 – 92) |
NPV |
99.6 (99.1 – 99.8) |
99.6 (98.9 – 99.8) |
Accuracy |
99.1 (98.1 – 99.6) |
98.7 (97.6 – 99.4) |
Kappa |
0.93 (0.87 – 0.97) |
0.9 (0.84 – 0.95) |
Positive likelihood ratio |
150 (56.4 -399.5) |
98.5 (44.3 – 219.3) |
Negative likelihood ratio |
0.07 (0.03 – 0.17) |
0.08 (0.04 – 0.19) |
*Se: sensitivity; Sp: specificity; CI: confidence interval; PPV: positive predictive value; NPV: negative predictive value.
In Conclusion, in-house iELISA outperformed the commercial ELISA kits and other conventional serological tests. The current brucellosis control program in India allows prevention by vaccination and country-wide surveillance of the disease in animals. However, the most important measures for the prevention of human brucellosis would be to educate artificial inseminators, animal handlers, and farmers, about the disease and to conduct routine testing to prevent the disease progression. These in-house assays are cost-effective and rapid than the commercial kits which make them suitable for large-scale serosurveillance in areas with high brucellosis prevalence. In addition, seropositive cases can be re-evaluated and confirmed with paired sera for both IgG and IgM antibodies.
ACKNOWLEDGMENTS
The authors would like to thank participants, scientists, and staff of the Indian Council for Agricultural Research-National Institute of Veterinary Epidemiology and Disease Informatics (ICAR- NIVEDI), Yelahanka, Bengaluru- 560064, India, for their support during the study.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
SP, MMS, GS, SSk and SSh carried out the experiment. SP contributed in statistical analysis. RS, BRS and NM supervised the experiment. SP and RS drafted the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This study was funded by the Institute Service Project ‘Sero-epidemiology of brucellosis’ (Project ID: IXX10708).
DATA AVAILABILITY
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
ETHICS STATEMENT
The study was approved by the Institutional Ethics Committee, Indian Council for Agricultural Research-National Institute of Veterinary Epidemiology and Disease Informatics (ICAR-NIVEDI), Bengaluru, Karnataka, India.
INFORMED CONSENT
Written informed consent was obtained from the participants before enrolling in the study.
- Seleem MN, Boyle SM, Sriranganathan N. Brucellosis: A re-emerging zoonosis. Vet Microbiol. 2010;140(3-4):392-398.
Crossref - Muma JB, Samui KL, Siamudaala VM, et al . Prevalence of antibodies to Brucella spp. and individual risk factors of infection in traditional cattle, goats and sheep reared in livestock-wildlife interface areas of Zambia. Trop Anim Hlth Prod. 2006;38(3):195-206.
Crossref - Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. The new global map of human brucellosis. Lancet Infect Dis. 2006;6(2):91-99.
Crossref - Nielsen K, Yu WL. Serological diagnosis of brucellosis. Prilozi. 2010;31:65-89. http://manu.edu.mk/prilozi/4kn.pdf.
- Ducrotoy MJ, Munoz PM, Conde-Alvarez R, Blasco JM, Moriyon I. A systematic review of current immunological tests for the diagnosis of cattle brucellosis. Prev Vet Med. 2018;151:57-72.
Crossref - Christopher S, Umapathy B, Ravikumar K. Brucellosis: Review of the recent trends in pathogenicity and laboratory diagnosis. J Lab Phys. 2010;2(2):55-60.
Crossref - Corbel MJ. Brucellosis in humans and animals. WHO. 2006. https://apps.who.int/iris/handle/10665/43597
- Moyer NP, Evins GM, Pigott NE, et al. Comparison of serologic screening tests for brucellosis. J Clin Microbiol. 1987;25(10):1969-1972.
Crossref - Alhajj M, Farhana A. Enzyme linked immunosorbent assay. Stat Pearls. 2022. https://www.ncbi.nlm.nih.gov/books/NBK555922/
- Gomez MC, Nieto JA, Rosa C, et al. Evaluation of seven tests for diagnosis of human brucellosis in an area where the disease is endemic. Clin Vaccine Immunol. 2008;15(6):1031-1033.
Crossref - Mantur B, Parande A, Amarnath S, et al. ELISA versus conventional methods of diagnosing endemic brucellosis. Am J Trop Med. 2010;83(2):314-318.
Crossref - Welch RJ, Litwin CMA. Comparison of Brucella IgG and IgM ELISA assays with agglutination methodology. J Clin Lab. 2010;24(3):160-162.
Crossref - Memish ZA, Almuneef M, Mah MW, Qassem LA, Osoba AO. Comparison of the Brucella standard agglutination test with the ELISA IgG and IgM in patients with Brucella bacteremia. Diagn Microbiol Infect Dis. 2002;44(2):129-132.
Crossref - World Organisation for Animal Health (OIE), Bovine brucellosis. 2009. In: The OIE Manual of diagnostic tests and vaccines for terrestrial animals (mammals, birds and bees), Paris. http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.04.03_BOVINE_BRUCELL.pd. Accessed 24 March 2012
- Shome R, Rao KN, Nagalingam M, et al. Comprehensive approaches for diagnosis of human brucellosis. Indian J Comp Microbiol Immunol Infect. 2013;34(2):30-38. https://indianjournals.com/ijor.aspx?target=ijor:ijcmiid&volume= 34&issue=2&article=006
- Alton GG, Jones LM, Angus RD, Verger JM. Techniques for the brucellosis laboratory, Paris, Institut National de la Recherche Agronomique. 1988:192.
- Altman D. Practical statistics for medical research. London: Chapman and Hall. 1991:611.
Crossref - Deka RP, Magnusson U, Grace D, Shome R, Lindahl JF. Knowledge and practices of dairy farmers relating to brucellosis in urban, peri-urban and rural areas of Assam and Bihar, India. Infect Ecol Epidemiol. 2020;10(1):1769531.
Crossref - Memish ZA, Mah MW, Al Mahmoud S, Al Shaalan M, Khan MY. Brucella bacteraemia: clinical and laboratory observations in 160 patients. J Infect. 2000;40(1):59-63.
Crossref - Yagupsky P. Detection of brucellae in blood cultures. J Clin Microbiol. 1999;37(11):3437-3442.
Crossref - Wright AE, Smith F. A Note on the occurrence of malta fever in india. Bri Med. J. 1897;1(1893):911.
Crossref - Tiwari HK, Proch V, Singh BB, et al. Brucellosis in India: comparing exposure amongst veterinarians, para-veterinarians and animal handlers. One Health. 2022;14:100367.
Crossref - Shome R, Kalleshamurthy T, Shankaranarayana PB, et al. Prevalence and risk factors of brucellosis among veterinary health care professionals. Pathog Glob Hlth. 2017;111(5):234-239.
Crossref - Mert A, Ozaras R, Tabak F, et al . The sensitivity and specificity of Brucella agglutination tests. Diagn Microbiol Infect Dis. 2003;46(4):241-243.
Crossref - Ertek M, Yazgi H, Ozkurt Z, Ayyildiz A, Parlak M. Comparison of the diagnostic value of the standard tube agglutination test and the ELISA IgG and IgM in patients with brucellosis. Turk J Med Sci. 2006;36(3):159-163.
Crossref - Al Dahouk S, Herbert T, Karsten N, Heinrich N, Dimitrios F. Laboratory-based diagnosis of brucellosis—A review of the literature. Part I: Techniques for direct detection and identification of Brucella spp. Clin Lab. 2003;49(9-10):487-505. https://pubmed.ncbi.nlm.nih.gov/14572205/
- Navarro-Soto MC, Morales-Loredo A, Alvarez-Ojeda G, et al . Recombinant proteins as antigens in serological diagnosis of brucellosis. In: Baddour MM editor. Updates on Brucellosis [Internet]. London: IntechOpen. 2015.
Crossref - Cardoso PG, Macedo GC, Azevedo V, Oliveira SC. Brucella spp. noncanonical LPS: structure, biosynthesis, and interaction with host immune system. Microb Cell Fact. 2006;5:13.
Crossref - Asaad AM, Alqahtani JM. Serological and molecular diagnosis of human brucellosis in Najran, Southwestern Saudi Arabia. J Infect Public Hlth. 2012;5(2):189-194.
Crossref - Ozdemir M, Feyzioglu B, Kurtoglu MG, et al. A Comparison of immuncapture agglutination and ELISA methods in serological diagnosis of brucellosis. Int J Med Sci. 2011;8(5):428-432.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.