ISSN: 0973-7510
E-ISSN: 2581-690X
Bacteria use different types of motilities in order to colonize and adapt to new environments. These motilities also play an important role in the formation of biofilm, allowing bacteria to develop resistance to antibiotics and host’s immune systems. The objective of this study was to evaluate the ability of essential oils of Lippia multiflora flowers to inhibit biofilm formation and motility in Pseudomonas aeruginosa PAO1. The capacity of essential oil of Lippia multiflora flowers to inhibit biofilm formation in Pseudomonas aeruginosa was evaluated spectrophotometrically by using the crystal violet method. The property of the essential oil to inhibit different types of motilities such as swimming, swarming and twitching was further evaluated by measuring the diameters of bacterial migration on liquid or semi-liquid Luria Bertani agar medium. The essential oil exhibited good anti-biofilm activity in Pseudomonas aeruginosa. At a concentration of 1%, essential oil presented a degree of biofilm inhibition similar to that of salicylic acid used as a reference (P > 0.05). In addition, the essential oil significantly inhibited swarming, twitching and swimming in Pseudomonas aeruginosa compared to the control (P < 0.001). The inhibition of biofilm formation as well as that of bacterial motility increases with the concentration of the essential oil. The essential oil of Lippia multiflora flowers possesses an anti-bacterial potential to fight against multi-resistant bacteria.
Biofilm, Essential Oil, Lippia multiflora, Motilities, Pseudomonas aeruginosa
The emergence of multi-resistant bacteria to the conventional antibiotic therapy is a global public health problem.1 This bacterial resistance could find its origin in the formation of biofilm. The eradication of a bacterial biofilm poses serious medical problems. Indeed, the biofilm is a bacterial population adhered to a surface and coated with an exopolysaccharide matrix, thus limiting the access of antibiotics and biocides to the cytoplasm of the bacteria.2
Pseudomonas aeruginosa is naturally resistant to numerous antibiotics, due to the low permeability of its cellular envelope and the presence of numerous efflux pumps which reject drugs that enter in small quantities out of the cell.3 This bacterial resistance increases with the formation of biofilm.4 Previous studies have shown that the formation of this bacterial biofilm needs a cell aggregation mechanism that requires bacterial mobilities, and not just a simple bacterial colonial growth5 This bacterial mobility involves several types of motilities including swarming, swimming and twitching, allowing the bacteria to escape from unfavorable conditions and to exploit new resources or opportunities.6 The swarming is a multicellular semi-solid surface movement powered by rotating helical flagella.7 Unlike swarming, the swimming is an individual movement in liquid powered by rotating flagella.8 Twitching is a type of bacterial motility that uses type IV pili to extend and adhere to the solid surface, then retract, dragging the bacteria along.9 Inhibition of bacterial biofilm formation and motilities in P. aeruginosa can therefore help prevent infections caused by this bacterium and improve the effectiveness of antibiotic treatments. Previous work has shown that certain synthetic compounds such as furanone derivatives can inhibit biofilm formation in P. aeruginosa but become ineffective face to the development of resistance of P. aeruginosa such as the genetic recombination (development of resistance genes), the enzymatic deactivation of the antibiofilm compounds (lactamases) and the virulence factors production (elastase, rhamnolipids, pyocyanin).10 It is therefore necessary to conduct research on other antibacterial compounds, preferably from plants, which will effectively combat bacterial resistance.
Lippia multiflora Moldenke is a woody aromatic shrub growing naturally in tropical ecologies (Figure 1).11 Lippia multiflora offers a diversity of uses in pharmacopoeia and medicine. Indeed, infusions of its flowers are traditionally used for the treatment of malaria, diabetes, fevers, gastrointestinal disorders, coughs and hypertension.12,13 In addition to its medical potential, the essential oil of L. multiflora provides cosmetic and pesticide adjuvants.14 The antibacterial properties of the essential oil of L. multiflora flowers demonstrated in previous studies were limited to its bactericidal properties.15–17 However, its ability to inhibit the formation of biofilm and the bacterial motility remains virtually unassessed. This present study aims to evaluate the capacity of the essential oil of L. multiflora flowers to inhibit P. aeruginosa PAO1 biofilm formation and motilities.
Chemicals
Luria Bertani (LB) agar, salicylic acid, crystal violet, acetic acid and dimethlsulfoxide were purchased by Sigma–Aldrich (St. Louis, USA). Methanol was purchased by Prolabo (Paris, France). All chemicals were analytical grade.
Plant collection
The flowers were harvested during the flowering of the plant in September 2022 at Loumbila (12° 31’5.39’’N; -1° 22’ 8.39’’W), a locality situated at 25 km of the Ouagadougou city. A sample of leafy and flowering stem was collected and transported to the plant biology and ecology laboratory of the University of Joseph KI-ZERBO, Burkina Faso for identification of the plant by Pr Amado Ouיdraogo, a full professor in plant biology and ecology. A herbarium has been deposited under the identification code IC-0922.
Extraction
The essential oil of L. multiflora flowers was obtained by the standard hydrodistillation. 200 g of plant material are subjected to hydrodistillation for 4 hours using a clevenger apparatus (LG-6655-100; USA). The essential oil obtained was separated from the water by decantation and stored in a sealed bottle and kept at 4°C until its use for the different antibacterial assays.
Anti-biofilm assay
The anti-biofilm activity of the essential oil was assessed by using the crystal violet method.18 This method is commonly used to quantify the biofilm formation due to its high sensibility and its simplicity of used unlike the utilization of the microscopy that remains purely qualitative. This method is based on the ability of the crystal violet to bind to components of the extracellular matrix of bacterial biofilms whose the intensity of the coloration is proportional to the quantity of the biofilm biomass.
P. aeruginosa was grown in 96-well microplate wells containing a Luria Bertani (LB) agar growth medium and treated with two non-bactericidal and non-bacteriostatic concentrations of L. multiflora essential oil (0.7% and 1%). The wells were washed with distilled water to eliminate non-adherent bacteria. The adherent bacteria were fixed with methanol for 15 minutes. The wells were stained with 0.1% (m/v) crystal violet for 30 minutes at room temperature. Excess dye was removed by washing with distilled water. The crystal violet linked to the biofilms was solubilized with acetic acid 33%. The optical density of the solution was measured at 590 nm using an ELISA microplate reader (BioTek Instruments, USA). The experiment was carried out in sixplicate and the results were expressed as percentage of inhibition of biofilm formation compared to a control without essential oil (DMSO 1%). Salicylic acid was used as reference compound.
Anti-motility assay
Swarming, twitching and swimming motilities assays were performed using the same Luria Bertani (LB) agar medium with different proportions of agar as described previously.19 The swarming medium was constituted by 8 g/L of LB and 0.5% of agar to form a semi-solid medium. The swimming and twitching media was constituted by a LB broth with 0.3 and 1% agar respectively to form a liquid medium. Bacterial culture were used to inoculate the swarming, twitching and swimming plates containing two non-bactericidal and non-bacteriostatic concentrations of essential oil of L. multiflora flowers (0.7% and 1%) and the plates were incubated overnight at 37°C. Plates inoculated with bacteria without essential oil were used as control. The areas of the bacterial migration were photographed using Photoshop CS3 Extended (Adobe) and the diameters of swimming, swarming and twitching motilities were measured. The experiments were performed in sixplicate.
Statistical analysis
The experiments were carried out in sixplicate and data were expressed as means values ± S.E.M. Analysis of variance (Newman keuls post-tests) was done to test significance and p < 0.05 was considered statistically significant.
Anti-biofilm activity of essential oil
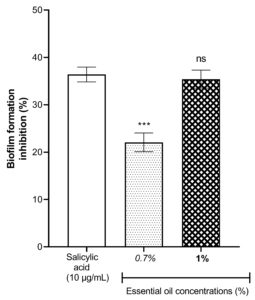
Considering the Figure 2, the essential oil of L. multiflora flowers exhibited good inhibitory activities on the biofilm formation of P. aeruginosa. The inhibitory activity of the biofilm formation increases when the concentration used of the essential oil is high. The essential oil at the concentration 1% presented a degree of biofilm inhibition similar to that of salicylic acid used as a reference (P > 0.05) and known as an inhibitor pure compound of the formation of biofilm in P. aeruginosa. This finding suggested that the essential oil at a concentration 1% is a potent inhibitor of the formation of biofilm in P. aeruginosa.
Figure 2. Effect of essential oil of Lippia multiflora on Pseudomonas aeruginosa biofilm formation. Data are expressed as the means±S.E.M, n=6. ns: no significant ; ***P < 0.001 versus the corresponding salicylic acid (ANOVA followed by Newman Keul’s test)
Anti-motilities activities of essential oil
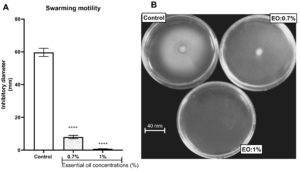
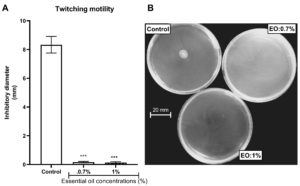
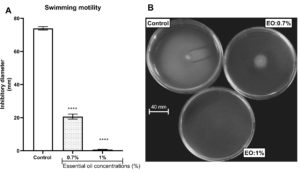
The property of the L. multiflora essential oil to inhibit P. aeruginosa motility was evaluated by measuring the ability of the essential oil to trap the swimming, swarming and twitching motilities. L. multiflora essential oil inhibited significantly the swarming (Figure 3), twitching (Figure 4) and swimming (Figure 5) motilities compared to the control (P < 0.001). When the inoculated bacteria were untreated with the essential oil, the diameter of bacterial migration was beyond 60 mm for swarming, 70 mm for swimming and 8 mm for twitching. However, when the inoculated bacteria were treated with the essential oil, the diameter of the bacterial migration was significantly reduced, below 10 mm for swarming, 20 mm for swimming and 1 mm for twitching. The essential oil at the concentration of 1% showed the highest swarming and swimming inhibitory activity compared to the essential oil at 0.7% while the twitching inhibitory activity did not vary depending on the essential oil concentration.
Figure 3. Effect of essential oil of Lippia multiflora on Pseudomonas aeruginosa swarming motility. A: histogram of inhibition diameters; B: Photo of bacterial swarming. Data are expressed as the means±S.E.M, n=6. ***P < 0.001 versus the control (ANOVA followed by Newman Keul’s test) ; EO: Essential Oil
Figure 4. Effect of essential oil of Lippia multiflora on Pseudomonas aeruginosa twitching motility. A: Histogram of inhibitory diameters; B: Photo of bacterial twitching. Data are expressed as the means±S.E.M, n=6. ***P < 0.001 versus the control (ANOVA followed by Newman Keul’s test) ; EO: Essential Oil
Figure 5. Effect of essential oil of Lippia multiflora on Pseudomonas aeruginosa swimming motility. A: Histogram of inhibitory diameters; B: Photo of bacterial swimming. Data are expressed as the means±S.E.M, n=6. ***P < 0.001 versus the control (ANOVA followed by Newman Keul’s test); EO: Essential Oil
The formation of biofilms plays an important role in several types of infections which remain difficult to treat.20 This sessile lifestyle, unlike planktonic cells (living in a liquid environment) found in a context of acute infection, allows better adaptation to situations of nutritional stress, biocides and persistence of the immune reaction.21 In this study, the essential oil of L. multiflora flowers exhibited good inhibitory activities against P. aeruginosa biofilm formation. This inhibition of the biofilm formation by the essential oil could be explained by several properties of the essential oil such as the prevention of the initial adhesion of bacteria, the inhibition of the interbacterial communication (quorum sensing), the inhibition of the N-acetyl-glucosamine polymer synthesis that constitutes the biofilm matrix or its degradation.22 Indeed, natural compounds from plants such as cis-2-decenoic acid are capable of inhibiting the development of the biofilm and inducing its dispersion in many gram+, gram- bacteria and in yeasts.23 Likewise, compounds from the coumarin family (esculetin), flavonoids (fisetin) and tannins (ellagic acid) have the capacity to inhibit the bacterial biofilm formation.24-26 Other studies have shown that essential oils are capable of inhibiting adhesin, a protein used by bacteria to adhere to surfaces, which is the basis for the formation of biofilms. Biofilm formation in P. aeruginosa is under the control of a mechanism called quorum sensing. Quorum sensing (QS) is a mechanism for acquiring information on cell density allowing bacteria to coordinate and modulate the expression of certain genes regulating several bacterial behaviors such as sporulation, production of virulence factors, biofilm formation and motility.27 The inhibitory activity of the biofilm formation of the essential oil demonstrated in this study could be the result of an inhibition of the bacterial quorum sensing, in particular the inhibition of the Las and RhL genes involved in the regulation of the quorum sensing.28 Although most of these antibiofilm products do not kill bacteria, they can make them more susceptible to the action of antibiotics or to the host’s immune defense.29 The formation of the bacterial biofilm (sessile form) requires prior movement of bacteria (planktonic form) according to several mechanisms called swimming, swarming and twitching. In this study the essential oil of L. multiflora flowers inhibited significantly the swarming, swimming and twitching motilities in P. aeruginosa. Swimming requires a motor flagellum while swarming requires the presence of a functional flagellum and the production of a surfactant.7,8 In P. aeruginosa, a surface-wetting surfactant composed of 3-(3-hydroxyalkanoyloxy) alkanoic acid (HAA) and rhamnolipids (mono- and di-rhamnolipids) promotes this type of motility.30 The essential oil of L. multiflora could prevent the production of the surfactant and rhamnolipids by the bacteria or quench the production of ATP which supplies the flagellum with energy, thus inhibiting bacterial motility. As for twitching type motility, it is due to type IV pili which are polar surface appendages present on the surface of bacteria. The movement by this type of motility involves the active extension and retraction of type IV pili located on either side of the poles of the bacterial cell.9 The involvement of type IV pili has been reported for several phenomena such as the formation of biofilms and fruiting bodies.31 Twitching-type motility generally involves a close cell-cell interaction which generates the formation of cellular “rafts”, thus allowing radial movement outwards from the center of the colony. Studies have shown that the movement of the flagellum and these pili requires a large amount of chemical energy.32 The essential oil could inhibit the production of ATP by the bacterial cells, thus preventing the extension and retraction of the pili which conditions this type of motility.
The essential oil of L. multiflora flowers has shown good anti-biofilms and anti-motilities activities. Biofilms and motilities are virulence factors of bacteria and such processes increase the severity of infections and pathogenicity. Therefore inhibiting biofilms and motilities are suitable strategies to combat multiresistant pathogens. Essential oil of L. multiflora could be an adjuvant to help with the increasingly threatening antibiotic resistance or to reduce adverse effects.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Chin KW, Tiong HLM, Luang-In V, Ma NL. An overview of antibiotic and antibiotic resistance. Environ Adv. 2023;11:100331.
Crossref - Vetrivel A, Ramasamy M, Vetrivel P, et al. Pseudomonas aeruginosa biofilm formation and its control. Biologics. 2021;1(3):312-336.
Crossref - Pang Z, Raudonis R, Glick BR, Lin T, Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol Adv. 2019;37(1):177-192.
Crossref - Vestby LK, Gronseth T, Simm R, Nesse LL. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics. 2020;9(2):59.
Crossref - Houry A, Briandet R, Aymerich S, Gohar M. Involvement of motility and flagella in Bacillus cereus biofilm formation. Microbiology. 2010;156(Pt 4):1009-1018.
Crossref - Zegadlo K, Gieron M, Zarnowiec P, et al. Bacterial motility and its role in skin and wound infections. Int J Mol Sci. 2023;24(2):1707.
Crossref - Yan J, Monaco H, Xavier JB. The ultimate guide to bacterial swarming:an experimental model to study the evolution of cooperative behavior. Annu Rev Microbiol. 2019;73:293-312.
Crossref - Hamby AE, Vig DK, Safonova S, Wolgemuth CW. Swimming bacteria power microspin cycles. Sci Adv. 2018;4(12):eaau0125.
Crossref - Kuhna MJ, Talaa L, Inclanc YF, et al. Mechanotaxis directs Pseudomonas aeruginosa twitching motility. Proc Natl Acad Sci U S A. 2021;118(30):e2101759118.
Crossref - Gomez A, Lyons T, Mamat U, et al. Synthesis and evaluation of novel furanones as biofilm inhibitors in opportunistic human pathogens. Eur J Med Chem. 2022;242:1-16.
Crossref - Folashade KO, Omoregie EH. Essential oil of Lippia multiflora Moldenke:A review. J Appl Pharm Sci. 2022;2(1):15-23.
- Goly KRC, Soro Y, Dadie A, Kassi ABB, Dje M. Antibacterial activity of essential oils and extracts from the leaves of Hyptis suaveolens and Lippia multiflora on multi-resistant bacteria. Rasayan J Chem. 2015;8(4):396-403.
- Masunda AT, Inkoto CL, Masengo CA, et al. Traditional uses, Physical properties, phytochemistry and bioactivity of Lippia multiflora Moldenke (Verbenaceae):a mini-review. Discovery Phytomedicine. 2020;7(1):19-26.
Crossref - Kobenan KC, Ochou GEC, Kouadio IS, et al. Chemical composition, antioxidant activity, cholinesterase inhibitor and in vitro insecticidal potentiality of essential oils of Lippia multiflora Moldenke and Eucalyptus globulus Labill. on the main parpophagous pests of cotton plant in Ivory Coast. Chem Biodivers. 2022;19(4):e202100993.
Crossref - Bonou J, Baba-Moussa F, AdAoti Z, et al. Antimicrobial activity of essential oils of Lippia multiflora, Eugenia caryophyllata, Mentha piperita and Zingiber officinale on five oral-dental microorganisms. J Pharmacogn Phytochem. 2016;5(5):271-276.
- Oladimeji FA, Akinkunmi EO, Raheem AI, Abiodun GO, Bankole VO. Evaluation of topical antimicrobial ointment formulations of essential oil of Lippia multiflora Moldenke. Afr J Tradit Complement Altern Med. 2015;12(5):135-144.
Crossref - Samba N, Aitfella-Lahlou R, Nelo M, et al. Chemical composition and antibacterial activity of Lippia multiflora Moldenke essential oil from different regions of Angola. Molecules. 2021;26(1):155.
Crossref - Kalbassi S, Yarahmadi M, Mohammadifard H, Ahmadi F. The antibiofilm and antibacterial effects of medicinal plant extracts on isolated sulfate-reducing bacteria from orthodontic appliances. Food Sci Technol Campinas. 2022;42(4):1-9.
Crossref - Fuente-Nunez C, Korolik V, Bains M, et al. Inhibition of bacterial biofilm formation and swarming motility by a small synthetic cationic peptide. Antimicrob Agents Chemother. 2012;56(5):2696-2704.
Crossref - Abebe GM. The role of bacterial biofilm in antibiotic resistance and food contamination. Int J Microbiol. 2020;1705814.
Crossref - Gondil VS, Subhadra B. Biofilms and their role on diseases. BMC Microbiol. 2023;23:203.
Crossref - Asma ST, Imre K, Morar A, et al. An overview of biofilm formation-combating strategies and mechanisms of action of antibiofilm agents. Life. 2022;12(8):1110.
Crossref - Marques CNH, Davies DG, Sauer K. Control of biofilms with the fatty acid signaling molecule cis-2-decenoic acid. Pharmaceuticals. 2015;8(4):816-835.
Crossref - Nejatbakhsh S, Ilkhanizadeh-Qomi M, Razzaghi-Abyaneh M, Jahanshiri Z. The effects of ellagic acid on growth and biofilm formation of Candida albicans. J Med Microbiol Infect Dis. 2020;8(1):14-18.
Crossref - Raorane CJ, Lee J, Kim Y, Rajasekharan SK, Garcia-Contreras R, Lee J. Antibiofilm and antivirulence efficacies of flavonoids and curcumin against Acinetobacter baumannii. Front Microbiol. 2019;10(990):1-12.
Crossref - Sun B, Luo H, Jiang H, Wang Z, Jia A. Inhibition of quorum sensing and biofilm formation of esculetin on Aeromonas hydrophila. Front Microbiol. 2021;12:1-9.
Crossref - Compaore E, Ouedraogo V, Compaore M, Rouamba A, Kiendrebeogo M. Anti-quorum sensing potential of Ageratum conyzoides L. (Asteraceae) extracts from Burkina Faso. J Med Plant Res. 2022;16(5):174-187.
Crossref - Ouedraogo V, Kiendrebeogo M. Methanol extract from Anogeissus leiocarpus (DC) Guill. and Perr. (Combretaceae) stem bark quenches the quorum sensing of Pseudomonas aeruginosa PAO1. Medicines. 2016;3(4):26.
Crossref - Karama K, Ouedraogo V, Rouamba A, Compaore M, Kiendrebeogo M. Anti-quorum sensing and anti-biofilm activities of Securidaca longepedunculata Fresen, an endangered species from Burkina Faso. Afr J Microbiol Res. 2021;15(3):146-151.
Crossref - Schellenberger R, Crouzet J, Nickzad A, et al. Bacterial rhamnolipids and their 3-hydroxyalkanoate precursors activate Arabidopsis innate immunity through two independent mechanisms. Proc Natl Acad Sci U S A. 2021;118 (39):e2101366118.
Crossref - David AC, Jozef A. Twitching motility in Legionella pneumophila. FEMS Microbiol Lett. 2009;293(2):271-277.
Crossref - Daichi T, Ikuko F, Yuya S, Akihiro N, Katsumi I, Makoto M. ATP dependent polymerization dynamics of bacterial actin proteins involved in Spiroplasma swimming. Open Biol. 2022;12(10):220083.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.