ISSN: 0973-7510
E-ISSN: 2581-690X
The in-vivo mouse bladder model was sought to determine the effect of virulent uropathogenic E. coli (UPEC) strain harboring hly, papC and cnf-1 genes on uroepithelium of diabetic mouse bladder infected transurethrally. The female BALB/c mice aged between 6-8 weeks were used in the study. The diabetes was induced by subcutaneous injection of alloxan hydrate (80mg / kg body weight) in mice. Two UPEC strains, one with hly, papC and cnf-1 virulent genes and the other (hypovirulent) without hly, cnf-1 and papC genes were selected for the study. The animals were anesthetized and 50 µl of bacterial inoculum was instilled in to bladder of DM and non-DM mice using specially devised mice catheter. The mice were sacrificed at 4 hrs, 24 hrs and 48 hrs of post infection, and the bladder was removed aseptically. One half of the bladder was homogenized and bacterial culture was performed. The other half of the bladder was used to document bacterial adhesion and invasion by histopathology and scanning electron microscopy. The exaggerated consequence of virulent UPEC strain on diabetic mouse bladder model was documented as enhanced adhesion and extensive damage of the uroepithelium of the bladder. However, hypovirulent UPEC strain failed to produce observable pathophysiological effect. Many of the UPEC were in the filamentous form and occasionally seen looping within and between adjacent superficial cells to escape from immune mechanism like micturation and exfoliation.
UPEC; Escherichia coli; Mouse UTI model; Diabetes mellitus.
The Escherichia coli is responsible for over 80% of the community-acquired urinary infections. The ability of the uropathogenic Escherichia coli (UPEC) to adhere to the host bladder epithelium is considered critical to cause the disease1,2. There is a wide array of virulence markers employed by UPEC, and the adherence is mostly caused by the presence of type 1, P, F1C and S pili. Other than pili, hemolysin, flagella, biofilm formation and iron acquisition/transport system play a crucial role in the colonization.3-5
Once the uropathogen adhere to the mucosal surface, a series of host defense pathways are activated. Within hours of initial adherence, uroepithelial cells exfoliate and infected cells are shed.6,7 An in-vivo study on mice has shown that exfoliation of uroepithelial cells prevents UPEC forming clusters and chances of biofilm formation by UPEC become high due to mild exfoliation process in the bladder.8 Experimental systems have also found that UPECs have an invasive ability, following which they form intracellular communities helping them to escape from both host innate and adaptive response and facilitate further dissemination.9
The diabetics are more prone to infection and these infections are more severe than in non-diabetics. In patients with poor glycemic control, cystitis, ascending infections leading to pyelonephritis, emphysematous complications and renal and perinephric abscesses are well recognized.10 Many hypotheses have attributed to the increased UTI in patients with diabetes mellitus, such as glycosuria, impaired function of neutrophils, functional abnormalities of the urinary tract; however, these theories have inadequately understood.11,12
In the present in-vivo mouse cystitis model study, we sought to determine the effect of virulent UPEC strain having hly, papC, and cnf-1 genes on the bladder uroepithelium of diabetic mouse infected transurethrally.
Ethical clearance
The present study was performed in accordance with the guidelines of National Institutes of Health for the care and use of experimental animals in the research, and the protocol of animal experiment has been approved by the Institutional Animal Ethical Committee, Navodaya Medical College, Raichur, Karnataka.
Maintenance of Mice
The female BALB/c mice aged between 6-8 weeks were housed five in each cage, fed with pellets and tap water. They were housed under controlled environment with temperature of 23oC (± 2°C), humidity 50% (± 5%) and 10-12 hours of light and dark cycles. For anatomical reasons, only female mice were used.
Induction of diabetes mellitus in mice
The diabetes was induced by subcutaneous injection of Alloxan hydrate (LOBA CHEMIE PVT LTD, Mumbai) in mice, 80mg / kg body weight, after 12 hrs of fasting. The fasting blood sugar level was evaluated by Glucometer (SD Fine chemicals) after 3 days of the induction. Following the initial injection of alloxan, if the mice blood glucose level falls below 300 mg/dl, a second dose of alloxan was given to maintain blood sugar level above 300 mg/dl during the study period.15
Selection of strains
The molecular characterization of UPEC strains were done by PCR amplification using the primers previously described by Johnson and Stell.16
Two UPEC strains selected for the study were from symptomatic UTI patients, and strains having following characteristics were selected for the study and inoculated in DM and non- DM mouse model transurethrally:
- Uropathogenic E. coli with hly, cnf-1 and papC genes, and belonging to (extraintestinal) phylogenetic group B2 (Virulent strain).
- Uropathogenic E. coli without hly, cnf-1 and papC genes, and belonging to (intestinal) phylogenetic group A (Hypovirulent strain).
Inoculum Preparation:
These Bacteria were inoculated from nutrient agar deeps in to BHI broth and incubated at 37°C.
Fresh bacterial cultures were pelleted by centrifugation (2000 x g for 20 minutes), and resuspending in PBS and diluted appropriately to yield 50 µl inocula of 1-2 x 107 Colony Forming Units (CFU)/ml.
Mouse model of UTI
The preparation of urethral catheter and inoculation procedures to induce UTI in mice model was followed as per Johnson DE et al protocol17. Briefly, the mouse urinary bladder was voided by gentle massage over bladder (lower abdomen) before infection. A drop of urine was collected directly at urethral orifice with a calibrated loop, and by spreading on the surface of Mac Conkey agar plate sterility was tested. The mice urine showing bacterial count >102 per ml were excluded from the study.
The animals were anesthetized by injecting 0.05 ml of Ketamine intraperitoneally (50mg/ml). The 50 µl of inoculum prepared was instilled in to urinary tract through a soft polyethylene catheter (Outer diameter 0.61mm) adapted to a 0.4 x 20 mm needle on a tuberculin syringe. After injection, the catheter was immediately withdrawn, and no further manipulation was performed.17 Control animals were injected with 50 µl of sterile PBS transurethrally.
Out of 26 female mice selected for the study, a group of 13 were injected with alloxan hydrate to induce diabetes (DM) and the other group of 13 were used as non-diabetic (non-DM) controls. The mice were tested for the presence of bacteriuria by collecting urine directly on calibrated loop by gentle abdominal massage, and the mice showing bacterial count d” 102 UFU/ml were included in the study.
Detection of adhesion and Invasion of UPEC to uroepithelium
Once the animals are sacrificed, the bladder was taken out aseptically. One half of the bladder was homogenized, and bacterial cultures were performed. The other half of the bladder is used to register bacterial adhesion by histopathology and scanning electron microscopy.
Histopathology
A bit of bladder tissue was placed in 10% formalin for 24 hrs. The resulting formalin-fixed tissue was embedded in paraffin and cut in to 3 µm thick sections. The slides were stained with haematoxylin and eosin stain. The histopathology slides were graded by a qualified pathologist in a blinded manner. For each slide, histopathological parameters recorded were – interstitial edema, hemorrhage, leucocyte infiltration, and uroepithelial cell damage. A semi quantitative severity scale (0-4 of each parameter) was used for the evaluation. For each of the four categories assessed, average score of less than one was considered mild effect, score of 2-3 as moderate effect and greater than 3 as severe effect of the strain on the histopathological feature.
Bacterial Culture
The viable count was performed by serial dilutions of homogenized bladder in PBS and plated on Mac Conkey’s agar plates. The number of bacteria was expressed as the number of CFU per gram tissue.
Tissue fixation for scanning electron microscopy (SEM)
The bladder tissue in its intact form was fixed overnight at 4oC in 0.1M cacodylate buffer (pH 7.2) containing 0.5% gluteraldehyde. The next day, these fixed tissues, were washed three times with cacodylate buffer and transported to Indian Institute of Chemical Technology (IICT), Hyderabad for SEM.
From each group of 13 mice, six were inoculated with virulent UPEC strains having hly, pap C and cnf-1 gene, another six with hypovirulent UPEC strains devoid of hly, papC and cnf-1 gene and one with sterile PBS transurethrally in to the bladder. Each set of four mice in combination of DM+ virulent strain, DM+ hypovirulent strain, non-DM + virulent strain and non- DM+ hypovirulent strain were sacrificed at 4 hrs, 24 hrs and 48hrs of post infection. Before sacrificing mice, urine was collected directly on to the loop and cultured for estimation of bacterial count.
Histological score of mice bladder at different time interval
To measure the in-vivo effect of UPEC strains on mouse bladder epithelial cells in different host conditions histological scoring was done (Table -1).
Table (1):
In-vivo effect of E.coli strains on bladder epithelium of DM and non- DM mice.
| Combination of E. coli strain & mice DM status | Histological Score | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adherence | Edema | Hemorrhage | Leucocyte infiltration | Epi. Damage | |||||||||||
| 4h | 24h | 48h | 4h | 24h | 48h | 4h | 24h | 48h | 4h | 24h | 48h | 4h | 24h | 48h | |
| DM + Virulent strain | 0 | 0 | 2 | 1 | 2 | 0.5 | 0 | 0 | 1 | 0.5 | 1.5 | 0.5 | 0 | 0.5 | 1.5 |
| DM + hypovirulent strain | 0 | 0.5 | 0 | 0 | 1 | 0.5 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0.5 |
| non DM + Virulent strain | 0 | 0.5 | 0.5 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 1 | 0.5 | 0.5 | 1 | 0 | 0.5 | 0.5 |
| non DM + hypovirulent strain | 0 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 |
Average score of < 1: mild histological effect
Average score of 1-3: moderate histological effect
Average score of >3: severe histological effect
Virulent UPEC strain: Moderate leucocyte infiltration (Grade 1.5) and sub mucous edema (Grade 2.0) of mouse bladder was seen DM mice after 24 hrs of challenge. In non-DM mice leucocyte infiltration and edema was shown a similar pattern with comparatively low histological grade. Hemorrhagic response (grade 1) was seen after 48 hrs of challenge in both DM and non-DM mice. Enhanced bacterial adherence (grade 1.5-2.0) and extensive epithelial cell damage was noticed in DM mice, after 48 hrs of post infection (Figure-1).
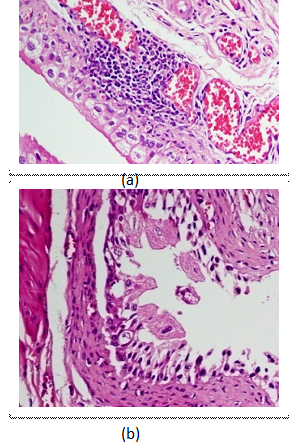 Fig. 1. Histopathological study showing a). sub epithelial edema, mild to moderate infiltration of neutrophils, congested blood vessels after 24 hrs, and b) patchy epithelial damage after 48 hrs of challenge with virulent strain in DM mouse bladder.
Fig. 1. Histopathological study showing a). sub epithelial edema, mild to moderate infiltration of neutrophils, congested blood vessels after 24 hrs, and b) patchy epithelial damage after 48 hrs of challenge with virulent strain in DM mouse bladder.Hypovirulent UPEC strain: Mild degree (Grade 0.5-1.0) of leucocytic infiltration and edema was seen after 24 hrs of post infection in DM mice and after 48 hrs of post infection in non-DM mice. No hemorrhage was noticed in both the study groups. Minimum amount of bacterial adherence to uroepithelial cells at 24 hrs and sparse epithelial cell damage at 48 hrs of post infection was noticed in DM mice. Neither bacterial adherence to epithelial cells nor epithelial damage was recorded at any time of post infection in non-DM mice.
Bacterial count from DM mouse bladder and urine
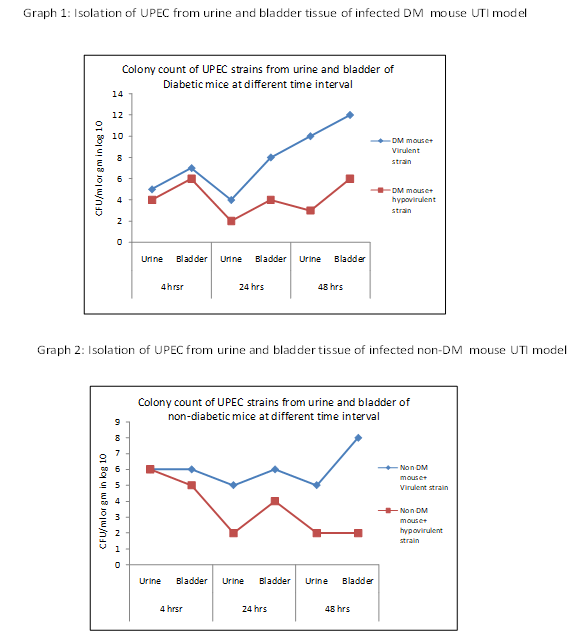
Virulent UPEC strain: Decreased colony count of 105 CFU/gm compared to initial bacterial inoculation of 107 CFU/gm was observed at 4 hrs of post infection and was gradually increased up to 1012 CFU after 48 hrs of post infection. UPEC colony count showed an increasing trend in the bladder culture compared to the urine culture.
Hypovirulent UPEC strain: The Colony count from urine was dropped from 104 to 103 CFU/gm after 48 hrs. However, the colony count from bladder was remained at 106CFU/gm at 4 hrs as well as at 48 hrs. of post infection. Similar to virulent strain, hypovirulent A strain has also showed higher colony count of UPEC in the bladder compared to that in the urine (Graph -1).
Bacterial count from non-DM mouse bladder and urine
Virulent UPEC strain: Colony count from bladder was increased gradually from 106 to 108 CFU/gm at 48 hrs of post infection. However, colony count from urine dropped from 106 to 105 CFU/ml.
Hypovirulent UPEC strain: Colony count from bladder dropped down from106 to 102 CFU/gm at 24 hrs. and maintained the same after 48 hrs of post infection. Bacterial count from urine gradually decreased from 105 CFU/ml at 4 hrs to 102 CFU/ml at 48 hrs of post infection (Graph – 2).
Scanning electron microscopic study of bacterial attachment
Non-DM mouse bladder challenged with the virulent strain
SEM of the bladder mucosa showed bacterial adherence after 4 hrs and 24 hrs of challenge. Moderate amount of discrete bacterial population was recorded after 48 hrs post infection (Fig. 2).
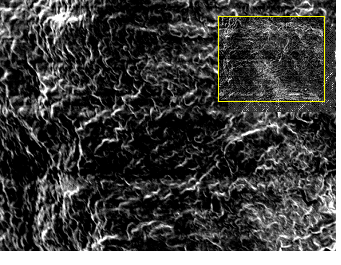
Fig. 2. Scanning electron micrograph showing bacterial adherence and moderate bacterial population in single and in clusters in non-DM mouse bladder. Bar, 20um (inset bar, 100 um)
DM mouse bladder challenged with the virulent strain
SEM of the bladder mucosa showed bacterial adherence in single and clusters after 4 hrs, Patchy epithelial damage and large population of bacteria adhering to all over the surface after 24 hrs , and epithelial damage with heavy population of bacteria in large clusters was seen after 48 hrs of post infection. Many bacteria appeared in elongated filamentous form. These filamentous bacteria were occasionally seen looping within and between adjacent superficial cells (Fig. 3).
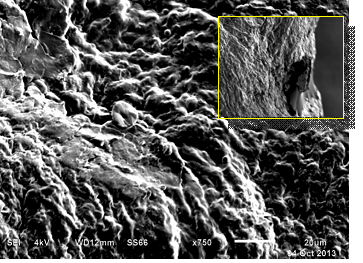
Fig. 3. Scanning electron micrograph shows patchy epithelial damage and heavy population of bacteria adhering all over the surface & most bacilli are filamentous in DM mouse bladder challenged with group B2 UPEC strain. Bar, 20 um (inset bar, 100um)
In the present study, mouse model was chosen, as the other experimental animals like rabbits, rats, and guinea pigs do not parallel urinary tract of humans in receptors for attaching bacteria.19 Also, it has been shown that the mouse diabetic UTI model is consistent with the features of diabetic human’s urinary tract with infection, and can be an important tool for understanding the UPEC pathogenicity in such subjects.13
In the study, diabetic mouse cystitis model showed enhanced inflammatory reaction, bacterial adhesion and destruction of uroepithelium compared to non-diabetic mouse cystitis model. Bacterial adherence and cell destruction was more pronounced by virulent UPEC strain (hly+, papC+ and cnf1+gene) in DM mice. The hypovirulent strain showed no appreciable inflammatory reaction and adherence after 48 hrs of post infection in non-DM mice.
The increased leucocyte infiltration and submucous edema of mouse bladder tissue was recorded with virulent strain compared to hypovirulent strain in both DM and non-DM mice at 24 hrs of post infection. Further, the virulent strain showed enhanced bacterial adherence and epithelial damage in DM mice. Hagberg et al selected bacterial growth after 24 h and found it as better measure of the early bacterial establishment in the urinary tract. The activation of host defense mechanism in the tissue was indicated by the accumulation of inflammatory cells in the bladder tissue.18
Previously, Smith and co-workers evaluated the relative impact of expression of CNF1 and Hly on bacterial colonization and histopathology in female mice, and reported that the hemolysin of UPEC evokes widespread flaking of the uroepithelium from the bladder and hemorrhage in bladder tissue within the first 24 hrs after intraurethral inoculation of mice. They have also observed that the combination of CNF1 and Hly contribute to leucocyte infiltration into the bladder at day 1 after infection.19
Mo et al hypothesized that the (Tamm-Horsefall protein) THP abnormalities have been associated with diabetes, and such subjects had shown profound reduction in urinary THP. Though granulocyte dysfunction in diabetic subjects could also render more susceptibility to UTI, THP dysfunction can also contribute significantly to the propensity to develop UTI in diabetics independent of the neutrophil status.20
When DM mice bladder model was challenged with virulent UPEC strain, decreased colony count of 105 CFU/gm compared to initial bacterial inoculation of 107 CFU/gm was observed in the early stage of infection and was gradually increased up to 1012 CFU/gm at 48 hrs of post infection. The kinetics of urinary tract colonization by E. coli was compared by Rosen et al between diabetic and healthy control mice. They observed that E. coli efficiently colonized mouse bladder as early as 6h post-infection in both healthy and diabetic mice. The bacterial count in infected bladders of healthy mice decreased to a geometric mean of less than 103 CFU per bladder by 72 hrs post infection. Diabetic mouse bladder, on the other hand, retained a high level of bacterial colonization at 72 hrs post infection.
Our study was in agreement with the report of Mulvey et al that the number of bacteria within the bladder decreased substantially (an average of 3 log units) during the first 12 hrs of inoculation via transurethral catheterization in to female mice. The reduction of bacteria correlates with considerable exfoliation of the superficial epithelial cells in mice bladder, and the incursion of neutrophils into the bladder tissue in response to infection.21
The scanning electron microscopy of diabetic mouse bladder showed bacterial adherence in single and in clusters at 4 hrs, patchy epithelial damage with a large population of bacteria adhering to all over the surface at 24 hrs of post infection and extensive epithelial damage and heavy population of bacteria in large clusters was observed at 48 hrs of challenge. Many of the bacteria were in the filamentous form after 48 hrs of challenge. Mulvey et al have also observed that the bacteria associated with the dying superficial cells at 6 hrs after inoculation were frequently elongated, some times reaching the length of above 50 µm. These filamentous bacteria were occasionally seen looping within and between adjacent superficial cells. These observations suggest that UPEC has the capacity to multiply within the superficial bladder cells. Consequently, the UPEC escape from micturation before the host cell absolutely gets exfoliated.21
To conclude, our findings suggest that the combined effect of virulent UPEC strain and diabetic status of mouse showed enhanced adhesion and extensive damage of the uroepithelium in the bladder. It was evident from the study that the majority of bacteria persisted within the mouse bladder at 48 hrs of post infection was protected from the host immunity. Based on these observations, it is possible that many recurrent UTIs may also occur due to revival of UPEC from dormant reservoirs status established within the bladder mucosa following an initial acute infection. Other pathogenic bacteria that are traditionally considered non-invasive may acquire similar strategies to that of UPEC to establish long-term bacterial reservoirs in other tissues, and this in-vivo experiment may help us to explain the recurrent nature of many infectious diseases.
- Foxman BR, Barlow H, D’Arcy, Gillespie B, Sobel JD. Urinary tract infection: self reported incidence and associated costs. Annal Epidemiol 2000; 10: 509-511.
- Fatima N, Agrawal M, Shukla I, Khan PA. Characterization of uropathogenic E. coli in relation to virulence factors. 20121:342, doi: 10.4172/Scientific reports. 342.
- Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol 1991; 4: 80-128.
- Wright KJ, Seed PC, Hultgren SJ. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect Immun 2005; 73: 7657-7668.
- Jadhav S, Hussain A, Devi S, Kumar A, Parveen S, Gandham N et al. Virulence characteristics and genetic affinities of multiple drug resistant uropathogenic Escherichia coli from se mi urban locality in India. PLOS One 2011; 6: e 18063.
- Mysorekar IU, Mulvey M A, Hultgren S J, Gordon J I. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J Biol Chem 2002; 277: 7412-9.
- Mulvey MA, Lopez-Boado YS, Wilson CL, RothR, Parks WC, Heuser J. Hultgren S J. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 1998; 282: 1494-7.
- Anderson GG, Martin SM, Hultgren SJ. Host subversion by formation of intracellular bacterial communities in the urinary tract. Microbes Infect 2004; 6, 1094-
101.
- Mulvey MA, Schilling JD, and Hultgren SJ. Establishment of persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun 2001; 69: 4572-79.
- Geerlings SE, Brouwer EC, Gaastra W, Stolk R, Diepersloot RJA, Hoepelman AIM. Virulence factors of Escherichia coli isolated from urine of diabetic women with asymptomatic bacteriuria: correlation with clinical characteristics. Antonie van Leeuwenhoek 2001; 80: 119–27.
- Saleem M & Daniel B. Prevalence of Urinary Tract Infection among patients with diabetes in Bangalore City. Int J Emerg Sci 2011; 1: 133-142.
- Geerlings SE. Urinary tract infections in patients with diabetes mellitus: epidemiology, pathogenesis and treatment. Int J Antimicrob Agents2008; 31: 118.
- Rosen DA, Hung CS, Kline KA, and Hultgren SJ. Strptozocin induced mouse model of urinary tract infection. Infect. Immun 2008; 76: 4290-98.
- Mo L, Zhu X, Huang H, Shapiro E, Hasty DL and Wu X. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. AJP-Renal Physiol 2004; 286: F795-802.
- Vindokumar CS, Srinivasa H. Basavarajappa KG, Umakanth Patil, Nitin Bandekar, Rajshree Patil. Effectiveness of bacteriophage in the treatment of Staphylococcus aureus wound infection in diabetic animal model. Asian j Pharm Clin Res 2012; 5: 123-127.
- Johnson JR & Stell AL. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J Infect Dis 2000; 181: 261-272.
- Johnson DE, Lockatell CV, Russel RG, Hebel JR, Island MD, Stapleton A, Stamm WE, and Warren JW. Comparicon of Escherichia coli strains recovered from human cystitis and pyelonephritis infections in transurethrally challenged mice. Infect. Immun1998; 66: 3059-65.
- Hagberg L, Engberg I, Freter R, Lam J, Olling S, and Eden CS. Ascending unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun 1983; 40: 273-83.
- Smith YC, Rasmussen SB, Grande KK, Conran RM, and O’Brien AD. Hemolysin of uropathogenic Escherichia coli evokes extensive shedding of the uroepithelium and hemorrhage in bladder tissue within the first 24 hours after intrauretharl inoculation of mice. Infect. Immun. 2008; 76: 2978-90.
- Mo L, Zhu X, Huang H, Shapiro E, Hasty DL and Wu X. Ablation of the Tamm-Horsfall protein gene increases susceptibility of mice to bladder colonization by type 1-fimbriated Escherichia coli. AJP-Renal Physiol 2004; 286: F795-802.
- Mulvey MA, Schilling JD, and Hultgren SJ. Establishment of persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun 2001; 69: 4572-79.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


