ISSN: 0973-7510
E-ISSN: 2581-690X
Acinetobacter baumannii is a clinically significant pathogen found in both environment and clinical samples. Its accurate identification is crucial for infection control and patient management. This study aims to assess the effectiveness of the commercially available Leeds Acinetobacter Medium (LAM) in the presumptive identification of A. baumannii, and compare its performance with that of multiplex PCR (mPCR) for accurate identification. A. baumannii was isolated using LAM from water (n = 100) and clinical respiratory samples (n = 100) which included endotracheal tube samples, sputum samples, etc. and the accuracy of identification was compared with mPCR. Additionally, the cultural characters of known A. baumannii strains (n = 42) were evaluated on LAM and the isolates were tested with mPCR. A. baumannii prevalence was 5.5% in water and 35.1% in clinical respiratory samples. LAM’s identification accuracy, compared to mPCR, showed 66.7% sensitivity and 76.5% specificity for water samples, and 88.5% sensitivity and 37.5% specificity for clinical respiratory samples. Positive predictive values (PPV) were 14.3% and 43.4%, while negative predictive values (NPV) were 97.5% and 85.7% for water and clinical respiratory samples, respectively. Sequential testing involving preliminary identification followed by LAM culture achieved perfect agreement (kappa = 1) and higher sensitivity/specificity (100%) between LAM and mPCR. The study established mPCR as a reliable standard for A. baumannii identification, while preliminary identification tests such as Gram’s stain, catalase, oxidase and motility test improved LAM’s accuracy by reducing false positives. Limitations in A. baumannii identification were observed with LAM, suggesting its use for screening samples rather than for definitive identification.
Acinetobacter baumannii, Multiplex Polymerase Chain Reaction (mPCR), Positive predictive value (PPV) and Negative predictive value (NPV), Leeds Acinetobacter Medium (LAM)
Acinetobacter baumannii, an aerobic, pleomorphic, non-motile Gram-negative bacillus, is implicated in severe infections.1 Notably, the World Health Organization (WHO) designated it as a critical priority pathogen in 2017 due to its resistance to prevalent antibiotics.2,3 It belongs to the group of ESKAPE pathogens.4 A. baumannii’s antibiotic resistance and adaptability in hospital settings, facilitates its nosocomial transmission and waterborne spread, make it highly concerning.5,6
Acinetobacter species, including A. baumannii, are known for their adaptability, metabolic versatility, and resistance to antibiotics. These characteristics make them model pathogens for studies in environmental microbiology. Their study enhances understanding of bacteria-environment interactions and potential biotechnological applications.7 Though extensively studied, A. baumannii strains obtained from clinical settings may not represent the diverse potential of this species. Therefore, studying this pathogen from environmental sources is essential to gain a comprehensive understanding of its disparate nature.8
Thus, there is a pressing need to understand their epidemiology by studying their presence in environments like sewage water, water bodies close to hospitals, hospital drainage dumps, etc. as well as their prevalence in clinical samples to curtail their spread. The accurate identification of bacteria is essential for understanding their epidemiology, virulence factors, and antimicrobial resistance patterns. Efficient detection and identification of A. baumannii is crucial for appropriate patient management and infection control measures. Though there has been a growing interest in developing rapid and reliable molecular methods for the identification of A. baumannii and other Acinetobacter species,9,10 traditional phenotypic methods for presumptive identification have been reported to be accurate and reliable in rapid identification of A. baumannii.11
Leeds Acinetobacter Medium (LAM) has been developed as a selective and differential medium. LAM contains casein acid hydrolysate, soya peptone, fructose, sucrose, and mannitol and antibiotics such as vancomycin, cefsulodin and cephradine specifically designed for the isolation of clinically important Acinetobacter species and inhibit other Gram-negative bacteria.12 The presence of fructose and sucrose, along with neutral red as a pH indicator in LAM, allows for the differentiation of bacterial colonies based on the metabolic byproducts produced during the utilization of these sugars. Bacteria that release acidic byproducts result in yellow colonies, whereas those that produce alkaline byproducts form pink colonies. LAM’s use in presumptive identification of Acinetobacter in clinical as well as environmental samples has been reported in literature.12,13 It is important to evaluate and compare the efficacy of LAM with PCR-based identification to identify Acinetobacter. Hence, we evaluated a multiplex PCR assay for identification of Acinetobacter baumannii at genus and species level using reported primers. The value of routine physiological tests used for presumptive identification of A. baumannii such as Gram stain morphology, catalase, oxidase, motility test, etc., was further validated. This evaluation is crucial for determining the reliability of LAM in presumptive identification of Acinetobacter and their comparison with PCR will help laboratories to handle a large number of, especially, environmental samples. Furthermore, we wanted to assess the utility of LAM in a medical diagnostic laboratory and its precise identification underscoring its value in environmental monitoring and infection control strategies.
This study aimed to determine whether LAM is a viable substitute or complementary tool to mPCR for detecting A. baumannii in clinical and environmental settings.
In this study, water and clinical respiratory samples were collected from November 2021 to July 2023. A total of 200 samples were cultured on LAM for isolation and identification of A. baumannii. These included environmental water (n = 100) and clinical respiratory samples (n = 100). LAM was prepared using Leeds Acinetobacter agar base (HiMedia, Mumbai, India, M1839) to which Selective Supplement (HiMedia, FD335) was added to help isolation of MDR Acinetobacter spp. from hospital environment.
For the collection of water samples, a stratified random sampling approach was employed, categorizing the sampling locations into different strata based on water source types: lakes (n = 24), ponds (n = 11), drainage water (n = 22), river water (n = 5), agricultural runoff (n = 08), stagnant water (n = 18) and Institutional Water Sources (n = 12). A proportionate number of samples were randomly selected from each stratum based on the availability of sources, ensuring homogeneous and representative coverage of all water types.
For clinical respiratory samples, selective sampling was employed which included endotracheal tube (ET) samples (n = 85), ET aspirates (n = 9), and sputum (n = 6).
Apart from this, a set of 42 Acinetobacter spp. cultures from microbiology laboratory stocks were streaked on LAM. An mPCR assay for identification of A. baumannii was established using ATCC A. baumannii 19606 strain.
Standardization of mPCR for A. baumannii
Overnight culture of ATCC A. baumannii 19606 in Luria Bertani broth (HiMedia, Mumbai, India) at 37 °C was subjected to genomic DNA extraction by phenol-chloroform method.14 The extracted DNA was then used to optimize mPCR assay. Two pairs of reported primers, one targeting the conserved sequence of rpoB, for genus level identification of Acinetobacter11,15 and another targeting species specific sequence of gyrB gene for A. baumannii9 for species identification were used (Table 1).
Table (1):
Primers used in the mPCR assay for identification of A. baumannii
| Targets | Gene | Primer Name | Primer (5’-3’) | Source |
|---|---|---|---|---|
| Genus Acinetobacter | rpoB | Ac696F | TAYCGYAAAGAYTTGAAAGAAG | 15 |
| Ac1093R | CMACACCYTTGTTMCCRTGA | |||
| A. baumannii | gyrB | SP4F | CACGCCGTAAGAGTGCATTA | 9 |
| SP4R | AACGGAGCTTGTCAGGGTTA |
Initially, monoplex PCR assays were standardized to determine the ideal conditions for amplification of the selected genes individually. Genomic DNA was extracted from reference strain by phenol-chloroform technique. The reaction was designed for 20 µl reaction volume containing ready to use 2X Taq PCR MasterMix (Maxome Lifesciences, MX-1103), bacterial genomic DNA and 10 pmol of each primer and water to make up the volume. The amplification conditions included initial denaturation at 94 °C for 2 min, 35 cycles of denaturation at 94 °C for 30 sec, annealing over a gradient of 55-60 °C for 30 sec, extension at 72 °C for 1 min followed by final extension at 72 °C for 7 min. Two thermal cyclers, Master Cycler (Eppendorf, Germany) and QB96 (Quanta Biotech Satellite, Banebio, Maryland, USA) were used for the work. Both gave reproducible results with the same protocol. The amplicons of monoplex PCR’s were confirmed by sequencing (Biokart India Pvt., Ltd.) for presence of the gene of interest.
After the standardization of monoplex PCR, an mPCR assay targeting both genes simultaneously was standardized. The reaction volume was set to 20 µl having 2X Taq PCR MasterMix, ATCC A. baumannii extracted DNA as template and 10 pmol of each genus and species-specific primers and water to make up the volume. To determine the optimum annealing temperature, temperature gradient of 55-60 °C was applied. The optimum temperature for this mPCR assay was found to be 57 °C. The PCR conditions for amplification were: initial denaturation at 94 °C for 2 min, 35 cycles of denaturation at 94 °C for 30 sec, 57 °C annealing for 30 sec, and extension at 72 °C for 1 min followed by final extension at 72 °C for 7 min. PCR was carried out in both the thermal cyclers mentioned above. The mPCR products were resolved on 1.5% agarose gels incorporating ethidium bromide (0.5 µg/ml), after electrophoresis in a 1X Tris-acetic acid-EDTA buffer at 100 V for 45 min. The amplicons were documented using GelDoc system (Vilber Gel Documentation system).
Isolation and identification of A. baumannii from water samples
Water samples from various water bodies were collected by following standard guidelines.16 One ml of freshly collected water sample was added to 9 ml peptone water. The samples were incubated for 24 hours at 37 °C. Loop full of these incubated tubes was sub-cultured onto LAM and incubated at 37 °C for up to 24 hours. Bacterial colonies having pink color with a pinkish mauve in the medium were presumptively identified as Acinetobacter spp. Both the ‘presumptive positive’ isolates and ‘presumptive negative’ colonies were subjected to mPCR for molecular confirmation.
Isolation and identification of A. baumannii from clinical respiratory samples
Clinical respiratory samples including sputum, endotracheal tube and endotracheal secretions which were received for culture and sensitivity at the clinical microbiology laboratory were also inoculated on LAM in addition to the standard laboratory media viz. blood agar and MacConkey agar plates. The cultures were incubated overnight and observed for growth. As described above, the bacterial isolates showing pink colonies with pink mauve colour in the medium were presumptively identified as Acinetobacter spp. These isolates along with presumptively negative isolates were subjected to mPCR assay for confirmation.
Characterization of known Acinetobacter isolates (Reported clinical isolates) using LAM
A total of 42 clinical Acinetobacter spp. isolates phenotypically confirmed by biochemical tests were sub-cultured on LAM. Isolates showing growth on LAM were subjected to the mPCR assay.
Statistical analysis
The data was entered in MS Excel and was presented as numbers and percentages. The data was analyzed using IBM SPSS statistics 20 software. The results were expressed as frequency and percentages. Using mPCR as reference standard, efficacy of LAM in identifying A. baumannii was presented in terms of sensitivity, specificity, positive and negative predictive values. Confidence intervals (95% CI) for these diagnostic measures were calculated using the Wilson score method.
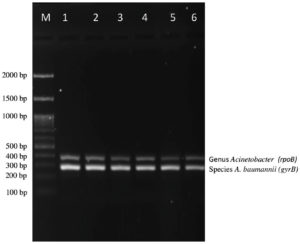
In this study, we established an mPCR assay for confirmatory identification of A. baumannii by targeting the genus as well as species-specific genes (Figure 1). From a total of 100 water samples, 54 showed bacterial growth on LAM whereas from 100 clinical respiratory samples, 74 showed bacterial growth on LAM (Table 2). All presumptive positive and presumptive negative (Figure 2 and 3) bacterial isolates were subjected to mPCR.
Table (2):
Total number of water and clinical respiratory samples processed with corresponding LAM culture and mPCR results for identification of A. baumannii
Type of Sample |
Total Numbers collected |
Growth on LAM both PP and PN |
mPCR Negative Result |
mPCR Positive Result |
|---|---|---|---|---|
Water samples |
100 |
54 |
51 |
3 |
Clinical Respiratory Samples |
100 |
74 |
48 |
26 |
Abbreviations: mPCR, multiplex polymerase chain reaction; LAM, Leeds Acinetobacter Medium; PP, Presumptive Positive; PN, Presumptive Negative
Figure 1. Agarose gel electrophoresis of mPCR for identification of A. baumannii
Lane M: 100 bp DNA Ladder, Lane 1-ATCC A. baumannii (19606), Lane 2 to 6: bacterial isolates showing positive for genus Acinetobacter and species A. baumannii
Among the bacterial isolates from water samples, 5.5% (3/54) were confirmed as A. baumannii by mPCR assay, whereas among clinical respiratory samples, 35.1% (26/74) of the bacterial isolates were confirmed as A. baumannii.
In case of water samples (Table 3), the overall sensitivity of LAM as compared to mPCR assay was 66.7% with a specificity of 76.5%. The positive predictive value and negative predictive value of LAM was 14.3% and 97.5%, respectively. There was only a slight agreement between the tests, i.e. LAM and mPCR assay (kappa = 0.15).
Table (3):
Comparison of LAM and mPCR assay results for identification of A. baumannii in water samples
Leeds Medium |
mPCR Positive |
mPCR Negative |
Total |
|---|---|---|---|
Presumptive Positive |
2 |
12 |
14 |
Presumptive Negative |
1 |
39 |
40 |
Total |
3 |
51 |
54 |
In case of clinical respiratory samples streaked on LAM (Table 4), the overall sensitivity of LAM as compared to mPCR assay was 88.5% with a specificity of 37.5%. The positive predictive value and negative predictive value of LAM was 43.4% and 85.7% respectively. For a comprehensive statistical analysis including 95% confidence intervals and kappa score here, there was a fair agreement between the tests, i.e. LAM and mPCR assay (kappa = 0.21)
Table (4):
Comparison of LAM and mPCR assay results for identification of A. baumannii in clinical respiratory samples
Leeds Medium |
mPCR Positive |
mPCR Negative |
Total |
|---|---|---|---|
Presumptive Positive |
23 |
30 |
53 |
Presumptive Negative |
3 |
18 |
21 |
Total |
26 |
48 |
74 |
We cultured 42 known Acinetobacter isolates on LAM. Only one isolate showed yellow colonies which was found to be negative by mPCR assay, whereas 41 isolates producing pink colonies with pinkish mauve on LAM were confirmed by mPCR assay as A. baumannii. The isolate giving yellow colony on LAM was subjected to reidentification and was found to be a contaminant. In this sequential identification (Table 5), the overall sensitivity and specificity of LAM when compared to mPCR assay was 100%. The PPV and NPV were also 100% with a strong agreement between the two tests (k = 1) (See Table 6 for 95% confidence intervals and diagnostic metrics).
Table (5):
Comparison of LAM and mPCR assay results for identification of A. baumannii in known clinical isolates using sequential testing
Leeds Medium |
mPCR Positive |
mPCR Negative |
Total |
|---|---|---|---|
Presumptive Positive |
41 |
0 |
41 |
Presumptive Negative |
0 |
1 |
1 |
Total |
41 |
1 |
42 |
Table (6):
Diagnostic performance of LAM compared to mPCR as reference standard in identifying A. baumannii
Culture type on LAM |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
Kappa Score |
|---|---|---|---|---|---|
Water Samples |
66.7 (95% CI: 20.8% to 93.9%) |
76.5 (95% CI: 63.2% to 86.0%) |
14.3 (95% CI: 4.0% to 39.9%) |
97.5 (95% CI: 87.1% to 99.6%) |
0.15 |
Clinical Respiratory Samples |
88.5 (95% CI: 71.0% to 96.0%) |
37.5 (95% CI: 25.2% to 51.6%) |
43.4 (95% CI: 30.9% to 56.7%) |
85.7 (95% CI: 65.4% to 95.0%) |
0.21 |
Reported Clinical isolates |
100.0 (95% CI: 91.4% to 100%) |
100.0 (95% CI: 20.7% to 100%) |
100.0 (95% CI: 91.4% to 100%) |
100.0 (95% CI: 20.7% to 100%) |
1.00 |
Abbreviations: CI = Confidence Interval; PPV = positive predictive value; NPV = negative predictive value; CI = computed using the Wilson score method. LAM = Leeds Acinetobacter Medium; mPCR = multiplex polymerase chain reaction
In this study, we evaluated a multiplex PCR (mPCR) assay for the confirmatory identification of Acinetobacter baumannii using reported primers and evaluated LAM as a selective medium for isolating and presumptively identifying A. baumannii from water and clinical respiratory samples. The prevalence of A. baumannii was found to be 5.5% in water samples and 35.1% in clinical respiratory samples.
The presence of multiple sugars in LAM allows not only Acinetobacter but also other bacteria such as Burkholderia cepacia, Stenotrophomonas maltophilia producing pink colonies and Citrobacter freundii producing yellow colonies also grow on this medium (HiMedia and Hardy Diagnostics literature). The former two bacteria most commonly encompass the non-fermenting Gram-negative bacteria.12 Differentiating A. baumannii from these isolates is usually quite obscure.17
This suboptimal selectivity of LAM, thus fails to inhibit non-target organisms,12 affecting its reliability for isolating Acinetobacter spp. in mixed flora like water or sputum samples. This drawback of LAM necessitates confirmatory identification of the isolates and mPCR ensures accurate differentiation between A. baumannii and other organisms. Thus, both presumptive positive (pink colonies) and presumptive negative isolates with different morphologies on LAM were further subjected to mPCR assay for confirmatory identification of A. baumannii.
An easy to use, accurate identification protocol will help clinical laboratories and research laboratories to identify A. baumannii from various specimens with high reliability and confidence.
Microbiological sampling of water is commonly used in environmental surveillance for monitoring and implementation of infection control practices. It is also useful in outbreak investigations. Emerging research indicates that numerous antimicrobial resistance genes (ARGs) of significance in clinical contexts were initially acquired from environmental microbes such as Acinetobacter and Pseudomonas species. The persistence of these bacteria creates avenues for horizontal gene transfer among other bacteria. Moreover, the culture techniques used to isolate P. aeruginosa and A. baumannii from environmental sources are typically designed for clinical specimens and have not been thoroughly validated for use with environmental samples.16,18 The respiratory samples often carry mixed flora; making it difficult to detect A. baumannii from the overgrowing commensal flora on common culture plates used for sample processing in clinical microbiology laboratory.19
Given these challenges, several investigators have used LAM for culture and identification of A. baumannii in environmental and other studies.20-24 However, to the best of our knowledge we are the first to evaluate its efficacy in identifying A. baumannii as compared to standard identification methods used in majority of medical laboratories. In this study, we could compare the efficacy of LAM to isolate and presumptively identify A. baumannii and assess its performance vis-à-vis confirmatory molecular identification.
On comparing the performance of LAM and mPCR, we found that LAM showed a sensitivity of 66.7% and 88.5% in detecting A. baumannii from water samples and clinical respiratory samples respectively. The sensitivity of medium is influenced by sample collection technique and further processing. Our study collected both water and clinical respiratory samples over a period from November 2021 to July 2023. For water samples, changes in temperature, rainfall, and other environmental conditions could have influenced bacterial contamination levels, potentially affecting the detection of A. baumannii. Similarly, for clinical respiratory samples, hospital-related factors, such as variations in patient admission rates, infection control practices, and antibiotic usage, could contribute to shifts in A. baumannii prevalence.
Doi et al. reported the sensitivity of LAM to range from as low as 21.7% to as high as 82.6%. The investigators however compared different collection methods and used a modified composite of LAM containing aztreonam and ceftazidime.25 In our study, the specificity of LAM compared to mPCR was 76.5% for water samples and only 37.5% for clinical respiratory samples. McConnell et al., reported a high PPV of 90.7% for isolating A. baumannii from ICU environmental surface samples however they speciated the bacterial isolates using MALDI-TOF analysis.26 In our study, the PPV was 14.3% and 43.4% for water samples and clinical respiratory samples respectively, whereas the NPV, was 97.5% and 85.7% respectively. Detailed 95% confidence intervals for these diagnostic metrics are presented in Table 6. Several other studies have evaluated A. baumannii isolation from environmental and clinical sources using LAM as selective media. Ahmad et al. and Fasuyi et al. reported the recovery of MDR and XDR strains using culture and molecular methods in Iraq and Nigeria, respectively.27,28 Hossain et al. detected A. baumannii in 32% of surface water samples in Bangladesh,29 while Suresh et al. demonstrated the effectiveness of LAM in isolating resistant strains from soil in India.23 Kitti et al. demonstrated genetic relatedness and shared resistance traits between clinical and environmental A. baumannii strains isolated from the same hospital ward in Thailand,24 and Gao et al. reported antibiotic-resistant Acinetobacter in swine farm groundwater in China.30 These findings collectively support the use of selective media across diverse settings and highlight the importance of integrating culture-based and molecular techniques for reliable detection.
The high NPV of LAM indicates that negative results obtained from this selective media can be considered reliable, making it a suitable method for initial screening to rule out the presence of A. baumannii. However, due to its low PPV, LAM may not be adequate for confirming the presence of A. baumannii. This suggests that LAM should not be used as the sole confirmation tool but rather needs to be complemented by mPCR or another confirmatory technique to mitigate the risk of misidentification. The kappa agreement between LAM culture and mPCR assay in both the scenarios was not satisfactory. These results indicate that LAM may not be reliable in accurately identifying A. baumannii, especially from clinical respiratory samples, where it had a lower specificity. The poor performance of LAM can be attributed to the phenotypic nature of this selective media, which may not be sufficiently specific to differentiate A. baumannii from phenotypically closer species. The mPCR assay, however, targets genus and species-specific genes, allowing more reliable and specific identification of A. baumannii.
Interestingly, the sensitivity and specificity of LAM improved when it was tested on known Acinetobacter isolates. This improvement can be attributed to a sequential identification process, wherein the isolates were presumptively identified based on phenotypic characteristics such as Gram stain, catalase, oxidase, and motility tests, and then confirmed using the mPCR assay. This validation step helped eliminate the false-positive result observed during the initial LAM screening, resulting in a perfect agreement (k = 1) between LAM and mPCR. These findings underscore the significance of the preliminary identification tests (Gram stain, catalase, oxidase, and motility test), as they exhibited a perfect concordance with the mPCR assay and enhanced the utility of LAM selective media. The efficacy of physiological and biochemical identification of Acinetobacter has already been reported. 11 The meticulous execution of these basic preliminary test protocols proved to be indispensable for accurate identification of Acinetobacter spp.
The implications of our findings extend beyond the accurate identification of A. baumannii. In clinical settings, the high sensitivity of mPCR, particularly for clinical respiratory samples, can significantly enhance infection control strategies by enabling early and accurate diagnosis, reducing cross-contamination risks in high-risk environments such as intensive care units and guiding more targeted antibiotic treatments. In environmental contexts, the detection of A. baumannii in various water sources, coupled with the utility of mPCR for confirmatory identification, underscores the importance of integrating mPCR into environmental monitoring programs. This combined approach can help track contamination sources, inform public health strategies, and prevent potential waterborne transmission risks, thereby contributing to both infection control and environmental health efforts.
Finally, it is important to note that using different sampling methods for environmental and clinical respiratory samples may have introduced some bias in our study. Stratified random sampling was used for environmental samples to ensure representation of diverse water sources, while selective sampling of clinical respiratory samples targeted high-risk patients. This selective sampling may have overestimated A. baumannii prevalence in clinical settings. Future studies should adopt random sampling for clinical specimens to achieve a more representative analysis of A. baumannii prevalence across a broader patient population.
Our study highlights the limited specificity of LAM selective medium in identification of A. baumannii, especially from clinical respiratory samples. A multiplex PCR assay (mPCR) for identification of clinically relevant and most common Acinetobacter spp., A. baumannii would be very useful for clinical laboratories. The sequential testing approach, incorporating preliminary phenotypic tests followed by mPCR substantially improves the accuracy of A. baumannii identification. Although the study considered relatively less water and clinical respiratory samples, they were carefully chosen to represent a diverse range of sources, reflecting both environmental and clinical contexts. The study’s primary objective was to conduct a preliminary evaluation of the LAM in comparison to mPCR, providing a foundation for further research. Although larger sample sizes would increase the robustness of the findings, the current dataset offers valuable insights into the diagnostic performance of LAM and its potential application for initial screening.
This approach aligns with the broader understanding of microbiological identification methods, where a combination of phenotypic and genotypic techniques enhances reliability. Future research can focus on improving selective as well as indicator media, PCR assays targeting ARGs and screening of environmental samples directly by mPCR. This study provides a foundational comparison between LAM and mPCR for A. baumannii detection and underscores the need for molecular confirmation in routine diagnostics.
ACKNOWLEDGMENTS
The authors are thankful to Rashtriya Uchchatar Shiksha Abhiyan (RUSA), Karnatak University Dharwad and Shri Dharmasthala Manjunatheshwara (SDM) University, Dharwad, Karnataka, for providing the necessary facilities for conducting the experiments.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
This study was supported by Karnatak University, Dharwad, for providing University Research Studentship vide grant number KU/MOBC/URS/2022/361 dated 18/09/2022.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This study was approved by the Institutional Ethics Committee, SDM College of Medical Sciences & Hospital, Karnataka, India, vide approval number SDMIEC/2022/162, dated 08/01/2022.
- Howard A, O’Donoghue M, Feeney A, Sleator RD. Acinetobacter baumannii An emerging opportunistic pathogen. Virulence. 2012;3(3):5.
Crossref - World Health Organization (WHO). WHO Bacterial Priority Pathogens List, 2024: Bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. Published 2024. Accessed December 12, 2024. https://www.who.int/publications/i/item/9789240093461
- Vazquez-Lopez R, Solano-Galvez SG, Vignon-Whaley JJJ, et al. Acinetobacter baumannii resistance: A real challenge for clinicians. Antibiotics. 2020;9(4):205.
Crossref - Rice LB. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J Infect Dis. 2008;197(8):1079-1081.
Crossref - Dijkshoorn L, Nemec A, Seifert H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol. 2007;5(12):939-951.
Crossref - Carvalheira A, Silva J, Teixeira P. Acinetobacter spp. in food and drinking water – A review. Food Microbiol. 2021;95:103675.
Crossref - Jung J, Park W. Acinetobacter species as model microorganisms in environmental microbiology: current state and perspectives. Appl Microbiol Biotechnol. 2015;99(6):2533-2548.
Crossref - Diancourt L, Passet V, Nemec A, Dijkshoorn L, Brisse S. The population structure of Acinetobacter baumannii: Expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One. 2010;5(4):e10034.
Crossref - Li XM, Choi JA, Choi IS, et al. Development and evaluation of species-specific PCR for detection of nine Acinetobacter species. Ann Clin Lab Sci. 2016;46(3):270-278.
- Chen TL, Lee YT, Kuo SC, Yang SP, Fung CP, Lee SD. Rapid identification of Acinetobacter baumannii, Acinetobacter nosocomialis and Acinetobacter pittii with a multiplex PCR assay. J Med Microbiol. 2014;63(PART 9):1154-1159.
Crossref - Kulkarni SS, Madalgi R, Ajantha GS, Kulkarni RD. Identification of genus Acinetobacter: Standardization of in house PCR and its comparison with conventional phenotypic methods. J Lab Physicians. 2017;9(4):279-282.
Crossref - Jawad A, Hawkey PM, Heritage J, Snelling AM. Description of Leeds acinetobacter medium, a new selective and differential medium for isolation of clinically important Acinetobacter spp., and comparison with herellea agar and Holton’s agar. J Clin Microbiol. 1994;32(10):2353-2358.
Crossref - Benoit T, Cloutier M, Schop R, Lowerison MW, Khan IUH. Comparative assessment of growth media and incubation conditions for enhanced recovery and isolation of Acinetobacter baumannii from aquatic matrices. J Microbiol Methods. 2020;176:106023.
Crossref - Sambrook J, Fritsch EF, Maniatis T. Plasmids and Their usefulness in Molecular Cloning. Molecular Cloning A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press. 1989:1.93-1.97.
- Gundi VAKB, Dijkshoorn L, Burignat S, Raoult D, La Scola B. Validation of partial rpoB gene sequence analysis for the identification of clinically important and emerging Acinetobacter species. Microbiology (Reading). 2009;155(7):2333-2341.
Crossref - Sehulster L, Chinn RYW. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 2003;52(RR-10):1-42.
- Rolston KV, Safdar A. Pseudomonas, Stenotrophomonas, Acinetobacter, and Other Nonfermentative Gram-Negative Bacteria and Medically Important Anaerobic Bacteria in Transplant Recipients. In: Safdar, A. (eds) Principles and Practice of Transplant Infectious Diseases. Springer, New York, NY. 2019:425-447.
Crossref - Milligan EG, Calarco J, Davis BC, et al. A Systematic Review of Culture-Based Methods for Monitoring Antibiotic-Resistant Acinetobacter, Aeromonas, and Pseudomonas as Environmentally Relevant Pathogens in Wastewater and Surface Water. Curr Environ Health Rpt. 2023;10(2):154-171.
Crossref - Alados JC, Serrano J, Garcia JA, Miranda C, Orellana M, de la Rosa M. Usefulness of Leeds Acinetobacter Medium Respiratory Specimens Collected in an Intensive Care Unit. Eur J Clin Microbiol Infect Dis. 1995;(2):474-476.
Crossref - El-Kholy AM, Zeinhom MA, Shinawy SHH, Gaber A. Detection of Adulteration in Milk and Some Dairy Products. Assiut Vet Med J. 2018;64(157):1-10.
Crossref - Safi M, Al-Hallab L, Al-Abras R, Khawajkiah M, Kherbik H, AL-Mariri A. Efficacy of Some Antibiotics and Essential Oils Against Acinetobacter baumannii: An In vitro Study. Avicenna J Clin Microbiol Infect. 2020;7(1):1-7.
Crossref - Hubeny J, Korzeniewska E, Buta-Hubeny M, Zielinski W, Rolbiecki D, Harnisz M. Characterization of carbapenem resistance in environmental samples and Acinetobacter spp. isolates from wastewater and river water in Poland. Sci Total Environ. 2022;822:153437.
Crossref - Suresh S, Aditya V, Deekshit VK, Manipura R, Premanath R. A rare occurrence of multidrug-resistant environmental Acinetobacter baumannii strains from the soil of Mangaluru, India. Arch Microbiol. 2022;204(7):1-12.
Crossref - Kitti T, Manrueang S, Leungtongkam U, et al. Genomic relatedness and dissemination of blaNDM-5 among Acinetobacter baumannii isolated from hospital environments and clinical specimens in Thailand. Peer J. 2023;11:1-19.
Crossref - Doi Y, Onuoha EO, Adams-Haduch JM, et al. Screening for Acinetobacter baumannii colonization by use of sponges. J Clin Microbiol. 2011;49(1):154-158.
Crossref - McConnell MJ, Perez-Romero P, Lepe JA, et al. Positive predictive value of leeds Acinetobacter medium for environmental surveillance of Acinetobacter baumannii. J Clin Microbiol. 2011;49(12):4416.
Crossref - Ahmad NH, Mohammad GA. Identification of Acinetobacter baumannii and Determination of MDR and XDR Strains. Baghdad Sci J. 2020;17(3):726-732.
Crossref - Fasuyi OC, Ike WE, Ojo DA, Adeboye AO. Plasmid profile of multidrug resistant Acinetobacter baumannii strains from wounds of patients attending Federal Medical Centre, Abeokuta, Southwest, Nigeria. Niger J Pharm Appl Sci Res. 2020;9(2):47-51.
- Hossain MA, Fatima NNE, Tushar JH, Mahmud H, Haque FKM. Isolation and characterization of Acinetobacter baumannii from environmental waters in Dhaka City, Bangladesh. BMC Microbiol. 2025;25(1):314.
Crossref - Gao FZ, He LY, Chen X, et al. Swine farm groundwater is a hidden hotspot for antibiotic-resistant pathogenic Acinetobacter. ISME Commun. 2023;3(1):34.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.