ISSN: 0973-7510
E-ISSN: 2581-690X
Polycyclic aromatic hydrocarbons (PAHs) are organic compounds, composed of two or more fused benzene rings sourced from unburned fossil fuels and petroleum, recognized for their wide presence in the environment and harmful impacts on human health and ecosystems. PAHs pose remarkable challenges to living habitats due to their mutagenic properties. Fluorene is a low-molecular-weight heterocyclic aromatic hydrocarbon with multiple industrial applications, for example, pigments, fluorescents, and pharmaceuticals. Its xenobiotic activities on living cells may lead to severe health concerns including cancer and organ damage. The degradation of PAHs through several physicochemical reactions is costly, labor-intensive, time-consuming, and detrimental to the environment. However, biodegradation of PAHs using microorganisms such as bacteria, fungi, and algae leads to a sustainable and cost-effective method. Despite ongoing research, finding potent microbial strains capable of degrading PAHs comprehensively is a significant challenge. This review highlights the toxicity of PAHs (especially fluorene) on the environment and summarizes effective microorganisms and their approaches for meaningful PAH bioremediation.
Polycyclic Aromatic Hydrocarbon, Fluorene, Microorganisms, Biodegradation, Bioremediation
The environment is consistently getting polluted by diverse anthropogenic causes, such as the overconsumption of fossil fuels and biomass. Petroleum is a vast reservoir of thousands of organic compounds, but some chemicals are directly and indirectly harmful to humans, animals, and plants. Without any concern, we are using these toxic organic pollutants in our daily lives, which lead to fatal diseases like cancer, lung and liver dysfunction, skin and brain disorders, kidney failure, reproductive infertility, etc.1,2
Polycyclic aromatic hydrocarbons (PAHs), composed of multiple benzene rings, like Naphthalene, Anthracene, Phenanthrene, Fluorene, Pyrene, Dibenzothiophene, and several thousand of their derivatives are present in soil, air, and water as a group of organic priority pollutants. They have direct and indirect toxic, genotoxic, mutagenic, or carcinogenic impacts on plants, animals, human health, and microorganisms.3-5 PAHs bind with environmental pollutants and become more rigid and toxic than before, as a result, they are not easily degradable. Most of the PAHs are hydrophobic, thermostable, and have heterocyclic aromatic ring configurations like fluorene, so they are resistant and highly persistent to the environment.6 PAHs are widely used for various purposes such as agrochemicals, dyes, electronics, fluorescent, liquid crystals, pigments, pesticides, pharmaceuticals, resins, roofing tar, thermoset functional plastic, and so on.7
The main sources of PAHs are fossil fuels such as petroleum (oil), coal, and gas, plant-biomass, garbage, and narcotic plants like tobacco. PAHs are exerted as byproducts when those organic compounds are combusted incompletely.8,9 Some PAHs are produced in the reservoir, but some are synthesized or converted from large organic polymers. Seepage and spillage of fossil fuels, industrial waste, stormwater runoff, and volcanic eruptions are also the key sources of fluorene and other PAHs in surface water, and groundwater.10,11
Polycyclic aromatic hydrocarbons enter humans and other organisms through direct inhalation of carbon engine exhaust, cigarette smoke, and wood smoke, consumption of grilled or charred meats, contaminated bread, processed and pickled foods, wastewater, and polluted cow milk.7 PAHs arrive in the body through skin absorption, or oral administration, then gather in adipose tissue, liver, and kidney through the chylomicron process, and subsequently lead to many known and unknown diseases at cellular and genomic levels.12,13 PAHs can alter gene regulation and lead to disorders in the future, and even this harmful impact may go to the next generation.2
Physical, chemical, and biological procedures can degrade the toxic PAHs.14 Anyways, physical, and chemical degradation processes are costly and time-consuming, moreover, both are not suitable for huge, contaminated areas like the sea, landfills, gasworks, and so on. Therefore, the biological process can be better than others. Bioremediation of PAHs refers mainly to microbial degradation by bacteria and fungi and phytoremediation by plants.15,16
In this study, we described to find how PAHs impact human health and the environment, the general mechanism of their degradation, and potential microbial sources that could biodegrade PAHs remarkably. The objective of this review is to lead to conclusive research in the future.
General properties of PAHs
PAHs are composed of two or more fused benzene cycles, as naphthalene is the smallest polycyclic aromatic hydrocarbon bearing only two benzene rings, and anthracene is composed of three rings. In the PAH structure, the common key elements are Hydrogen, Carbon, and Oxygen, and the structure is stable due to the ring’s resonance ability of pi (π) bonds, however, some other chemical elements or compounds can bind with or between carbon rings to initiate new PAHs, for instance, Dibenzothiophene has a sulfur.14 Several thousands of PAH derivatives exist on the earth, according to their structures and molecular weights PAHs can be classified into low molecular weight (LMW: <202 g/mol, 2-3 fused benzene rings), middle molecular weight (MMW: <228 g/mol, 4 rings), and higher molecular weight (HMW: >228 g/mol, >4 rings).17 The physicochemical properties of PAHs differ from one to another based on their molecular weight, nevertheless, almost all PAHs form crystals, and a few are soluble in water, while most of them are soluble in organic solvents like acetone or ethanol.
Fluorene is a white crystal organic compound obtained from coal tar that emits an aromatic smell like naphthalene. It is flammable and has a violet fluorescence. It is normally insoluble in water, but a small amount (1.69 mg/L) is soluble at 25 °C. It is widely used in dyes, polymers, electronic devices, sensors, and photochromic materials, and as a talented blue emitter for organic light-emitting diodes.18
Toxicity of Fluorene and other PAHs
Fluorene is one of the highly toxic polycyclic aromatic hydrocarbons, broadly scattered in the contaminated water and dry land ecosystems. Its toxicity affects plants, animals, algae, fungi, and even some bacteria; human beings are the most vulnerable victims of it.19 Hsieh et al. discovered the reproductive, developmental toxicity and immunotoxicity of fluorene and other PAHs through in silico, in vitro, and in vivo methods. They found that polycyclic aromatic compounds (PACs) like fluorene could have diverse toxicity profiles, for instance, genotoxicity or carcinogenicity (in silico toxicity), xenobiotic homeostasis and stress response (in vitro activity), and enriched toxicity in conjunction with the availability of carcinogenicity (in vivo activity).20
The terminal differentiation of mouse embryonic stem cells (mESCs), and embryonic bodies (EBs) was affected by Fluorene-9-bisphenol (BHPF), a derivative of fluorene. The study revealed that BHPF exposure led to loss of self-renewal and pluripotency in mESCs by increasing the expression of the inflammatory gene IL6. Moreover, BHPF altered the terminal differentiation pathway, controlling the expression of 16 genes that related to different cell types, for example, cardiomyocytes, keratinocyte epithelium, lymphatic endothelium, macrophages, monocytes, nephrons proximal tubule cells, neurons, pancreatic beta cells, retinal ganglion cells, and T-cells.21
Jia et al. demonstrated that fluorene had strong antiestrogenic adverse effects on female development. They conducted some tests on CD-1 mice and found endocrine-disrupting properties in adolescent mice by multiple toxicological bioassays.22 Benzo[b]fluoranthene (B[b]F), a derivative of fluorene, synthesized glutathione (GSH) in HepG2 cells (liver cells). GSH levels were increased up to 3-fold by B[b]F than control levels, and constant cytotoxicity IC50 > 100 µM after 24, 48, and 72 hours.23
Fluorene-9-bisphenol also had a toxic effect on freshwater algae, Chlorella vulgaris, which was sensitive to BHPF at a concentration of >0.1 mg/L, and lipid peroxidation was remarkably increased. Besides, the oxidative stress caused by BHPF, the activities of superoxide dismutase (SOD) were notably declined in algal cells by >0.5 mg/L of BHPF.24
Polycyclic aromatic hydrocarbon contamination is widespread, particularly in countries involved in oil production and processing. Saudi Arabia is one of the petroleum-producing countries, contaminated with several PAHs. Al-Daghri et al. reported that they found at least 6 PAHs, ranging between 54.5% and 90.9% positive in the blood serum of some experienced teenagers (195 children of 17 years and below from 11 locations in Saudi Arabia were examined).25 Another research disclosed that 16 different PAHs were determined from 50 locations in Riyadh City. The concentrations of PAHs in road dust were from 0.01-126 ng/g. Children at high risk for asthma suffered more from PAH exposure dose by ingestion and dermal absorption than adults in the city.26
The abundant presence and toxicity of fluorene and other PAHs in our environment remind us to mitigate them through a harmless and cost-effective process like biodegradation. Numerous studies have identified several bacteria, archaea, fungi, and algae, and their co-cultures, for PAH bioremediation. However, the key procedure of the degradation is poorly understood (Table 1).
Table (1):
Toxicity of PAHs on human health and other species
PAHs |
Implementation |
Toxicity / Dose |
Impact |
Ref. |
|---|---|---|---|---|
Naphthalene Fluorene Phenanthrene Pyrene |
Healthy and non-smoking participants (n = 8), serum, and urinary metabolites. |
0.02 mg/Kg, 0.04 mg/Kg, 0.02 mg/Kg, and 0.03 mg/Kg |
The PAHs were absorbed and eliminated from the participants. |
[27] |
C3-Fluorene, Dibenzothiophene, and Naphthalene |
Skeletal muscle and liver samples from stranded Bigg’s Killer Whales and Southern Resident Killer Whales, in British Columbia. |
Mean: 632 ng/g (lipid weight) |
Efficient and preferential contaminant exposure to the fetus |
[28] |
2-Fluorene 3-Fluorene 9-Fluorene 1-Phenanthrene 1-Pyrene |
Non-smoker adults in the USA (n = 2691), urinary PAH metabolites. |
176.7 ng/L 68.7 ng/L 236.8 ng/L 112.2 ng/L 77.7 ng/L |
Most of the PAHs had inverse impacts on skin disease and obesity. |
[29] |
9,9-bis[4-(2-hydroxyethoxy) phenyl] Fluorene (BPEF) |
Immature female CD-1 mice. |
10 mg/kg, 30 mg/kg, and 90 mg/kg (body weight/day) BPEF in peanut oil |
BPEF had higher antiestrogenic effects that disrupted CD-1 mice female development |
[22] |
Fluorene |
Healthy and non-smoking Air Force employees in Denmark (n = 79), skin wipes, and urinary metabolites. |
15.9 ± 23.7 ng/g per 24h, where the reference was 5.28 ± 7.87 ng/g per 24h, P = 0.007) |
The exposure level of Fluorene was significantly higher than the reference group (Biomarker) |
[30] |
Fluorene-9-bisphenol (BHPF) |
Toxic effect of BHPF on green algae Chlorella vulgaris and its metabolites. |
>1.0 mg/L >0.1mg/L >0.5 mg/L |
C. vulgaris was sensitive to BHPF. Lipid peroxidation was increased. |
[24] |
Fluorene-9-bisphenol (BHPF) |
The livers of 20-day-old female CD-1 mice. |
2 mg/kg, 10 mg/kg, and 50 mg/kg (bw/3day) |
Leukocyte infiltration and cytoplasmic vacuolation were detected in the liver of the mouse. |
[31] |
Fluorene, and 4-OH -Phenanthrene (4-OH-PHE) |
In Sweden, chimney sweeps, Creosote-exposed, and unexposed workers (n = 151, 19, 152), urinary monohydroxylated metabolites. |
0.32 and 0.37 µg/g (Chimney), 53 and 1051 µg/g (Creosote), and 0.15 and 15 µg/g (healthy samples) |
PAH-exposed workers had a higher risk of cancer, and AHRR genes were markers for lung cancer risk. |
[32] |
Naphthalene, Phenanthrene, Benzo(a)Pyrene, Fluorene |
Asthmatic and healthy children with PAHs in serum (n = 195), in Saudi Arabia. |
26.2 and 10.7ng/ml 20.3 and 6.2 ng/ml 4.8 and 2.1 ng/ml 3.6 and 2.5 ng/ml |
PAH pollution strongly influences childhood asthma. |
[25] |
The biodegradation of PAHs by microorganisms
Microorganisms, mainly bacteria and fungi can adapt themselves to various environmental conditions. They can tolerate many toxic molecules by changing their adaptation efficiency and gaining essential exogenous DNA from the environment. Mohapatra and Phale discovered several species of Proteobacteria, Firmicutes, and Actinobacteria from soil flora, which degrade several polycyclic aromatic hydrocarbons.33
Another group of researchers reported that Pseudomonas aeruginosa san ai degraded 96%, 50%, and 41% of 20 mg/L of fluorene, phenanthrene, and pyrene respectively, after seven days.34 Desta et al. pointed out that SMT-1 Pseudomonas sp. had a fluorene-degrading 4921-dioxygenase gene confirmed by primer-specific PCR. The 4921-dioxygenase enzyme exhibited the optimum activity at pH 7.5 and 25 °C in Tris-HCl buffer after 1 minute, as indicated by the reaction velocity.19 Some species of Nocardia showed potential bioremediation on polycyclic aromatic hydrocarbons, phenol, and sodium sulfate; among them, N. farcinica was the most prevalent contributor in PAHs biodegradation.35
A marine fungus named Aspergillus sydowii BOBA1 was studied to degrade spent engine oil and PAHs. The fungus carried several genes such as dioxygenase, decarboxylase, hydrolase, reductase, and peroxidase which were integrated into PAHs and xenobiotic metabolism.36 Another filamentous fungus, Mucor irregularis (strain bpo1), had degraded fluorene efficiently through the Box–Behnken Design (BBD) process with optimum parameters (pH-7, temperature-32.5 °C, substrate concentration 100 mg/L, and dry weight 2 g), and resulted in 81.50% fluorene degradation on 5th day.37
Struszczyk-wita et al. described that creosote, composed of aromatic hydrocarbons, is normally an undegradable compound. However, Bjerkandera adusta DSM-3375 mycelium contained Mold cells that obtained enzyme was used in the bioremediation of soil contaminated with creosote (2% w/w). The B. adusta degraded 35% of creosote and almost 73%, 79%, and 72% of fluoranthene, pyrene, and fluorene, respectively, after 15 weeks (Table 2).38
Table (2):
Bacterial and fungal species for biodegradation of PAHs
Species and strain |
Source of PAHs |
Degrade PAHs |
Level of degradation |
Method and Materials |
Ref. |
|---|---|---|---|---|---|
Rhodococcus sp. |
Previously purified strain |
PAHs (2 to 5 benzene rings) at 20mM concentration |
PAHs at 20mM level had no toxicity after 21 days. |
Luria–Bertani Broth (LB), GC–MS analysis. |
[39] |
Pseudomonas aeruginosa RS1 (MTCC 25391) |
Oily sludge, India |
Fluorene and Dibenzothiophene (25-500 mg/L) |
0.14mg Fluorene and 0.18 mg Dibenzothiophene utilization per day. |
Nutrient broth. PDA detection at 254nm wavelength |
[40] |
Pseudomonas sp. (SMT-1) |
Previously studied strain |
9-Fluorenone, (0.1-0.5 mM) |
The highest cell growth of SMT-1 was below 0.5mM fluorene. |
Mineral salt medium (MSM), HPLC, and LC-MS analysis |
[19] |
Mucor irregularis (bpo) (Fungus) |
Marine-soil, Nigeria |
Fluorene (50mg/L) |
Height 79.80% fluorene degradation after 7 days. |
MSM, Potato dextrose agar, Malt extract agar, GC–MS analysis. HPLC at 255 nm. |
[37] |
Nocardia fuminea N. farcinica and N. kroppenstedtii |
Wastewater, salt water, fresh water, and soil samples, in Iran |
1% mix of 16 PAHs including fluorene (0.2mg/ml) |
The strains degraded 70% – 90% of PAHs. |
Mineral Salt Medium, HPLC analysis at 254nm |
[35]
|
Pseudomonas aeruginosa (san ai) |
Alkaline cutting oil |
Fluorene, pyrene, phenanthrene (20mg/L) |
96%, 41%, and 50% degradation respectively after 7 days. |
MSM, 2D GC-MS analysis |
[34]
|
Marasmiellus sp. (CBMAI 1062) (Fungus) |
Marine-derived basidiomycetes |
Pyrene and B[a]P (0.04 and 0.02 mg/ml) |
Degraded more than 90% of both pollutants after 7 days. |
Malt extract agar (MA2), GC–MS analyses |
[41] |
Cladosporium sp. (CBMAI 1237) (Fungus) |
Marine sponges |
PAHs (50mg/ml) |
Anthracene (71%), Fluorene (70%), and Pyrene (62%) degradation after 14 days. |
Malt 2% Medium HPLC, and GC–MS analysis. |
[42] |
Coriolopsis byrsina (APC5) (Fungus) |
Decayed wood surface |
Pyrene |
Efficiently 96.1% pyrene degraded within 18 days. |
Mineral Salt Broth, FTIR, and GC–MS analysis. |
[43] |
Microbial culture for PAHs biodegradation
According to previous research, bacteria and fungi were collected from contaminated soil, wastewater, sediments, and marine water, and sponges. It seemed that the microbes grown in PAH-contaminated environments can degrade PAHs. The studies targeted to find out the most efficient species of microbes to degrade PAHs. However, the strains of microbes were developed by repeated subcultures on a suitable medium and abiotic parameter or stress (pH, temperature, salt concentration, and metal or compound tolerance). In most cases, we noticed that microbes were cultured in Mineral Salt Medium (MSM) with different PAH doses, moreover, other media like marine agar, nutrient broth, etc. were also used.44,45 To detect the PAH metabolism by microorganisms, sophisticated chromatographic techniques like High-Performance Liquid Chromatography (HPLC), Gas Chromatography (GC), Thin Layer Chromatography (TLC), Mass Spectrophotometry (MS), and Fourier-Transform Infrared Spectroscopy (FTIS) were used (Table 3), and to execute these experiments some essential chemicals like Anhydrous Na2SO4, methanol, n-hexane, and nitrogen gas were also used.14,19,45
Table (3):
Characteristics of biodegrading microbial species
| Species (Strain) | Sources | Media | Physical properties (tolerance) | Ref. | ||
|---|---|---|---|---|---|---|
| pH | Temp. | Salt/Metal | ||||
| Rhodococcus sp. | Purified strain | LB with PAHs | 7.0 | 28°C | 0.5% | [39] |
| Bacterial isolates (Sp1, Sp2, Sp3, and Sp4) | Seawater and Marine Sponge | Marine Agar with PAHs | 6.8 – 7.2 | 29 – 31°C | 30 – 31% | [14] |
| Aspergillus sydowii (BOBA1) Fungus | Marine sediment | MSM with SE | 4.0 – 10.0 (Opt.: 5.0) | 10 – 40°C (Opt.: 25°C) | 1-10% (w/v) (Opt.: 4%) | [36] |
| Mucor irregularis (bpo) Fungus | Marin soil | MSM with FLU | 7.0 | 29°C | 0.5% | [37] |
| Pseudomonas sp. SMT-1 | Previously studied | MSM with FLU | 7.0 | 20 – 42°C (Opt.: 30°C) | 0.5% | [19] |
| Nocardia sp. | Wastewater, Soil, and Sediment | Sauton’s Medium with PAH | 6.8 – 8.2 6.2 – 7.8 6.4 – 8.0 | 5 – 28°C 6 – 32°C 4 – 29°C | CPC 0.005% | [35] |
| Pseudomonas aeruginosa | Alkaline cutting oil | MSM with FLU, PYR, and PHE | up to 9.8 | 30°C | NaCl 0.5% Cd 7.3 mM, Cr 5.0 mM | [34] |
| Coriolopsis byrsina (APC5) (Fungus) | decayed wood surface | Mineral Salt Broth with PYR | 3.0–8.0 (Opt.: 6.0) | 15–55 °C (Opt.: 25°C) | 1 – 3.2% | [43] |
| Pleurotus pulmonarius (FO43) (Fungus) | Tropical rain forest | MMB with PYR | 2.0 – 10.0 (Opt.: 5.0) | 04–60 °C (Opt.: 25°C) | 0.5 % Tween 80 | [8] |
| Penicillium janthinellum, and P. Terrestre, (Fungi) | Soil from a gasworks site | BSM with PYR | 5.0 | 22°C | 0.5% | [46] |
General pathways of PAH degradation
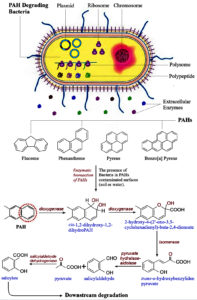
The mechanism of PAH degradation needs several catabolic reactions in the presence of specific enzymes (Table 4). Some bacteria such as Pseudomonas sp., Rhodococcus sp., Mycobacterium sp., and Staphylococcus sp., for example, start PAH catabolism with ring hydroxylation and oxygenation to activate benzene rings.47 The hydroxylated and dihydrodiol intermediates undergo dehydrogenation and yield the central intermediates catechols or protocatechuates. Enzyme, dioxygenase performs an ortho and a meta-cleavage of catechols to muconic acids by completing the tricarboxylic acid (TCA) cycle and finally degrades the PAH into the end-product as carbon dioxide (CO2).48 On the other hand, most fungi cannot use PAHs as their main carbon sources but can co-metabolize PAHs to other products. However, ligninolytic fungi like Aspergillus sp., Cladosporium sp., Cunninghamella sp., and Penicillium sp., have PAHs catabolic ability (2-5 rings). They primarily oxidize PAH by the cytochrome P450 and oxygen to form unstable arene oxide intermediates that are later converted into tarns-dihydrodiols and phenols, and finally to glucosides, glucuronides, xylosides, methoxyls, and sulfates (Figure). Whereas the white-rot fungi produce extracellular ligninolytic enzymes, such as lignin peroxidases, manganese, and laccases that oxidize PAHs in the presence of H2O2 to quinones. Finally, the fungi cleave quinones to CO2.49,50
Table (4):
The sources and function Enzymes for PAH bioremediation
Species |
Enzyme(s) |
Activities |
Ref. |
|---|---|---|---|
Pseudomonas sp. (SMT-1) |
4921-Dioxygenase |
The metal salt (FeCl3) exhibited the enzyme’s low activity in Fluorene degradation; the enzyme was most active at pH 7.5 and 25 °C in Tris–HCl buffer. |
[19] |
Aspergillus sydowii (BOBA1) Fungus |
Dioxygenase, Decarboxylase, Hydrolase, Reductase, and Peroxidase |
These enzymes have a potential role in PAHs and xenobiotic compound metabolism. |
[36] |
Nocardioides sp. (KP7) |
Dihydrodioldehydrogenase |
The enzyme is involved in phenanthrene degradation. |
[51] |
Mycobacterium vanbaalenii (PYR-1, and 6PY1) |
Ring-hydroxylating oxygenase (α and β subunits), Aldehyde dehydrogenase, Dihydrodiol dehydrogenase, Ring cleavage dioxygenase, and Hydratase-aldolase |
The enzymes are the parts of the o-phthalate and the beta-ketoadipate pathway for PAH degradation. |
[51-
53] |
Coriolopsis byrsina (APC5) Fungus |
Ligninolytic enzyme |
C. byrsina produced a significant amount of enzyme. |
[43] |
Cycloclasticus sp. (78-ME) |
Dioxygenase (pahA1-4 gene cluster) |
The enzyme and gene products had significant PAHs degrading capacities up to 5 rings. |
[54] |
Alteromonas sp. (SN2) |
Naphtalene dioxygenase (nahAc/NDO) |
The enzyme functions in gentisate and catechol metabolic pathways and degrades naphthalene (PAH). |
[55] |
Polycyclic aromatic hydrocarbons are naturally occurring organic molecules originating in the mine of petroleum, discharged into the environment with industrial wastes and incomplete combustion of fossil fuels.58 The toxicity of PAHs is directly proportional to their molecular weight, which means the heavier, the more toxic to the victims. Nevertheless, low molecular weight PAHs like fluorene and its derivatives are also harmful.19 Fluorene can affect embryonic stem cells, neurons, lymphatic cells, and T-cells and can regulate several genes abnormally.21
The common procedures to detect the presence and quantity of PAHs, even their degradation level and byproducts production, HPLC, TLC, paper chromatography, GC-MS, SFC-MS, and FTIR UV-spectrophotometer are used and analyzed.59 The optimum wavelength for PAH detection was examined at 254-255 nm, and other chemicals like n-hexane, methanol, liquid nitrogen, etc. were used to prepare samples for analysis.35,37
The key components of PAH biodegradation are enzymes that enhance the rate of biochemical reactions for stepwise degradation of PAHs. The most crucial enzymes for degradation are the Ligninolytic enzymes like decarboxylase, dioxygenase, hydrolase, oxidase, and reductase. During PAH catabolism several intermediates are produced in different steps; finally, the TCA cycles are completed by yielding simpler molecules and some byproducts.36,43
The bioremediation of PAHs is executed by biological tools like microorganisms and plants. The degradation by plants is known as phytoremediation, and degradation by microbes is well-known as biodegradation. A lot of bacteria and fungi are around us which can combat from LMW PAHs to HMW PAHs using their metabolites. PAHs contaminated soil and marine resources are the main targets for collecting samples of biodegradable species. After a fruitful selection by lab experiments, the strains are identified through DNA sequencing and analyzing alignment with the previous database via Bioinformatic tools.34,35
According to previous studies some efficient species of bacteria are Sphingobium sp., Bacillus licheniformis, Sphingobacterium sp., Pseudomonas aeruginosa, Massili, Bacillus, Coriolopsis byrsina, which could degrade PHAs for more than 50%-95% from the culture. On the other hand, some potential fungi are Penicillium janthinellum, Pleurotus pulmonarius, Coriolopsis byrsina, Cladosporium sp., Marasmiellus sp, Aspergillus sydowii, etc; they could degrade more than 70%-98% PAHs from the culture media.8,46
The suitable media for biodegradation were MSM, LB, Marin agar, Nutrient broth, Sauton’s broth, BSM, and MMB; both solid and broth were used in various previous studies. Most of the strains had better growth at around pH 7.0 and temperature 22-25 °C, though some strains could grow a wide range of pH (2.0-10.0) and temperature 4.0-60.0 °C, and the overall time range was between 7 and 30 days for degradation.41,42,60
This study focuses on PAHs, their toxicity, degradation mechanisms, necessary methods, materials, and microbial species that excellently degrade PAHs. The future perspective is to search for and develop a novel microbe that can degrade many PAHs significantly, to mitigate toxic PAHs from the environment.
The use of PAHs is increasing regularly from the industries to the consumers which consequently poses a permanent danger to human beings as well as other species. It is high time to make sensitization about the safe use of toxic organic compounds like fluorene. PAHs pollute the environment in three directions (soil, air, and water). Contaminated soil is dangerous for plants, crops, and animals, whereas air pollution with PAHs increases the risk of liver cancer and respiratory disorders especially in children. It has been uncovered that long-time exposure to high molecular weight PAHs can alter DNA in some organisms, CD-1 mice, for instance. Hopefully, many marine symbiont bacteria and fungi have the potential to degrade PAHs significantly, such as Aspergillus sydowii, Mucor irregularis, and Marasmiellus sp. However, some species degrade one or two PAHs separately, but our focus should be to find a unique species that can realistically mitigate multiple PAHs.
ACKNOWLEDGMENTS
The project was funded by KAU Endowment (WAQF) at King Abdulaziz University, Jeddah, Saudi Arabia. The authors, therefore, acknowledge with thanks WAQF and the Deanship of Scientific Research (DSR) for technical and financial support.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
The study was funded by KAU Endowment (WAQF) at King Abdulaziz University, Jeddah, Saudi Arabia.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Patel AB, Shaikh S, Jain KR, Desai C, Madamwar D. Polycyclic Aromatic Hydrocarbons: Sources, Toxicity, and Remediation Approaches. Front Microbiol. 2020;11:562813.
Crossref - Zhang J, Wang K, Guo J, et al. Study on the mechanism of liver toxicity induced by acenaphthene in zebrafish. Ecotoxicol Environ Saf. 2023;249:114441.
Crossref - Ghosal D, Ghosh S, Dutta TK, Ahn Y. Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs) A review. Front Microbiol. 2016;7:1369.
Crossref - Marzooghi S, Toro DMD. A critical review of polycyclic aromatic hydrocarbon phototoxicity models. Environ Toxicol Chem. 2017;36(5):1138-1148.
Crossref - Parhamfar M, Abtahi H, Godini K, et al. Biodegradation of heavy oily sludge by a two-step inoculation composting process using synergistic effect of indigenous isolated bacteria. Process Biochem. 2020;91:223-230.
Crossref - Barathi SJG, Rathinasamy G, Sabapathi N, et al. Recent trends in polycyclic aromatic hydrocarbons pollution distribution and counteracting bioremediation strategies. Chemosphere. 2023;337:139396.
Crossref - Abdel-Shafy HI, Mansour MSM. A review on polycyclic aromatic hydrocarbons:Source, environmental impact, effect on human health and remediation. Egypt J Petrol. 2016;25(1):107-123.
Crossref - Hadibarata T, Teh ZC. Optimization of pyrene degradation by white-rot fungus Pleurotus pulmonarius F043 and characterization of its metabolites. Bioprocess Biosyst Eng. 2014;37(8):1679-1684.
Crossref - Wang Z, Ren P, Su, Y, et al. Gas/particle partitioning of polycyclic aromatic hydrocarbons in coastal atmosphere of the north Yellow Sea, China. Environ Sci Pollut Res. 2013;20(8):5753-5763.
Crossref - Akinyeye RO, Adebawore AA, Awokunmi EE, Olanipekunl EO. Evaluation of polycyclic aromatic hydrocarbons in water from hand-dug wells at Ile-Oluji, Nigeria. IOSR J Environ Sci Toxicol Food Technol. 2016;10(9):112-119.
Crossref - Salam LB, Ilori MO, Amund OO. Properties, environmental fate and biodegradation of carbazole. 3 Biotech. 2017;7(2):111.
Crossref - Harris KL, Banks LD, Mantey JA, et al. Bioaccessibility of polycyclic aromatic hydrocarbons: relevance to toxicity and carcinogenesis. Expert Opin Drug Metab Toxicol. 2013;9(11):1465-8140.
Crossref - Kozak K, Ruman M, Kosek K, Karasinski G, Stachnik L, Polkowska Z. Impact of Volcanic Eruptions on the Occurrence of PAHs Compounds in the Aquatic Ecosystem of the Southern Part of West Spitsbergen (Hornsund Fjord, Svalbard). Water. 2017;9(1):42.
Crossref - Marzuki I, Asaf R, Paena M, et al. Anthracene and Pyrene Biodegradation Performance of Marine Sponge Symbiont Bacteria Consortium. Molecules. 2021;26(22):6851.
Crossref - Liu Q, Li Q, Wang N, Liu D, et al. Bioremediation of petroleum-contaminated soil using aged refuse from landfills. Waste Manage. 2018;77:576-585.
Crossref - Sayed K, Baloo L, Sharma NK. Bioremediation of Total Petroleum Hydrocarbons (TPH) by Bioaugmentation and Biostimulation in Water with Floating Oil Spill Containment Booms as Bioreactor Basin. Int J Environ Res Public Health. 2021;18(5):2226.
Crossref - Brozman O, Novak J, Bauer AK, Babica P. Airborne PAHs inhibit gap junctional intercellular communication and activate MAPKs in human bronchial epithelial cell line. Environ Toxicol Pharmacol. 2020;79(S3):103422.
Crrossref - Ziarani GM, Moradi R, Lashgari N Kruger HG. Degradation of Polycyclic Aromatic Hydrocarbons by Fungi . In: Timmis KN. (eds) Handbook of Hydrocarbon and Lipid Microbiology. Springer, Berlin, Heidelberg. 2018:153-164.
Crossref - Desta M, Zhang L, Wang W, Xu P, Tang H. Molecular mechanisms and biochemical analysis of fluorene degradation by the Pseudomonas sp. SMT-1 strain. 3 Biotech. 2021;11(9):416.
Crossref - Hsieh JH, Sedykh A, Mutlu E, Germolec DR, Auerbach SS, Rider CV. Harnessing In Silico, In Vitro, and In Vivo Data to Understand the Toxicity Landscape of Polycyclic Aromatic Compounds (PACs). Chem Res Toxicol. 2021;34(2):268-285.
Crossref - McLaughlin AJ, Kaniski AI, Matti DI, Monear NC, Tischler JL, Xhabija B. Fluorene-9-bisphenol affects the terminal differentiation of mouse embryonic bodies. Curr Res Toxicol. 2023;5:100133.
Crossref - Jia X, Mao X, Zhou Y, et al. Antiestrogenic property of 9,9-bis[4-(2-hydroxyethoxy) phenyl] fluorene (BPEF) and its effects on female development in CD-1 mice. Ecotoxicol Environ Saf. 2022;242(1):113906.
Crossref - Branco V, Matos B, Mourato C, Diniz M, Carvalho C, Martins M. Synthesis of glutathione as a central aspect of PAH toxicity in liver cells:a comparison between phenanthrene, Benzo[b]Fluoranthene and their mixtures. Ecotoxicol Environ Saf. 2021;208:111637.
Crossref - Zhang H, Ding T, Luo X, Li J. Toxic effect of fluorene-9-bisphenol to green algae Chlorella vulgaris and its metabolic fate. Ecotoxicol Environ Saf. 2021;216:112158.
Crossref - Al-Daghri NM, Alokail MS, Abd-Alrahman SH, Draz HM. Polycyclic aromatic hydrocarbon distribution in serum of Saudi children using HPLC-FLD:marker elevations in children with asthma. Environ Sci Pollut Res. 2014;21(20):12085-12090.
Crossref - El-Saeid MH, Alghamdi AG, Alzahrani AJ. Impact of Atmospheric Polycyclic Aromatic Hydrocarbons (PAHs) of Falling Dust in Urban Area Settings:Status, Chemical Composition, Sources and Potential Human Health Risks. Int J Environ Res Public Health. 2023;20(2):1216.
Crossref - Choi JW, Kim M, Song G, et al. Toxicokinetic analyses of naphthalene, fluorene, phenanthrene, and pyrene in humans after single oral administration. Sci Total Environ. 2023;870:161899.
Crossref - Lee K, Raverty S, Cottrell P, et al. Polycyclic aromatic hydrocarbon (PAH) source identifcation and a maternal transfer case study in threatened killer whales (Orcinus orca) of British Columbia, Canada. Sci Rep. 2023;13(1):22580.
Crossref - Wang Y, Zhu L, James-Todd T, Sun Q. Urinary polycyclic aromatic hydrocarbon excretion and regional body fat distribution:evidence from the U.S. National Health and Nutrition Examination Survey 2001-2016. Environ Health. 2022;21(1):75.
Crossref - Andersen MHG, Saber AT, Frederiksen M, et al. Occupational exposure and markers of genetic damage, systemic inflammation and lung function:a Danish cross-sectional study among air force personnel. Sci Rep. 2021;11(1):17998.
Crossref - Yang L, Guo X, Mao X, et al. Hepatic toxicity of fluorene-9-bisphenol (BHPF) on CD-1 mice. Ecotoxicol Environ Saf. 2021;219:112298.
Crossref - Alhamdow A, Essig YJ, Krais AM, et al. Fluorene exposure among PAH-exposed workers is associated with epigenetic markers related to lung cancer. Occup Environl Med. 2020;77(7):488-495.
Crossref - Mohapatra B, Phale PS. Microbial Degradation of Naphthalene and Substituted Naphthalenes:Metabolic Diversity and Genomic Insight for Bioremediation. Front Bioeng Biotechnol. 2021;9:602445.
Crossref - Medic A, Ljesevic M, Inui H, et al. Efficient biodegradation of petroleum n-alkanes and polycyclic aromatic hydrocarbons by polyextremophilic Pseudomonas aeruginosa san ai with multidegradative capacity. RSC Adv. 2020;10(24):14060-14070.
Crossref - Azadi D, Shojaei H. Biodegradation of polycyclic aromatic hydrocarbons, phenol, and sodium sulfate by Nocardia species isolated and characterized from Iranian ecosystems. Sci Rep. 2020;10(1):21860.
Crossref - Kumar, AG, Manisha D, Sujitha K, Peter DM, Kirubagaran R, Dharani G. Genome sequence analysis of deep-sea Aspergillus sydowii BOBA1 and effect of high pressure on biodegradation of spent engine oil. Sci Rep. 2021;11(1):9347.
Crossref - Bankole PO, Semple KT, Jeon BH, Govindwar SP. Biodegradation of fluorene by the newly isolated marine-derived fungus, Mucor irregularis strain bpo1 using response surface methodology. Ecotoxicol Environ Saf. 2021;208:111619.
Crossref - Struszczyk-Swita K, Drozdzynski P, Murawska K, Marchut-Mikolajczyk O. PUF-Immobilized Bjerkandera adusta DSM 3375 as a Tool for Bioremediation of Creosote Oil Contaminated Soil. Int J Mol Sci. 2022;23(20):12441.
Crossref - Ivshina IB, Krivoruchko AV, Kuyukina MS, Peshkur TA, Cunningham CJ . Adhesion of Rhodococcus bacteria to solid hydrocarbons and enhanced biodegradation of these compounds. Sci Rep. 2022;12(1):21559.
Crossref - Ghosh P, Mukherji S. Growth kinetics of Pseudomonas aeruginosa RS1 on fuorene and dibenzothiophene, concomitant degradation kinetics and uptake mechanism. 3 Biotech. 2021;11(4):195.
Crossref - Vieira GAL, Magrini MJ, Bonugli-Santos RC, Rodrigues MVN, Sette LD. Polycyclic aromatic hydrocarbons degradation by marine-derived basidiomycetes:optimization of the degradation process. Braz J Microbiol. 2018;49(4):749-756.
Crossref - Birolli WG, Santos DdeA, Alvarenga N, Garcia ACFS, Romao LPC, Porto ALM. Biodegradation of anthracene and several PAHs by the marine-derived fungus Cladosporium sp. CBMAI 1237. Mar Pollut Bull. 2018;129(2):525-533.
Crossref - Agrawal N, Shahi SK. Degradation of polycyclic aromatic hydrocarbon (pyrene) using novel fungal strain Coriolopsis byrsina strain APC5. Int Biodeterior Biodegrad. 2017;122:69-81.
Crossref - Jimenez-Volkerink SN, Vila J, Jordan M, Minguillon C, Smidt H, Grifoll M. Multi-Omic Profiling of a Newly Isolated Oxy-PAH Degrading Specialist from PAH-Contaminated Soil Reveals Bacterial Mechanisms to Mitigate the Risk Posed by Polar Transformation Products. Environ Sci Technol. 2023;57(1):139-149.
Crossref - Marzuki I, Ali MY, Syarif HU, et al. Investigation of Biodegradable Bacteria as Bio indicators of the Presence of PAHs Contaminants in Marine Waters in the Marine Tourism Area of Makassar City. IOP Conf Ser Earth Environ Sci. 2021;750:012006.
Crossref - Saraswathy A, Hallberg R. Degradation of pyrene by indigenous fungi from a former gasworks site. FEMS Microbiol Lett. 2002;210(2):227-232.
Crossref - Cerniglia CE. Biodegradation of polycyclic aromatic hydrocarbons. Curr Opin Biotechnol. 1993;4(3):331-338.
Crossref - Kanaly RA, Harayama S. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J Bacteriol. 2000;182(8):2059-2067.
Crossref - Capotorti G, Cesti P, Lombardi A, Guglielmetti G. Formation of sulfate conjugates metabolites in the degradation of phenanthrene, anthracene, pyrene and benzo[a]pyrene by the ascomycete Aspergillus terreus. Polycycl Aromat Compd. 2005;25(3):197-213.
Crossref - Cerniglia CE, Sutherland JB. Degradation of Polycyclic Aromatic Hydrocarbons by Fungi. In: K.N. Timmis ed. Handbook of Hydrocarbon and Lipid Microbiology, Berlin, Heidelberg. 2010:2079-2110.
Crossref - Elyamine AM, Kan J, Meng S, Tao P, Wang H, Hu Z. Aerobic and Anaerobic Bacterial and Fungal Degradation of Pyrene: Mechanism Pathway Including Biochemical Reaction and Catabolic Genes. Int J Mol Sci. 2021;22(15):8202.
Crossref - Kweon O, Kim SJ, Holland RD, et al. Polycyclic aromatic hydrocarbon metabolic network in Mycobacterium vanbaalenii PYR-1. J Bacteriol. 2011;193(17):4326-4337.
Crossref - Kim SJ, Kweon O, Jones RC, Freeman JP, Edmondson RD, Cerniglia CE. Complete and integrated pyrene degradation pathway in Mycobacterium vanbaalenii PYR-1 based on systems biology. J Bacteriol. 2011;189(2):464-72.
Crossref - Messina E, Denaro R, Crisafi F, et al. Genome sequence of obligate marine polycyclic aromatic hydrocarbons-degrading bacterium Cycloclasticus sp. 78-ME, isolated from petroleum deposits of the sunken tanker Amoco Milford Haven, Mediterranean Sea. Mar Genomics. 2016;25:11-13.
Crossref - Jin HM, Kim JM, Lee HJ, Madsen EL, Jeon CO. Alteromonas as a key agent of polycyclic aromatic hydrocarbon biodegradation in crude oil-contaminated coastal sediment. Environ Sci Technol. 2012;46(14):7731-7740.
Crossref - Liang C, Huang Y, Wang H. pahE, a functional marker gene for polycyclic aromatic hydrocarbon-degrading bacteria. Appl Environ Microbiol. 2019;85(3):e02399-18.
Crossref - Zhang L, Qiao J, Cui H, Wang M, Li X. Using Low Molecular Weight Organic Acids to Enhance Microbial Degradation of Polycyclic Aromatic Hydrocarbons: Current Understanding and Future Perspectives. Water. 2021;13(4):446.
Crossref - Ambade B, Sethi SS, Kumar A, Sankar TK, Kurwadkar S. Health Risk Assessment, Composition, and Distribution of Polycyclic Aromatic Hydrocarbons (PAHs) in drinking water of Southern Jharkhand, East India. Arch Environ Contam Toxicol. 2021;80(1):120-133.
Crossref - Peng PL, Lim LH. Polycyclic aromatic hydrocarbons (PAHs) sample preparation and analysis in beverages:a review. Food Anal Methods. 2022;15(1):1042-1061.
Crossref - Lu L, Zhang J, Peng C. Shift of Soil Polycyclic Aromatic Hydrocarbons (PAHs) Dissipation Pattern and Microbial Community Composition due to Rhamnolipid Supplementation. Water Air Soil Pollut. 2019;230(5):107.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.