Emerging zoonotic viral diseases pose significant public health challenges due to their high fatality rates, potential for widespread outbreaks, and profound socioeconomic impact. Human, animal, and environmental health interconnectedness highlights the need for a collaborative One Health (OH) approach to control and prevent these diseases. With its diverse ecosystems and rapid urbanization, India has witnessed several major zoonotic outbreaks over the past two decades, including Nipah virus, Kyasanur Forest Disease (KFD), H1N1 influenza, and the global COVID-19 pandemic. These outbreaks underscore the urgent need for integrated surveillance systems, early detection strategies, and sustainable interventions to mitigate future risks. Contributing factors such as deforestation, climate change, unregulated wildlife trade, and intensive farming practices exacerbate the spread of zoonotic diseases. This manuscript emphasizes the importance of a multidisciplinary OH approach, drawing on evidence-based strategies for disease surveillance, vaccination, vector control, and community engagement. By addressing these challenges through coordinated efforts, India can strengthen its preparedness and response to emerging zoonotic viral diseases while promoting public and ecological health.
Zoonotic Diseases, One Health, Nipah Virus, Kyasanur Forest Disease, Surveillance, India, Climate Change
Human activities and stressed ecosystems create opportunities for the emergence and spread of viral diseases. The stress factors such as habitat fragmentation, animal trafficking, agriculture, cattle farming, urbanization, mining industries, climate change, and environmental degradation.1,2 In recent years, the global community has borne witness to the profound ramifications of emergent viral diseases such as Zika, COVID-19, and Ebola. These diseases not only engender significant threats to human health but also exert considerable impacts on the environmental and animal well-being.3 This paradigm embodies a collaborative attempt to acknowledge the intricate interrelations among human, animal, and environmental health domains. Through interdisciplinary cooperation, experts from diverse fields can identify and address the fundamental causes of emergent viral diseases, including factors such as deforestation, climate perturbations, and the proliferation of globalized networks.4 The efficacy of the OH framework has been substantiated through its successful implementation across numerous nations, bringing advancements in disease surveillance, prophylaxis, and mitigation strategies.5
Antimicrobial resistance (AMR) stands out as one of the most significant global health challenges within the OH framework.6 AMR is linked to all three components due to the excessive use of antimicrobials across diverse sectors, including human medicine, livestock farming, and agriculture. Bacteria acquire resistance genes and mobile genetic elements in response to selection pressure from antimicrobials. These genes can be transferred to other bacteria within the same genus or to new species.7 Due to factors such as mismanagement, lack of infection control, agricultural waste, environmental pollution, and the migration of persons and animals, antimicrobial-resistant bacteria have an increased capability to spread to humans, animals, and the environment.8 This study aims to evaluate the historical and contemporary impact of emerging viral diseases on human and animal populations, considering the potential for zoonotic transmission.
Review
Search strategy
Inclusion criteria
Inclusion criteria were (i) studies that focus on zoonosis, emerging viral diseases, and one health approach, (ii) studies published in the past decade, (iii) studies that match the objective of this study, and (iv) studies written in the English language.
Exclusion criteria
Exclusion criteria were (i) Studies written as editorials, book chapters, case series, short communication, or letter to editor; (ii) studies unavailable in full-text; (iii) studies published are not in English language; and (iv) studies whose findings were considered does not match the objective of the study.
Data Extraction
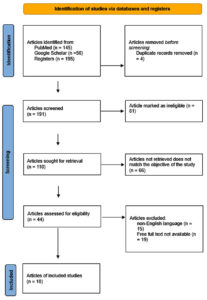
PubMed and Google Scholar search engines were used, Mesh terms were ((“zoonoses”[MeSH Terms] OR “zoonoses”[All Fields] OR (“zoonotic”[All Fields] AND “diseases”[All Fields]) OR “zoonotic diseases”[All Fields]) AND (“one health”[MeSH Terms] OR (“one”[All Fields] AND “health”[All Fields]) OR “one health”[All Fields]) AND (“virus diseases”[MeSH Terms] OR (“virus”[All Fields] AND “diseases”[All Fields]) OR “virus diseases”[All Fields]) AND (“collaborate”[All Fields] OR “collaborated”[All Fields] OR “collaborates”[All Fields] OR “collaborating”[All Fields] OR “collaboration”[All Fields] OR “collaborations”[All Fields] OR “collaborative”[All Fields] OR “collaborative s”[All Fields] OR “collaboratively”[All Fields] OR “collaboratives”[All Fields] OR “collaborator”[All Fields] OR “collaborators”[All Fields]) AND (“india”[MeSH Terms] OR “india”[All Fields] OR “india s”[All Fields] OR “indias”[All Fields]))(Figure 1) (Table 1).
Table (1):
Summary of research on one health strategies and their impact
Sr no. |
Author(s) |
Year |
Population |
Intervention/Exposure |
Outcome Measures |
Main Findings |
Strengths |
Limitations |
Relevance to Review |
|---|---|---|---|---|---|---|---|---|---|
1 |
Deepak et al.10 |
2022 |
Various animal species |
Infection with SARS-CoV-2 |
SARS-CoV-2 infection rates and transmission among animals |
The review highlights the zoonotic origin, its transmission, and the need for a OH approach. |
A comprehensive review of various animal studies on SARS-CoV-2 |
No new experimental data; relies on existing studies |
Provides a thorough understanding of the role of animals in COVID-19 transmission and emphasizes the need for an OH approach |
2 |
Filho et al.11 |
2022 |
Not specific to any population |
Impact of climate change on zoonotic diseases |
Analysis of literature and bibliometric trends |
Climate change alters the conditions for pathogens and vectors, leading to increased spread of zoonotic diseases such as West Nile and Usutu viruses; the USA, UK, Canada, Australia, Italy, and Germany are the most active in research. |
Comprehensive bibliometric analysis, and visualization of data using VOSviewer |
Relies on existing literature, no new experimental data |
Highlights the complex impact of climate change on zoonotic diseases and the need for OH approach |
3 |
Mourya et al.12 |
2021 |
Various populations in India |
Outbreak investigations and OH approach implementation |
Disease spread, outbreak response, and control measures |
Collaboration and coordination among stakeholders were crucial in managing outbreaks and; a significant reduction in impact through early detection and preventive measures. |
Detailed review but no new experimental data; relies on existing cases and outbreak reports |
Detailed review but no new experimental data; relies on existing cases and outbreak reports |
Provides insights into the practice of the OH approach in managing zoonotic diseases. |
4 |
Prejit et al.13 |
2022 |
Population of Wayana, Kerala |
OH approach for Kyasanur Forest Disease (KFD) control |
Degree of OH-ness, outbreak control effectiveness |
The OH approach was effective in controlling the KFD outbreak with high scores in a systemic organization and OH thinking; and identified areas for improvement in OH sharing and learning. |
Comprehensive evaluation of the OH approach using the Network for Evaluation of One Health (NEOH) framework |
Limited to one district and one disease; results may not be generalizable |
Demonstrates the effectiveness of the OH approach and provides a model for future implementation in similar contexts |
5 |
Chatterjee et al.14 |
2016 |
Various stakeholders in India |
Policy analysis and review |
Policy gaps and recommendations |
The paper discusses the missed opportunities in integrating One Health approaches in India’s national health policies, emphasizing the need for intersectoral coordination to address emerging infectious diseases. |
Critical analysis of policy framework, highlighting gaps and missed opportunities |
Focuses on policy analysis, lacks empirical data |
Provides a policy perspective on the importance and integration in national health strategies. |
6 |
de la Rocque et al.15 |
2021 |
Various countries |
One Health roadmap implementation |
Evaluation of IHR capacities and health security |
The study highlights the importance of operationalizing OH roadmaps to improve IHR capacities and health security, identifying gaps and recommending improvements for better coordination at the human-animal-environment interface. |
Comprehensive analysis of IHR capacities and OH implementation |
Limited to analysis, lacks implementation data |
Provides a framework for improving OH implementation and enhancing global health security |
7 |
Tazerji et al.16 |
2020 |
Various animal species |
Transmission of SARS-CoV-2 from humans to animals |
SARS-CoV-2 infection rates among animals, genomic and phylogenetic analyses |
The study reviews reported cases of SARS-CoV-2 transmission to animals, emphasizing the need for further research to understand the dynamics of the disease in humans and animals. |
Detailed genomic and phylogenetic analysis of SARS-CoV-2 isolates from animals |
Relies on existing reports and studies, no new experimental data |
Provides insights into the transmission dynamics of SARS-CoV-2 between humans and animals, highlighting the need for a OH approach |
8 |
Dasgupta et al.17 |
2021 |
Various populations in India |
OH policy implementation |
Policy recommendations, framework for OH Committees |
The study emphasizes the need for intersectoral collaboration and the establishment of OH Committees at state and district levels to address public health challenges effectively. |
Comprehensive policy recommendations, practical framework for implementation |
Focuses on policy analysis, lacks empirical data |
The importance of a coordinated OH approach in addressing zoonotic diseases and other public health challenges in India |
9 |
Wacharapluesadee et al.18 |
2018 |
Various animal species in Thailand |
Surveillance of Nipah virus |
Nipah virus infection rates, surveillance effectiveness |
The study reviews two decades of OH surveillance of Nipah virus in Thailand, highlighting the effectiveness of a coordinated surveillance approach in detecting and managing outbreaks. |
Long-term surveillance data, comprehensive analysis of Nipah virus trends |
Limited to surveillance data, no new experimental data |
Demonstrates the effectiveness of long-term OH surveillance in managing zoonotic disease outbreaks |
10 |
Taaffe et al.19 |
2023 |
Various populations in India |
Intersectoral coordination and collaboration |
Local intersectoral activities and collaboration examples |
The review identifies key themes and examples of local OH activities, demonstrating that intersectoral collaboration primarily occurs through specific research activities, during outbreaks, and community outreach efforts. Limited formal coordination among veterinary, medical, and environmental professionals at the district/sub-district levels is noted. |
Comprehensive landscape assessment, highlighting real-world examples of intersectoral coordination |
Limited to published literature and personal experiences, potential bias |
Provides insights into the current landscape of OH activities at the local level in India, emphasizing the need for formalized and sustained intersectoral coordination |
Study included
The reviewed studies collectively highlight the significant role of the OH approach in addressing complex health challenges at the human-animal-environment interface. Kumar et al. emphasized the zoological origins of SARS-CoV-2 and stressed the need for a one-health strategy to mitigate future epidemics.10 Likewise, Filho et al. explain the increasing impact of climate change on the spread of zoonotic diseases and emphasize the need for integrated health interventions.11 Mourya et al. further demonstrated the effectiveness of one-health interventions. Prejit et al. documented the management and control of successful epidemics of zoonotic diseases in India through cooperation efforts.12,13 Chatterjee et al. and de la Rocque et al. highlighted the critical gap in policy and the importance of implementing a health plan to improve global health security.14,15 The detailed study by Tazerji et al. on the transmission of SARS-CoV-2 in animals and Dasgupta et al. on the need for inter-disciplinary cooperation in India, we further highlight the importance of a multidisciplinary approach to the management of zoonotic diseases.16,17 Finally, Wacharapluesadee et al. long-term surveillance study of Nipah virus in Thailand illustrates the sustained benefits of coordinating.
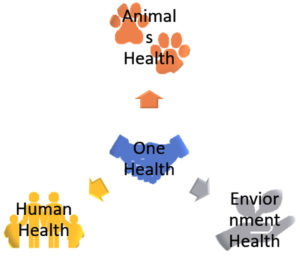
One health approach
One Health approach is a team effort to recognize that the health of animals, humans, and the environment are all connected and that one affects the others.20 Experts in human health, animal health, environmental science, ecology, and public health work together to find and solve the root causes of new viral diseases, which is why the One Health approach includes specialists from different fields.21
The OH approach is not a new concept. It has been recognized since the early 20th century, but it gained renewed attention in the 1990s following the outbreak of zoonotic diseases such as SARS and avian influenza. This approach is now widely recognized as an effective way to tackle emerging viral diseases and other health issues that affect multiple species.22
This approach has several advantages, including improved disease surveillance, prevention, and control. It also encourages a more complete and integrated approach to health, acknowledging the connection between the health of people, animals, and the environment1 (Figure 2).
The link between emerging viral diseases and the environment
Emerging viral diseases are interconnected with changes in the environment, a relationship grounded in the complex relationship between ecological systems, human activities, and infectious agents.23 Scientific evidence highlights the pivotal role of environmental alterations, including deforestation and climate change, in driving the emergence and transmission of zoonotic diseases those that originate in animals and spill over into human populations.24
Deforestation, often driven by agricultural expansion and urbanization, disrupts natural habitats and ecological balance, bringing humans into closer contact with wildlife species that may harbor novel pathogens.25 This increased proximity heightens the risk of zoonotic spillover events, wherein pathogens transfer from animals to humans, potentially resulting in outbreaks or pandemics. Similarly, climate change influences the distribution and behavior of vector species, such as mosquitoes and ticks, altering the transmission dynamics of vector-borne diseases and expanding their geographical range.26
Globalization and intensified travel patterns have profoundly transformed the landscape of disease transmission, facilitating the rapid spread of infectious agents across borders and continents. The COVID-19 pandemic serves as a poignant illustration of how interconnectedness and international mobility can amplify the dissemination of a novel virus.27 The emerging evidence suggests that the SARS-CoV-2 virus, which is responsible for COVID-19, probably originates from bats and may be transmitted to humans by the intermediate host, possibly pangolin, on a wet market in Wuhan, China.28 Subsequent international travel facilitated the global spread of the virus, leading to a devastating pandemic.29
The approach of one health has become a comprehensive framework for understanding and addressing the complex interdependence between human health, animal health, and environmental health. The goal of a health approach is to identify and mitigate the root causes of emerging viral diseases by promoting cooperation between experts from a variety of fields, such as medicine, veterinary sciences, ecology, and public health.1 This collaborative effort involves proactive surveillance, early detection, and preventive interventions aimed at mitigating the risks posed by environmental factors, thereby reducing the likelihood of future outbreaks and pandemics.
In short, the bridge between emerging viral diseases and the environment highlights the urgent need for interdisciplinary cooperation, evidence-based interventions, and sustainable practices to safeguard human health and ecological integrity.30 By adopting a comprehensive and integrated approach, informed by scientific and ethical studies, we can address the complex challenges associated with new infectious diseases and promote the resilience of the global health system to environmental changes.31
Indian Government
The Government of India plays an important role in the implementation of a one health approach that addresses the interdependence of human, animal, and environmental health and mitigates the risks of emerging infectious diseases. As a diverse and populous nation facing numerous health and environmental challenges, the Indian government’s actions and policies have significant implications for public health and ecological integrity.32
In terms of public health, the Indian government is tasked with enhancing disease surveillance, early detection, and response capabilities to combat infectious pathogens. This involves strengthening laboratory infrastructure, expanding diagnostic capacity, and developing robust public health systems capable of rapidly detecting and containing outbreaks. Additionally, promoting research and innovation in fields such as epidemiology and virology is essential for understanding the dynamics of disease emergence and transmission.33
Furthermore, the Indian government plays an important role in environmental governance and biodiversity conservation, addressing factors such as habitat destruction and deforestation that contribute to the emergence of zoonotic diseases. Policies aimed at conserving ecosystems, preserving wildlife habitats, and promoting sustainable land-use practices are integral to a OH approach. Initiatives to regulate the wildlife trade, promote responsible agriculture, and mitigate climate change impacts are also essential for minimizing ecological drivers of disease emergence.34
In terms of international collaboration, the Indian government engages with global health organizations and regional alliances to exchange knowledge and resources related to OH initiatives. Participation in forums such as the World Health Assembly and the Convention on Biological Diversity allows India to contribute to collective efforts to address emerging health and environmental challenges.35
However, challenges such as resource constraints, infrastructure gaps, and socio-economic disparities pose obstacles to effective OH implementation in India. To tackle these challenges, strong political support is needed, along with investment in building skills and community involvement. This will help ensure fair access to healthcare services and environmental resources for everyone.36
The Indian government’s role in implementing a OH approach is multifaceted, encompassing public health, environmental management, and international collaboration. By prioritizing interdisciplinary cooperation, evidence-based policymaking, and community participation, the Indian government can help in the prevention, detection, and control of emerging viral diseases while promoting the health and resilience of ecosystems and communities across the country (Figure 3).35
History of Viral diseases outbreak reported in India
Zoonotic viral diseases have a long history of impacting humans and animals, with notable outbreaks occurring throughout the ages. Rabies, a disease that dates back to ancient times, affects dogs, bats, and wild carnivores and is primarily transmitted by saliva.41 In the 1950s, Japanese Encephalitis emerged, affecting birds and pigs, transmitted by Culex mosquitoes.42 Kyasanur Forest Disease, discovered in 1957, primarily affects monkeys and is transmitted by ticks.43
The 1960s saw the emergence of Dengue Fever, transmitted by Aedes mosquitoes and affecting non-human primates.44 Chikungunya, another mosquito-borne disease, caused a major outbreak in 2006, primarily affecting non-human primates.45 Avian Influenza, or Bird Flu, emerged in 2006, impacting poultry and wild birds through direct transmission.46 The Nipah Virus, identified in 2001 and 2018, is associated with fruit bats and direct contact with bat secretions.47
In 2009, the H1N1 Influenza, or Swine Flu, emerged, primarily affecting mainly pigs and spreading through respiratory droplets.48 Crimean-Congo Hemorrhagic Fever, identified in 2011, affects livestock and is transmitted by ticks.49 Zika Virus gained prominence in 2017, transmitted by Aedes mosquitoes and impacting non-human primates.50 Recently in 2020, SARS-CoV-2, which causes COVID-19, emerged, possibly from bats and pangolins, spreading through respiratory droplets in humans.51 These diseases underscore the ongoing challenge of zoonotic infections and the importance of understanding their dynamics for effective prevention and control measures (Table 2).
Table (2):
Emerging, remerging zoonotic viral diseases in India
Zoonotic Viral Disease |
Year of Detection/Outbreak |
Affected Animals |
Vector |
|---|---|---|---|
Rabies |
Historical (ancient) |
Dogs, bats, wild carnivores |
Direct transmission (saliva/bite)41 |
Japanese Encephalitis (JE) |
1950s |
Pigs, birds |
Mosquito (culex species)42 |
Kyasanur Forest Disease (KFD) |
1957 |
Monkeys |
Ticks (Haemaphysalis spinner)43 |
Chandipura Virus |
1965 |
Not specific |
Sandflies (Phlebotomus species)53 |
Dengue Fever |
1960s |
Non-human primates |
Mosquito (aedes albopictus, aedes aegypti)44 |
Chikungunya |
2006 (major outbreak) |
Non-human primates |
Mosquito (aedes albopictus, aedes aegypti)45 |
Avian Influenza (Bird Flu) |
2006 |
Poultry, wild birds |
Direct transmission (Bird secretions)46 |
Nipah Virus |
2001 (West Bengal), 2018 (Kerala) |
Bats (fruit bats) |
Direct transmission (bat secretions)54 |
H1N1 Influenza (Swine Flu) |
2009 |
Pigs |
Direct transmission (Respiratory droplets)48 |
Crimean-Congo Hemorrhagic Fever (CCHF) |
2011 |
Livestock (cattle, sheep, goats) |
Ticks (Hyalomma species)49 |
Zika Virus |
2017 |
Non-human Primates |
Mosquito (Aedes aegypti, Aedes albopictus)50 |
SARS-CoV-2 (COVID-19) |
2020 |
Bats, Pangolins (Suspected) |
Direct transmission (Respiratory droplets)51 |
Collaboration between veterinary, environmental, and public health sectors to monitor bat populations, educate communities, and implement surveillance and control measures to prevent future outbreaks come under a OH approach.52
Emerging, remerging zoonotic viral diseases in India
Implications for future
The imperative for active disease management necessitates a collaborative approach to surveillance, vaccination, vector control, wildlife management, environmental stewardship, community engagement, and policy formulation, all supported by research and capacity-building initiatives.55 Integrated monitoring systems are necessary for human, animal, and vector surveillance to detect disease emergence easily. Simplified by enhanced data sharing among health, veterinary, and environmental agencies, timely dissemination of information is essential for effective response.56
Vaccination campaigns targeting both animals and high-risk human associates joined with improved access to antiviral therapeutics, establishment is an essential component of disease management strategies. Together, vector control measures, including source reduction and biological agents, aim to restrain vector-borne disease transmission.57 Implementation of zoning protocols and biosecurity measures in livestock and wildlife management, alongside community education on safe animal handling, foster disease containment.58
Environmental management interferences, such as deforestation control and water sanitation measures, moderate habitat disruption and minimize pathogen spillover. Community engagement initiatives, emphasizing preventive practices and culturally adapted messaging, increase public awareness and adopt behavioural change.59 Policy frameworks, surrounding disease control, wildlife trade, and interdisciplinary cooperation, provide the regulatory support necessary for effective implementation.60
The research aims to explain disease ecology dynamics across species and capacity-building initiatives in epidemiology, veterinary science, and environmental health to support institutional resilience against emergent threats. This collaborative approach, supported by scientific accuracy and interdisciplinary collaboration, embodies a positive attitude toward the protection of public health and ecological integrity in the evolving infectious disease challenges (Table 3).61
Table (3):
Key strategies for one health approach in disease prevention
Area |
Strategies |
|---|---|
Surveillance and Early Detection |
• Integrated monitoring means creating systems to keep track of diseases in people, animals, and insects that spread diseases. • Data sharing means improving teamwork between agencies that focus on human health, animal health, and the environment so they can quickly share important information.62 |
Vaccination and Treatment |
• Vaccination Campaigns can conduct vaccination programs in animals (e.g., rabies, avian influenza) and at-risk human populations. • Access to antiviral drugs can improve healthcare through early treatment.63 |
Wildlife and Livestock Management |
• Zoning and biosecurity can be implemented in livestock biosecurity measures and wildlife management strategies. • Educate communities on the safe handling of domestic and wild animals.64 |
Vector Control and Management |
• Source reduction of breeding sites for vectors such as mosquitoes or ticks. • Biological control by using biological agents or natural predators to reduce vector populations.55 |
Community Engagement and Education |
• Public Awareness to educate communities on preventive practices, safe animal handling, and recognizing disease symptoms. • Cultural Adaptation to give messages and strategies to local cultural practices for better adoption.65 |
Environmental and Ecological Management |
• Deforestation control can limit habitat disruption and manage land use changes to minimize wildlife-human contact. • Water management to reduce contamination and ensure proper waste disposal.26 |
Policy and Legislation |
• Establish strong legal regulatory frameworks for disease control, wildlife trade, and livestock management. • Cross-sector cooperation to promote multi-disciplinary coordination between health, agriculture, and environmental sectors.60 |
Research and Capacity Building |
• Research on disease ecology helps us understand how diseases spread between different species. • Training programs to build capacity in epidemiology, veterinary science, and environmental health for sustained expertise.66 |
The one health approach connects the health of people, animals, and the environment, making it essential for managing new diseases that can spread from animals to humans in India. Success requires teamwork across various fields, effective monitoring, vaccination efforts, controlling disease-carrying insects, managing wildlife, and engaging communities. The Indian government plays a crucial role by creating policies, collaborating with other countries, and building the necessary skills to address these health issues. Tackling challenges like antibiotic resistance and environmental damage through sustainable practices can help prevent future outbreaks of zoonotic diseases. By adopting a comprehensive and evidence-based strategy, India can enhance public health and foster a healthier balance between people and nature.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Mackenzie JS, Jeggo M. The One Health Approach-Why Is It So Important? Trop Med Infect Dis. 2019;4(2):88.
Crossref - Rabinowitz PM, Kock R, Kachani M, et al. Toward Proof of Concept of a One Health Approach to Disease Prediction and Control. Emerg Infect Dis. 2013;19(12):130265.
Crossref - Han JJ, Song HA, Pierson SL, Shen-Gunther J, Xia Q. Emerging Infectious Diseases Are Virulent Viruses-Are We Prepared? An Overview. Microorganisms. 2023;11(11):2618.
Crossref - Mubareka S, Amuasi J, Banerjee A, et al. Strengthening a One Health approach to emerging zoonoses. MacDonald R, ed. FACETS. 2023;8:1-64.
Crossref - Ghai RR, Wallace RM, Kile JC, et al. A generalizable one health framework for the control of zoonotic diseases. Sci Rep. 2022;12(1):8588.
Crossref - World Health Organization (WHO). Antimicrobial Resistance: Global Report on Surveillance. World Health Organization; 2014. https://iris.who.int/handle/10665/112642. Accessed March 19, 2024.
- Salam MA, Al-Amin MY, Salam MT, et al. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare. 2023;11(13):1946.
Crossref - Velazquez-Meza ME, Galarde-Lopez M, Carrillo-Quiroz B, Alpuche-Aranda CM. Antimicrobial resistance: One Health approach. Vet World. 2022;15(3):743-749.
Crossref - Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Crossref - Kumar D, Bayry J, Hegde NR. COVID-19: A Veterinary and One Health Perspective. J Indian Inst Sci. 2022;102(2):689-709.
Crossref - Filho WL, Ternova L, Parasnis SA, Kovaleva M, Nagy GJ. Climate Change and Zoonoses: A Review of Concepts, Definitions, and Bibliometrics. Int J Environ Res Public Health. 2022;19(2):893.
Crossref - Mourya DT, Yadav PD, Patil DY, Sahay RR, Rahi M. Experiences of Indian Council of Medical Research with tick-borne zoonotic infections: Kyasanur Forest disease & Crimean-Congo haemorrhagic fever in India with One Health focus. Indian J Med Res. 2021;153(3):339-347.
Crossref - Prejit, Hitziger M, Asha K. Effectiveness of One Health approach for control of Kyasanur Forest Disease in Wayanad, Kerala, India. J Vector Borne Dis. 2022;59(1):70-78.
Crossref - Chatterjee P, Kakkar M, Chaturvedi S. Integrating one health in national health policies of developing countries: India’s lost opportunities. Infect Dis Poverty. 2016;5(1):87.
Crossref - de la Rocque S, Belot G, Errecaborde KMM, et al. Operationalisation of consensual One Health roadmaps in countries for improved IHR capacities and health security. BMJ Glob Health. 2021;6(7):e005275.
Crossref - Tazerji SS, Duarte PM, Rahimi P, et al. Transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to animals: an updated review. J Transl Med. 2020;18(1):358.
Crossref - Dasgupta R, Tomley F, Alders R, Barbuddhe SB, Kotwani A. Adopting an intersectoral One Health approach in India: Time for One Health Committees. Indian J Med Res. 2021;153(3):281-286.
Crossref - Wacharapluesadee S, Ghai S, Duengkae P, et al. Two decades of one health surveillance of Nipah virus in Thailand. One Health Outlook. 2021;3(1):12.
Crossref - Taaffe J, Sharma R, Parthiban ABR, et al. One Health activities to reinforce intersectoral coordination at local levels in India. Front Public Health. 2023;11:1041447.
Crossref - Garg S, Banerjee B. One world, one health. Indian J Community Med. 2021;46(4):581-583.
Crossref - Aggarwal D, Ramachandran A. One health approach to address zoonotic diseases. Indian J Community Med. 2020;45(5):6-8.
Crossref - Osterhaus ADME, Vanlangendonck C, Barbeschi M, et al. Make science evolve into a One Health approach to improve health and security: a white paper. One Health Outlook. 2020;2(1):6.
Crossref - Bankar NJ, Tidake AA, Bandre GR, Ambad R, Makade JG, Hawale DV. Emerging and Re-Emerging Viral Infections: An Indian Perspective. Cureus. 2022;14(10):e30062.
Crossref - Vora NM, Hannah L, Walzer C, et al. Interventions to Reduce Risk for Pathogen Spillover and Early Disease Spread to Prevent Outbreaks, Epidemics, and Pandemics. Emerg Infect Dis. 2023;29(3):1-9.
Crossref - Ortiz DI, Piche-Ovares M, Romero-Vega LM, Wagman J, Troyo A. The Impact of Deforestation, Urbanization, and Changing Land Use Patterns on the Ecology of Mosquito and Tick-Borne Diseases in Central America. Insects. 2021;13(1):20.
Crossref - Esposito MM, Turku S, Lehrfield L, Shoman A. The Impact of Human Activities on Zoonotic Infection Transmissions. Animals. 2023;13(10):1646.
Crossref - Shrestha N, Shad MY, Ulvi O, et al. The impact of COVID-19 on globalization. One Health. 2020;11:100180.
Crossref - Sparrer MN, Hodges NF, Sherman T, VandeWoude S, Bosco-Lauth AM, Mayo CE. Role of Spillover and Spillback in SARS-CoV-2 Transmission and the Importance of One Health in Understanding the Dynamics of the COVID-19 Pandemic. Humphries RM, ed. J Clin Microbiol. 2023;61(7):e01610-22.
Crossref - Dhama K, Khan S, Tiwari R, et al. Coronavirus Disease 2019-COVID-19. Clin Microbiol Rev. 2020;33(4):e00028-20.
Crossref - Parkes MW, Bienen L, Breilh J, et al. All Hands on Deck: Transdisciplinary Approaches to Emerging Infectious Disease. EcoHealth. 2005;2(4):258-272.
Crossref - Piscitelli P, Miani A. Climate Change and Infectious Diseases: Navigating the Intersection through Innovation and Interdisciplinary Approaches. Int J Environ Res Public Health. 2024;21(3):314.
Crossref - Degeling C, Johnson J, Kerridge I, et al. Implementing a One Health approach to emerging infectious disease: reflections on the socio-political, ethical and legal dimensions. BMC Public Health. 2015;15(1):1307.
Crossref - Garg S, Bhatnagar N, Singh MM, et al. Strengthening public healthcare systems in India; Learning lessons in COVID-19 pandemic. J Fam Med Prim Care. 2020;9(12):5853.
Crossref - Singh BB, Gajadhar AA. Role of India’s wildlife in the emergence and re-emergence of zoonotic pathogens, risk factors and public health implications. Acta Trop. 2014;138:67-77.
Crossref - Yasobant S, Bruchhausen W, Saxena D, Falkenberg T. One health collaboration for a resilient health system in India: Learnings from global initiatives. One Health. 2019;8:100096.
Crossref - Kalita A, Carton-Rossen N, Joseph L, Chhetri D, Patel V. The Barriers to Universal Health Coverage in India and the Strategies to Address Them: A Key Informant Study. Ann Glob Health. 2023;89(1):69.
Crossref - Cella E, Giovanetti M, Benedetti F, et al. Joining Forces against Antibiotic Resistance: The One Health Solution. Pathogens. 2023;12(9):1074.
Crossref - International partnership to address human-animal-environment health risks gets a boost | FAO in Geneva | Food and Agriculture Organization of the United Nations. https://www.fao.org/geneva/news/detail/en/c/1136679/. Accessed April 5, 2024.
- Kumar HBC, Hiremath J, Yogisharadhya R, et al. Animal disease surveillance: Its importance & present status in India. Indian J Med Res. 2021;153(3):299-310.
Crossref - Ranjalkar J, Chandy, Sujith J. India’s National Action Plan for antimicrobial resistance – An overview of the context, status, and way ahead. J Fam Med Prim Care. 2019;8(6):1828-1834.
Crossref - Williams EP, Spruill-Harrell BM, Taylor MK, et al. Common Themes in Zoonotic Spillover and Disease Emergence: Lessons Learned from Bat- and Rodent-Borne RNA Viruses. Viruses. 2021;13(8):1509.
Crossref - Mulvey P, Duong V, Boyer S, et al. The Ecology and Evolution of Japanese Encephalitis Virus. Pathogens. 2021;10(12):1534.
Crossref - Pattnaik S, Agrawal R, Murmu J, Kanungo S, Pati S. Does the rise in cases of Kyasanur forest disease call for the implementation of One Health in India? IJID Reg. 2023;7:18-21.
Crossref - Moncayo AC, Fernandez Z, Ortiz D, et al. Dengue Emergence and Adaptation to Peridomestic Mosquitoes. Emerg Infect Dis. 2004;10(10):1790-1796.
Crossref - Broeckel R, Haese N, Messaoudi I, Streblow DN. Nonhuman Primate Models of Chikungunya Virus Infection and Disease (CHIKV NHP Model). Pathogens. 2015;4(3):662-681.
Crossref - Blagodatski A, Trutneva K, Glazova O, et al. Avian Influenza in Wild Birds and Poultry: Dissemination Pathways, Monitoring Methods, and Virus Ecology. Pathogens. 2021;10(5):630.
Crossref - Thomas B, Chandran P, Lilabi MP, et al. Nipah virus infection in Kozhikode, Kerala, South India, in 2018: Epidemiology of an outbreak of an emerging disease. Indian J Community Med. 2019;44(4):383-387.
Crossref - Al-Muharrmi Z. Understanding the Influenza A H1N1 2009 Pandemic. Sultan Qaboos Univ Med J. 2010;10(2):187-195.
- Akuffo R, Brandful JAM, Zayed A, et al. Crimean-Congo hemorrhagic fever virus in livestock ticks and animal handler seroprevalence at an abattoir in Ghana. BMC Infect Dis. 2016;16(1):324.
Crossref - Haese NN, Roberts VHJ, Chen A, Streblow DN, Morgan TK, Hirsch AJ. Nonhuman Primate Models of Zika Virus Infection and Disease during Pregnancy. Viruses. 2021;13(10):2088.
Crossref - Do Vale B, Lopes AP, de Conceicao FM, Silvestre M, Cardoso L, Coelho AC. Bats, pangolins, minks and other animals – villains or victims of SARS-CoV-2? Vet Res Commun. 2021;45(1):1-19.
Crossref - Alimi Y, Wabacha J. Strengthening coordination and collaboration of one health approach for zoonotic diseases in Africa. One Health Outlook. 2023;5(1):10.
Crossref - Sapkal GN, Sawant PM, Mourya DT. Chandipura Viral Encephalitis: A Brief Review. Open Virol J. 2018;12(1):44-51.
Crossref - Skowron K, Bauza-Kaszewska J, Grudlewska-Buda K, et al. Nipah Virus-Another Threat From the World of Zoonotic Viruses. Front Microbiol. 2022;12:811157.
Crossref - Wilson AL, Courtenay O, Kelly-Hope LA, et al. The importance of vector control for the control and elimination of vector-borne diseases. Barrera R, ed. PLoS Negl Trop Dis. 2020;14(1):e0007831.
Crossref - Sharan M, Vijay D, Yadav JP, Bedi JS, Dhaka P. Surveillance and response strategies for zoonotic diseases: a comprehensive review. Sci One Health. 2023;2:100050.
Crossref - Shaw WR, Catteruccia F. Vector biology meets disease control: using basic research to fight vector-borne diseases. Nat Microbiol. 2018;4(1):20-34.
Crossref - Karmacharya D, Herrero-García G, Luitel B, Rajbhandari R, Balseiro A. Shared infections at the wildlife-livestock interface and their impact on public health, economy, and biodiversity. Anim Front. 2024;14(1):20-29.
Crossref - Singh A, Srivastava R. Sustainable Resource Management through Innovative Management Practices. Bharti Publications. 2019:174.
- Li H, Chen Y, Machalaba CC, et al. Wild animal and zoonotic disease risk management and regulation in China: Examining gaps and One Health opportunities in scope, mandates, and monitoring systems. One Health. 2021;13:100301.
Crossref - Zyoud S. Global Mapping and Visualization Analysis of One Health Knowledge in the COVID-19 Context. Environ Health Insights. 2024;18.
Crossref - Belay ED, Kile JC, Hall AJ, et al. Zoonotic Disease Programs for Enhancing Global Health Security. Emerg Infect Dis. 2017;23(13).
Crossref - Tselis A, Booss J. Viral Vaccines and Antiviral Therapy. En Neurol Sci. 2014:659-669.
Crossref - Jori F, Hernandez-Jover M, Magouras I, Durr S, Brookes VJ. Wildlife-livestock interactions in animal production systems: what are the biosecurity and health implications? Anim Front. 2021;11(5):8-19.
Crossref - Hasanov E, Zeynalova S, Geleishvili M, et al. Assessing the impact of public education on a preventable zoonotic disease: rabies. Epidemiol Infect. 2018;146(2):227-235.
Crossref - Garchitorena A, Sokolow SH, Roche B, et al. Disease ecology, health and the environment: a framework to account for ecological and socio-economic drivers in the control of neglected tropical diseases. Philos Trans R Soc B Biol Sci. 2017;372(1722):20160128.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.