ISSN: 0973-7510
E-ISSN: 2581-690X
The dynamic drug-resistance characteristics of bacterial strains are excruciating, and various antibiotics have no discernible effect against life-threatening infections. The current study focuses on a strategy to increase the susceptibility of Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa, and Klebsiella pneumoniae against different antibiotics. Polyamines are the essential biogenic compounds found in prokaryotic and eukaryotic organisms. They carry out pivotal roles in the survival, growth, and tissue development of organisms. These small hydrocarbon molecules were found to be positively charged at physiological pH. Several studies have identified the role of polyamines in enhancing the sensitivity of bacteria towards antibiotics. The present study evaluates the desirable use of polyamines and their precursor, ornithine as potential synergistic agents to increase bacterial susceptibility. Putrescine and spermine supplementations showed the best effect on Gram-negative and Gram-positive bacteria, respectively. Results from the present study confirm the enhanced efficacy of antibiotics against bacteria when supplemented with polyamines.
Polyamines, Antibiotics, Antimicrobial Activity, Drug-resistance
Antibiotic resistance is precariously escalating to alarming levels. Increasing the efficacy of antibiotics, or new combinations which can improve the mode of action of the drugs, can give a solution to this problem. An expanding list of infections – such as tuberculosis, blood poisoning, pneumonia, gonorrhea, urinary tract infections, and foodborne diseases – are becoming tougher, and sometimes impractical to treat, as antibiotics turn out to be less efficient.1 Antibiotic resistance leads to elevated medical costs, extended hospital stays, and enhanced mortality.2 Antibiotic resistance can have dire consequences as it potentially affects industries such as veterinary, healthcare, and agriculture. However, it is essential to develop a revival strategy to amplify the antibiotic sensitivity of various antibacterial agents to beat the menace of antibiotic resistance.
The increase in untreatable infections and multidrug resistance can apparently damage our healthcare system catastrophically.3 Biotechnological interventions, such as genetic engineering, synthetic biology, and metagenomics, are increasingly used to discover and develop novel antimicrobial compounds from natural and engineered sources. These approaches enable targeted drug design, enhance production yields, and help overcome antimicrobial resistance.
Every living organism naturally produces polyamines such as cadaverine (Cad), ornithine (Orn), putrescine (Put), spermidine (Spd), and spermine (Spm). Polyamines are biogenic amines, displaying crucial roles in biological systems.4 Polyamines take part in major cellular activities like replication, membrane stability, cell proliferation, transcription, translation, apoptosis, differentiation, and tumorigenesis.5
Polyamines were shown to be effective against bacteria in different studies. Polyamines decrease Escherichia coli (E. coli) outer membrane permeability by inhibiting porin-mediated ion flux.6 Polyamine homeostasis plays a vital role in Streptococcus pneumoniae pathogenesis by regulating central metabolism, virulence factors like the capsule, and stress responses crucial for survival in the host. Targeting polyamine transport proteins shows promise as an effective immunization strategy against pneumococcal colonization and disease, highlighting polyamine pathways as potential therapeutic and preventive targets.7 Spd-capped carbon quantum dots were tested and found to be effective at minimal concentrations against multidrug-resistant and non-multidrug-resistant bacteria due to damage to the bacterial membrane.8 Studies have shown that the synthetic polyamine analogues such as methoctramine and naphthylacetyl spermine can disrupt lipopolysaccharide integrity and increase the penetrability of the external membrane of E. coli to increase the susceptibility towards hydrophobic antibiotics.9 Most of the previous microbial susceptibility study was carried out with the β-lactam, chloramphenicol, nalidixic acid, and trimethoprim antibiotics synergistic effect of polyamines and exogenous application of polyamines enhanced the vulnerability of Pseudomonas aeruginosa to various antibiotics.10,11 A study reported that synthetic polyamine AHA-1394 showed a 128-fold increase in inhibition against S. aureus compared to another natural polyamine.12 Substituted diamines were also reported to be operative against several Gram-positive and negative bacteria, such as stationary-phase bacteria and methicillin-resistant Staphylococcus aureus.13 Norspermidine, like other polyamines, displayed biofilm inhibitory and disseminative activities of clinically important multidrug-resistant bacterial isolates.14 Norspermidine was shown to inhibit P. aeruginosa biofilms.15 Cad pretreatment makes the eukaryotic cells less susceptible to Shigella enterotoxins.16 Antibiotic, chloramphenicol, along with Spm and Spd, has shown increased susceptibility in E. coli.10 Polyamine and ethanolamine metabolism in bacteria plays a crucial role in nitrogen assimilation, supporting both their survival and pathogenicity. Bacteria show that gamma-glutamylation pathways aid in polyamine detoxification, with specialized enzymes identified in P. aeruginosa and S. coelicolor.17 Aroma chemicals like trans-geraniol, citronellyl formate, and l-linalool also have a broad spectrum of antimicrobial activity.18 Polyamines exhibit antimicrobial activity through multiple mechanisms that enhance their overall efficacy. One of the primary actions involves disruption of bacterial cell membranes; polyamines, being positively charged, interact with negatively charged membrane components, leading to increased permeability and eventual cell lysis.19 Additionally, polyamines inhibit biofilm formation by interfering with quorum-sensing pathways and biofilm matrix synthesis, thereby reducing bacterial colonization and persistence.20 These molecules also bind to bacterial DNA and RNA, disrupting replication, transcription, and translation processes essential for bacterial survival.21 Moreover, polyamines can act synergistically with conventional antibiotics by enhancing membrane permeability and inhibiting bacterial efflux pumps, resulting in increased antibiotic uptake and efficacy.11 Some polyamines are also known to promote the generation of reactive oxygen species (ROS), inducing oxidative stress that damages key cellular components and contributes to bacterial killing.22 Collectively, these multifaceted mechanisms underline the potential of polyamines as effective antimicrobial agents or adjuvants in the fight against resistant pathogens.23
Recent research shows that by focusing on bacterial membranes and vital ribosomal processes, polyamines can greatly enhance the effects of antibiotics. Putrescine has been demonstrated to increase the sensitivity of Klebsiella pneumoniae to azithromycin by concurrently permeabilizing the outer and inner membranes and preventing protein synthesis at ribosomal sites. This prolongs the effectiveness of a macrolide that is typically ineffective against Gram-negative bacteria.24 Furthermore, in animal infection models, synthetic polyamine analogues such as d-LANA-14 have demonstrated in vivo effectiveness against multidrug-resistant A. baumannii and P. aeruginosa by demonstrating strong synergy with tetracycline and rifampicin, mainly through disruption of outer membranes and biofilm disintegration. E. coli and S. aureus indicated a strong synergistic effect of spermine in combination with β-lactams and chloramphenicol.10,25
Studies have shown a few polyamines as potential candidates for antimicrobial activity. The precursor amine in the polyamine biosynthetic pathway, Orn, and the important polyamines Put, Spd and Spm, were not methodically studied for their antimicrobial effect. Consequently, the current study was performed to check the impact of Orn, Put, Spd, and Spm on Gram-positive and Gram-negative bacteria. Orn, Put, Spd, and Spm were also checked for antibacterial susceptibility when supplemented along with tetracycline, rifampicin, carbenicillin, and streptomycin antibiotics. This study aimed to check the efficacy of antibiotics against bacteria supplemented with polyamines.
Bacterial strains, polyamines, and antibiotics
Bacterial strains, antibiotics, and culture conditions: Gram-positive bacterial strains Bacillus cereus (MCC 2243), Staphylococcus aureus (MCC 2408) and Gram-negative bacterial strains Pseudomonas aeruginosa (MCC 2328) and Klebsiella pneumoniae (MCC 2716) were purchased from the National Centre for Cell Science (NCCS), Pune, India. Polyamines, Orn (RM057-25G), Put (Cat. No.-RM5438-5G), Spd (Cat. No.-RM443-5G), and Spm (RM7506-5G) were purchased from Himedia Laboratories. Antibiotic discs of Tetracycline (30 µg), Rifampicin (5 µg), Carbenicillin (100 µg), and Streptomycin (10 µg) were purchased from Himedia Laboratories. All these concentrations of antibiotics were selected based on the minimal inhibitory concentrations mentioned in the literature.26-28
Preparation of polyamine impregnated discs
Solutions of Orn, Put, Spd, and Spm (50 µg/ml) were set by dissolving in sterile double-distilled H2O. Whatman no.1 filter paper was used to make discs with a diameter of 6 mm, sterilized and were further soaked with polyamines and their precursor Orn (100 µl) separately and air dried under sterile conditions. A similar process was carried out with different concentrations of (25 µM, 50 µM, 75 µM, 100 µM, 250 µM, and 500 µM) of Orn, Put, Spd, and Spm.
Culture conditions and bacterial susceptibility testing
The bacterial growth inhibition by the antibiotics and polyamines were assessed separately and compared with the combination of antibiotics and polyamines.10 All the bacterial strains used for the study were grown on nutrient agar slants and broth cultures at 37 °C and maintained by sub-culturing. Mueller Hinton (MH) agar plates were used to perform antimicrobial susceptibility tests by the disc diffusion method. Overnight broth cultures of each bacterial inoculum (200 µl) were evenly spread over the MH agar surface. Commercial antibiotic discs and discs impregnated with polyamine were placed over the MH agar surface carefully, and then the plates were subjected to incubation at 37 °C for 12 hours.29 The bacterial susceptibility was assessed by gauging the diameters of inhibition zones in centimeters of only the antibiotic, only the polyamine, and the antibiotic with polyamine. The experiment was formed with three biological replicates.
Statistical analysis
The measurements of zones of inhibition were compared between the control (antibiotic alone) and the antibiotic with polyamine of each bacterial strain. Statistical analysis by ANOVA and Student’s t-test was performed using GraphPad version 10.2.3. The values labeled with * are significant (p <0.05), and ** are highly significant (p <0.01).
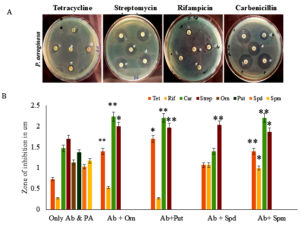
Effect of different polyamines on antibacterial sensitivity of Pseudomonas aeruginosa
P. aeruginosa is a pathogen that causes dreadful nosocomial infections in human beings. Its rapid emergence as a resistant bacterium against antibiotics like cephalosporins, penicillin, tetracyclines, fluoroquinolones, and macrolides is grievous. The multidrug efflux system and low-permeable outer membrane of P. aeruginosa contribute to its drug-resistance. The current study analyzed the effect of supplementation of Orn and polyamines (Put, Spd, Spm) along with antibiotics on the antibacterial sensitivity. Supplementation of Orn and polyamines with antibiotics showed a decrease in the zone of inhibition when compared to antibiotics alone, which was taken as a control. Out of four antibiotics tested individually, streptomycin was most effective against P. aeruginosa (Figure 1). The results have shown that carbenicillin supplemented with Orn proved to be the best combination for effective inhibition of P. aeruginosa (p <0.01) (Table S1).
Figure 1. Antimicrobial analysis against Pseudomonas aeruginosa. (A) Effect of antibiotics (Ab) along with different polyamines on antibiotic susceptibility of Pseudomonas aeruginosa. Only antibiotic was the control (a), different polyamines, Orn (b), Put (c), Spd (d), and Spm (e) (50 µg/ml) were tested along with antibiotics, tetracycline, streptomycin, rifampicin, and carbenicillin for antimicrobial activity against P. aeruginosa. (B) Antimicrobial activity of antibiotics (Ab) alone, polyamine (PA) alone, and the combination of Ab and PA was analyzed. ** indicates a highly significant difference (p <0.01) between Ab alone and Ab with PA antimicrobial activities * indicates a significant difference (p <0.05)
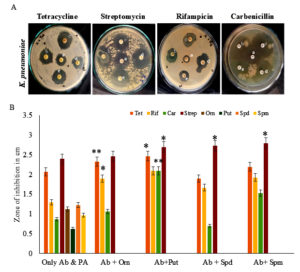
Effect of different polyamines on the antibacterial sensitivity of Klebsiella pneumoniae
K. pneumoniae uses various nosocomial infections like pneumonia, infections of the urinary tract, abdominal, and skin. They colonize in the skin, gastrointestinal tract, and nasopharynx. Delayed and ineffective antimicrobial therapy increases the mortality rate. K. pneumoniae produces β-lactamase enzymes, which bring forth antibiotic resistance to this organism. Its resistance towards third-generation cephalosporins and fluoroquinolones were studied earlier.30 Of the four antibiotics tested individually, streptomycin was most effective against K. pneumoniae (Figure 2). Spm supplemented streptomycin proved to be the best combination for effective inhibition of K. pneumoniae (Table S2).
Figure 2. Antimicrobial analysis against Klebsiella pneumoniae. (A) Effect of antibiotics (Ab) along with different polyamines on the antibiotic susceptibility of K. pneumoniae. Only the antibiotic was the control (a), different polyamines, Orn (b), Put (c), Spd (d), and Spm (e) (50 µg/ml) were tested along with antibiotics, tetracycline, streptomycin, rifampicin, and carbenicillin for antimicrobial activity against K. pneumoniae. (B) Antimicrobial activity of antibiotics (Ab) alone, polyamine (PA) alone, and the combination of Ab and PA was analyzed. ** indicates a highly significant difference (p <0.01) between Ab alone and Ab with PA antimicrobial activities * indicates a significant difference (p <0.05)
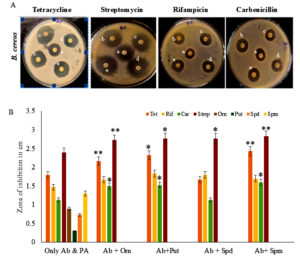
Effect of different polyamines on the antibacterial sensitivity of Bacillus cereus
B. cereus is a highly thermally resistant bacterium associated with foodborne outbreaks. It causes food-poisoning conditions with intoxications and infections.31 B. cereus is a spore-forming bacterium which is resilient to penicillin & other antibiotics of β-lactam.31 Several antimicrobial compounds have been tested to find out its sensitivity. Nevertheless, it is found that these bacteria are resistant to tetracycline, streptomycin, erythromycin, ciprofloxacin, and cloxacillin.32 Out of four antibiotics tested individually, streptomycin was most effective against B. cereus (Figure 3). Spm supplemented with streptomycin proved to be the best combination for effective inhibition of B. cereus (Table S3).
Figure 3. Antimicrobial analysis against Bacillus cereus. (A) Effect of antibiotics along with different polyamines on antibiotic susceptibility of Bacillus cereus. Only the antibiotic was the control (a), different polyamines, Orn (b), Put (c), Spd (d), and Spm (e) (50 µg/ml) were tested along with antibiotics, tetracycline, streptomycin, rifampicin, and carbenicillin for antimicrobial activity against K. pneumoniae. (B) Antimicrobial activity of antibiotics (Ab) alone, polyamine (PA) alone, and the combination of Ab and PA was analyzed. ** indicates a highly significant difference (p <0.01) between Ab alone and Ab with PA antimicrobial activities * indicates a significant difference (p <0.05)
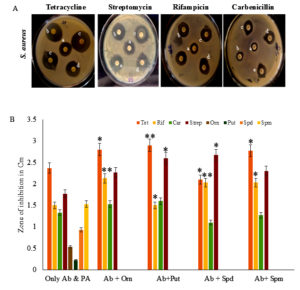
Effect of different polyamines on the antibacterial sensitivity of Staphylococcus aureus
S. aureus is a commensal organism which will turn into an opportunistic pathogen, causing skin, gastrointestinal & respiratory infections.33 They attain a swift resistance against antibiotics effortlessly as a consequence of antibiotic-resistant plasmids and intrinsic resistance mechanisms.34 Their exceptional ability to advance various resistance mechanisms contributes to the antibiotic resistance. Supplementation of Orn and polyamines with antibiotics showed an increase in the zone of inhibition when compared to antibiotics alone, which was taken as control (Figure 4). Out of four antibiotics tested individually, tetracycline was most effective against S. aureus. The results have showed that Put supplemented tetracycline proved to be the best combination for effective inhibition of S. aureus (p <0.01) (Table S4).
Figure 4. Antimicrobial analysis against Staphylococcus aureus. (A) Effect of antibiotics along with different polyamines on antibiotic susceptibility of S. aureus. Only antibiotic was the control (a), different polyamines, Orn (b), Put (c), Spd (d), and Spm (e) (50 µg/ml) were tested along with antibiotics, tetracycline, streptomycin, rifampicin, and carbenicillin for antimicrobial activity against S. aureus. (B) Antimicrobial activity of antibiotics (Ab) alone, polyamine (PA) alone, and the combination of Ab and PA was analyzed. ** indicates a highly significant difference (p <0.01) in antimicrobial activities between Ab alone and Ab with PA * indicates a significant difference (p <0.05)
The results of the present study showed enhanced susceptibility of the selected microbes upon administration of antibiotics with polyamines. The results support the findings of polyamines alone or polyamines in combination with other compounds or antibiotics as antimicrobial agents.8,10 The effective concentration of the antibiotic was also found to be lowered when administered along with polyamines. Orn significantly improved the antibacterial effect of tetracycline against all four selected strains. Literature on the effect of Orn is limited with regard to the enhancement of bacterial susceptibility to antibiotics. Put showed a positive effect with tetracycline and streptomycin against all four strains. A similar effect was seen with B. cenocepacia to polymyxin B.
Put decreased significantly the oxidative stress induced by the antimicrobial peptide and by multiple mechanisms.35 Spd was most effective with streptomycin, where the increased susceptibility was observed. Spd was shown to be effective against E. coli, P. aeruginosa, S. aureus, B. subtilis, and methicillin-resistant S. aureus (MRSA) bacteria.11 Streptomycin caused inhibition of protein synthesis in polyamine-supplemented E. coli.36 Spm gave a mixed response when supplemented with antibiotics against the four selected strains. Studies showed that Spm enhanced the combined activity of isoniazid (INH) and rifamycin (RIF) against M. tuberculosis.37 Exogenous Spm and Spd enhanced the susceptibility of P. aeruginosa to β-lactam antibiotics.10 The results showed that maximum susceptibility was observed with carbenicillin and Orn against P. aeruginosa; streptomycin and Spm against K. pneumoniae and B. cereus; and tetracycline and Put against S. aureus. The present study showed that polyamines enhanced the susceptibility of bacteria against all the antibiotics tested. Notable changes were observed with Spm, which could be due to the greater positive charge on Spm that could have helped in the easy entry of the antibiotic through the membrane. Therefore, the study shows that polyamines could be promising candidates and can be further tested to understand the underlying mechanism in detail.
This study demonstrates that supplementation of antibiotics with polyamines significantly enhances bacterial susceptibility in both Gram-positive and Gram-negative strains. Specific combinations, such as carbenicillin with ornithine against Pseudomonas aeruginosa, streptomycin with spermine against Klebsiella pneumoniae and Bacillus cereus, and tetracycline with putrescine against Staphylococcus aureus, exhibited the most prominent synergistic effects. These findings highlight the potential of polyamines as effective adjuvants to conventional antibiotics, offering a promising strategy to counteract antimicrobial resistance. Further studies and in vivo evaluations are required to understand their mode of action.
Additional file: Additional Table S1-S4.
ACKNOWLEDGMENTS
The authors acknowledge the infrastructure support provided by GITAM Deemed to be University, Andhra Pradesh, India.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
AM conceptualized the study. AL and BGLD performed bacterial cultures and polyamine disc preparation. RR performed the experiments. AM supervised the experiments. MHR analyzed the data. RR and MHR wrote the manuscript. VP reviewed the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This work was supported by the University Grants Commission (UGC) as UGC – MRP grant (Grant Number: BT/PR15319/TDS/121/12/2015) to the author (AM).
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229-241.
Crossref - Ahmed SK, Hussein S, Qurbani K, et al. Antimicrobial resistance: Impacts, challenges, and future prospects. Journal of Medicine, Surgery, and Public Health. 2024; 2:100081.
Crossref - Aslam B, Wang W, Arshad MI, et al. Antibiotic resistance: a rundown of a global crisis. Infection and drug resistance. 2018;11:1645-1658.
Crossref - Medina MA, Urdiales JL, Rodriguez-Caso C, Ramirez FJ, Sanchez-Jimenez F. Biogenic Amines and Polyamines: Similar Biochemistry for Different Physiological Missions and Biomedical Applications. Crit Rev Biochem Mol Biol. 2003;38(1):23-59.
Crossref - Igarashi K, Kashiwagi K. Effects of polyamines on protein synthesis and growth of Escherichia coli. J Biol Chem. 2018;293(48):18702-18709.
Crossref - Dela Vega AL, Delcour AH. Polyamines decrease Escherichia coli outer membrane permeability. J Bacteriol. 1996;178(13):3715-3721.
Crossref - Nanduri B, Swiatlo E. The expansive effects of polyamines on the metabolism and virulence of Streptococcus pneumoniae. Pneumonia. 2021;13(1):4.
Crossref - Li Y, Harroun SG, Su Y, et al. Synthesis of Self Assembled Spermidine Carbon Quantum Dots Effective against Multidrug Resistant Bacteria. Adv Healthc Mater. 2016;5(19):2545-2554.
Crossref - Sukupolvi S, Vaara M. Salmonella typhimurium and Escherichia coli mutants with increased outer membrane permeability to hydrophobic compounds. Biochim Biophys Acta BBA – Rev Biomembr. 1989;988(3):377-387.
Crossref - Kwon DH, Lu CD. Polyamine Effects on Antibiotic Susceptibility in Bacteria. Antimicrob Agents Chemother. 2007;51(6):2070-2077.
Crossref - Kwon DH, Lu CD. Polyamines Increase Antibiotic Susceptibility in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50(5):1623-1627.
Crossref - Douglas EJA, Alkhzem AH, Wonfor T, et al. Antibacterial activity of novel linear polyamines against Staphylococcus aureus. Front Microbiol. 2022;13:948343.
Crossref - Wang B, Pachaiyappan B, Gruber JD, Schmidt MG, Zhang YM, Woster PM. Antibacterial Diamines Targeting Bacterial Membranes. J Med Chem. 2016;59(7):3140-3151.
Crossref - Cardile AP, Woodbury RL, Sanchez CJ, et al. Activity of Norspermidine on Bacterial Biofilms of Multidrug-Resistant Clinical Isolates Associated with Persistent Extremity Wound Infections. In: Donelli G, ed. Advances in Microbiology, Infectious Diseases and Public Health. Vol 973. Springer Cham. 2016:53-70.
Crossref - Qu L, She P, Wang Y, et al. Effects of norspermidine on Pseudomonas aeruginosa biofilm formation and eradication. Microbiologyopen. 2016;5(3):402-412.
Crossref - Maurelli AT, Fernandez RE, Bloch CA, Rode CK, Fasano A. “Black holes” and bacterial pathogenicity: A large genomic deletion that enhances the virulence of Shigella spp. and enteroinvasive Escherichia coli. Proc Natl Acad Sci USA. 1998;95(7):3943-3948.
Crossref - Krysenko S, Wohlleben W. Polyamine and Ethanolamine Metabolism in Bacteria as an Important Component of Nitrogen Assimilation for Survival and Pathogenicity. Med Sci. 2022;10(3):40.
Crossref - Malik T, Singh P. Antimicrobial activity of aroma chemicals against uropathogens. Journal of Environmental and applied bioreseach, 2015;3(2):86-91
- Yasuda K, Ohmizo C, Katsu T. Mode of action of novel polyamines increasing the permeability of bacterial outer membrane. Int J Antimicrob Agents. 2004;24(1):67-71.
Crossref - Wortham BW, Oliveira MA, Patel CN. Polyamines in Bacteria: Pleiotropic Effects yet Specific Mechanisms. In: Perry RD, Fetherston JD. (eds) The Genus Yersinia. Advances In Experimental Medicine And Biology, vol 603. Springer, New York, NY. 2007;603:106-115.
Crossref - Miller CS, Foley JD, Bailey AL, et al. Current Developments in Salivary Diagnostics. Biomark Med. 2010;4(1):171-189.
Crossref - Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA. The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci U S A. 1998;95(19):11140-11145.
Crossref - Shah P, Swiatlo E. A multifaceted role for polyamines in bacterial pathogens. Mol Microbiol. 2008;68(1):4-16.
Crossref - Adams JME, Moulding PB, El-Halfawy OM. Polyamine-Mediated Sensitization of Klebsiella pneumoniae to Macrolides through a Dual Mode of Action. ACS Infect Dis. 2024;10(6):2183-2195.
Crossref - Atta S, Waseem D, Fatima H, Naz I, Rasheed F, Kanwal N. Antibacterial potential and synergistic interaction between natural polyphenolic extracts and synthetic antibiotic on clinical isolates. Saudi J Biol Sci. 2023;30(3):103576.
Crossref - Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(suppl_1):5-16.
Crossref - Zhou W, Shan W, Ma X, et al. Molecular characterization of rifampicin-resistant Staphylococcus aureus isolates in a Chinese teaching hospital from Anhui, China. BMC Microbiol. 2012;12(1):240.
Crossref - Matsen JM, Lund ME, Brooker DC. Comparison and Evaluation of Carbenicillin Disks in Diffusion Susceptibility Testing. Antimicrob Agents Chemother. 1974;5(6):599-606.
Crossref - Kirby-Bauer Disk Diffusion Susceptibility Test Protocol. ASM.org. https://asm.org:443/protocols/kirby-bauer-disk-diffusion-susceptibility-test-pro. Accessed July 11, 2025.
- Nakamura-Silva R, Cerdeira L, Oliveira-Silva M, et al. Multidrug-resistant Klebsiella pneumoniae: a retrospective study in Manaus, Brazil. Arch Microbiol. 2022;204(4):202.
Crossref - Tewari A, Abdullah S. Bacillus cereus food poisoning: international and Indian perspective. J Food Sci Technol. 2015;52(5):2500-2511.
Crossref - Citron DM, Appleman MD. In Vitro Activities of Daptomycin, Ciprofloxacin, and Other Antimicrobial Agents against the Cells and Spores of Clinical Isolates of Bacillus Species. J Clin Microbiol. 2006;44(10):3814-3818.
Crossref - Cheung GY, Bae JS, Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence. 2021;12(1):547-569.
Crossref - Shuval H. Estimating the global burden of thalassogenic diseases: human infectious diseases caused by wastewater pollution of the marine environment. J Water Health. 2003;1(2):53-64.
Crossref - El-Halfawy OM, Valvano MA. Putrescine Reduces Antibiotic-Induced Oxidative Stress as a Mechanism of Modulation of Antibiotic Resistance in Burkholderia cenocepacia. Antimicrob Agents Chemother. 2014;58(7):4162-4171.
Crossref - Goldemberg SH, Algranati ID. Poly amine Requirement for Streptomycin Action on Protein Synthesis in Bacteria. Eur J Biochem. 1981;117(2):251-255.
Crossref - Sao Emani C, Reiling N. Spermine enhances the activity of anti-tuberculosis drugs. Microbiol Spectr. 2024;12(1):e03568-23.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.