Leprosy is a chronic infectious disease caused by the Gram-positive Mycobacterium leprae or M. lepromatosis. Leprosy is a significant public health concern in many parts of the world. It remains a persistent global health challenge, particularly in low-resource settings, despite the success of multidrug therapy (MDT) in reducing disease prevalence. The increasing number of drug-resistant case patients, non-adherence to treatment, and the failure of existing regimens to completely eradicate Mycobacterium leprae underscore the pressing need for novel therapeutic research toward effective treatment. In this review, we explore current conventional therapies. This essay critically examines the challenges posed by prolonged treatment regimens and medication resistance. This review also discusses new developments in leprosy treatment, including the study of new chemical entities in preclinical and clinical settings, as well as promising medication prospects. New drug discovery methods, including high-throughput screening, artificial intelligence, and genomics-guided target identification, are also covered. In addition to new drug discovery, innovative drug delivery methods are also crucial for targeting drug delivery, such as transdermal patches, nanocarriers, and long-acting injectables, which are developed with a focus on improving patient adherence, decreasing the frequency of doses, and enhancing therapeutic efficacy. Collectively, these emerging approaches show promise for a shift toward more efficient, targeted, and patient-friendly leprosy treatments, potentially paving the way for the eventual elimination of the disease.
Leprosy, Treatment, Resistance, Drug Discovery, Delivery Systems
Leprosy is a chronic infectious disease caused by the bacterium Mycobacterium leprae and M. lepromatosis. It is a rod-shaped, non-motile, aerobic, and acid-fast bacterium discovered by G.H. Armauer Hansen in 1873. Leprosy can be traced back thousands of years in ancient texts from Egypt, India, China, and the Mediterranean world, where it was often associated with social isolation and moral judgment.1 Leprosy colonies were established and isolation practices continued far into the 20th century because of the widespread ostracization of leprosy patients throughout centuries due to apparent abnormalities and false beliefs about the disease’s contagiousness. Leprosy is still a public health issue in many low and middle-income nations today, despite tremendous advancements in our knowledge of and ability to treat it.2 According to the World Health Organization (WHO), over 200,000 new cases of leprosy are recorded each year, indicating that the disease’s worldwide impact is still significant. More than 120 nations still have endemic instances of the illness, with Brazil, Indonesia, and India accounting for the majority of new cases. Nearly 75% of all cases worldwide are from these three nations alone. A significant number of new cases are also reported annually by other countries, including Bangladesh, Nepal, Myanmar, and numerous African states.3 Due to worldwide travel and migration, imported cases from endemic areas continue to be a concern, even in affluent nations, which report far lower numbers (Figure 1). Leprosy’s protracted incubation phase, which can last anywhere from a few years to over 20 years, is one of its most challenging features.4
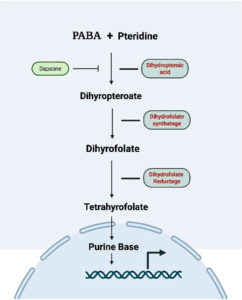
Figure 1. The folate biosynthesis pathway begins with the combination of p-aminobenzoic acid (PABA) and pteridine to form dihydropteroic acid, a step inhibited by dapsone. Dihydropteroic acid is then converted into dihydropteroate, which is acted upon by dihydrofolate synthetase to form dihydrofolate. Dihydrofolate is subsequently reduced to tetrahydrofolate by dihydrofolate reductase. Tetrahydrofolate serves as a critical cofactor for purine base synthesis, which is essential for DNA replication. Inhibition of any of these enzymatic steps disrupts nucleotide production and hinders bacterial growth
The primary obstacle with leprosy is the period between infection and clinical manifestation, which makes early diagnosis and effective therapy more complicated. The progressive cause of leprosy, such as nerve damage, skin sores, muscular weakness, and long-term impairments like blindness and facial, foot, and hand abnormalities, can result from untreated leprosy.5 The illness itself is not as devastating as the cycles of poverty, social exclusion, and stigma that these disabilities intensify. The importance of effective treatment in controlling leprosy cannot be emphasized. When the WHO launched MDT in 1982, leprosy treatment was revolutionized, marking a significant milestone in the fight against this disease.6 MDT is a combination of clofazimine, rifampicin, and dapsone that successfully cures patients and prevents the disease from spreading.7
The WHO’s MDT initiative has significantly decreased the burden of leprosy worldwide and prevented millions of disabilities caused by leprosy. Despite the successes, the early diagnosis and prompt MDT treatment initiation remain significant challenges, especially in remote and rural places of developing and underdeveloped countries. The untreated cases of the disease contribute to spread it throughout communities, and patients who do not receive treatment face the risk of suffering irreversible repercussions. The WHO launched “Zero Leprosy Global Leprosy Strategy 2021-2030”. The main aim is to eradicate leprosy-related stigma and prejudice, as well as infection and disability due to the illness.
To achieve this challenging aim, strong political unity and international collaboration are both required and necessary. There are consistent efforts in case identification, contact tracking, public health education, and rehabilitation programs. This review aims to provide a historical overview of leprosy, highlighting its social, cultural, and medical significance throughout time, and to describe the current worldwide burden of leprosy, with a focus on endemic countries, recent epidemiological trends, and regional disparities.
Current standard treatments
The management of leprosy has undergone significant development over the last century. Initially managed with single-agent therapies such as dapsone, the treatment of the disease necessitated the introduction of combination regimens due to the emergence of drug-resistance.8 Currently, MDT, as recommended by the WHO, is the globally accepted standard for treating leprosy. MDT demonstrated high efficacy in achieving bacteriological cure, preventing complications, and reducing disease transmission in the community (Table 1). Since 1982, the WHO has classified leprosy into two clinical categories: paucibacillary (PB) and multibacillary (MB), based on the number of skin lesions and the results of slit-skin smears.9 Treatment regimens are determined accordingly.
Table (1):
Treatment of leprosy
| Paucibacillary Leprosy | Multibacillary leprosy | ||
|---|---|---|---|
| Rifampicin | 600 mg once monthly | Rifampicin | 600 mg once monthly |
| Dapsone | 100 mg daily | Clofazimine | 300 mg once monthly |
| Duration | 6 months | Dapsone | 100 mg daily |
| Duration | 12 months | ||
The WHO recommends that contacts of diagnosed cases can be managed with a single dose of Rifampicin (SDR) to lower the risk of transmission among close contacts of leprosy patients. Based on the study results, when SDR is administered soon after exposure, it can reduce the likelihood of contacts contracting leprosy by approximately 60%, and it helps to decrease new cases of leprosy. Other antileprotic medicines, such as ofloxacin, minocycline, clarithromycin, and moxifloxacin, have been tested in situations of intolerance, adverse events, or documented drug resistance. Although their use is typically restricted to specific clinical settings, these medications are regarded as second-line options. They are invaluable in cases when the bacillus is resistant to dapsone and rifampicin.8
Mechanism of action of anti-leprosy drugs
Dapsone
Dapsone is a sulfone derivative drug; it has played a critical role in the treatment of leprosy for several decades. It acts by interfering with the folic acid synthesis in M. leprae. Folic acid is essential for purine base synthesis in DNA replication and the multiplication of bacteria.10 Dapsone is a structural analog of para-aminobenzoic acid (PABA), a key substrate in bacterial folate synthesis. It works primarily by competitively inhibiting the enzyme dihydropteroate synthase (DHPS), which catalyzes the condensation of dihydrofolic acid (DHFA) and para-aminobenzoic acid (PABA). This disrupts the production of tetrahydrofolate (THF), an essential cofactor for DNA and protein synthesis in bacteria.11 Dapsone acts by preventing this reaction and lowering the amount of tetrahydrofolate present in the bacterial cell, thereby inhibiting nucleic acid synthesis and bacterial growth (Figure 1). The Dapsone stops M. leprae from multiplying but does not kill the bacteria directly because its action is bacteriostatic rather than bactericidal. This was confirmed through both animal studies and clinical observations. In experimental models, such as the mouse footpad technique, dapsone has demonstrated the ability to suppress M. leprae multiplication at very low concentrations, with a minimum inhibitory concentration (MIC) reported to be around 0.01 µg/mL. However, its slow action and lack of bactericidal effect necessitated the introduction of combination therapy to prevent drug-resistance and enhance treatment outcomes.12
Rifampicin
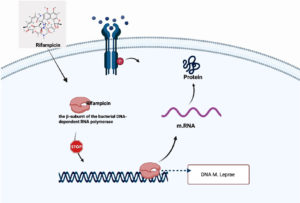
Rifampin is a semisynthetic derivative of rifampicin B; it is the most powerful bactericidal drug used in the WHO’s MDR-TB regimen for leprosy. Rifampicin primarily targets the β-subunit of the bacterial DNA-dependent RNA polymerase enzyme.13 This enzyme is essential for the transcription process, during which genetic information from DNA is converted into messenger RNA (mRNA).14 Rifampicin works by binding to the β-subunit of RNA polymerase, blocking its ability to attach to DNA, which results in the stoppage of mRNA production (Figure 2), a process necessary for protein synthesis and bacterial survival. Without mRNA, the bacteria cannot produce the proteins needed for growth and maintenance, ultimately leading to the death of Mycobacterium leprae, which is why the drug is known for its rapid and strong bactericidal activity. Hu et al. found that High-dose rifampicin (20-35 mg/kg) eradicated RPF-dependent Mycobacterium tuberculosis persisters in vitro and in murine models, enabling faster organ clearance and preventing relapse. This dose-dependent effect supports shortening TB therapy duration and reducing resistance, offering potential for improved human tuberculosis treatment.15
Figure 2. Rifampicin enters the bacterial cell and binds specifically to the α-subunit of the DNA-dependent RNA polymerase enzyme in Mycobacterium leprae. This binding inhibits the initiation of transcription by preventing the polymerase from synthesizing messenger RNA (mRNA) from the bacterial DNA template. As a result, the production of mRNA is halted, leading to suppression of protein synthesis and interruption of essential cellular functions, ultimately inhibiting bacterial replication
Clofazimine
Clofazimine is a highly lipophilic dye belonging to the riminophenazine group and has been widely used as part of multidrug therapy (MDT) for the treatment of leprosy. Although the exact mechanism of action against M. leprae is not fully understood, several studies have provided insights into how this drug works.16 The highly lipophilic character of the drug allows it to accumulate within bacterial cells. Few studies show the mechanism of action via binding strongly to the bacterial DNA, particularly at regions rich in guanine-cytosine base pairs.17 This interaction may interfere with essential DNA functions, ultimately disrupting bacterial growth and survival. The selective binding to the guanine-cytosine base-rich genomes of mycobacteria, as opposed to human DNA, contributes to its targeted antimycobacterial activity.18 Additionally, clofazimine may generate reactive oxygen species (ROS) during its metabolism within bacterial cells, leading to oxidative damage and contributing to its bactericidal effect.19 Few evidence also indicate that clofazimine promotes the accumulation of lysophospholipids, which act as membrane-damaging agents in certain gram-positive aerobic bacteria.20 Although the precise mechanism of action of clofazimine is incompletely understood, its proven clinical effectiveness in reducing bacterial load and controlling leprosy symptoms has made it an essential component of standard leprosy treatment.
Challenges in Current Therapy
Although MDT has helped bring leprosy under control in many parts of the world, several significant challenges remain in its management. These problems impact both the success of treatment and the ultimate goal of eradicating the disease.
Drug-resistance
One of the significant problems in current leprosy treatment is the appearance of drug-resistant M. leprae. A study by Williams et al. found that resistance to dapsone has been linked explicitly to missense mutations in the drug resistance-determining region (DRDR) of the folP1 gene, which encodes the DHPS enzyme. One of the most common observed mutations associated with dapsone resistance is at codons 53 and 55 of the folP1 gene. These types of mutations change the structure of the DHPS enzyme and also reduce the drug’s ability to bind effectively, thereby rendering the bacteria resistant to dapsone.10,21 Whole-gene sequencing of rpoB in 175 M. tuberculosis isolates revealed 34 mutations, with 25 altering rpoB-rifampin binding. Key mutations (S450L, H445D/Y/R) conferred high-level resistance. Structural analysis supports their role in resistance, aiding novel drug and diagnostic development.22
Long duration of treatment
Leprosy is a chronic bacterial infectious disease that requires long-term treatment therapy. Patients with Multibacillary (MB) leprosy need to take medicines for at least 12 months,23 while those with Paucibacillary (PB) leprosy need at least 6 months of continuous treatment.24 Since this disease commonly affects people living in poor and remote areas, it becomes difficult for many patients to continue taking the medicines for such a long time. Many of them stop taking the medication when they start feeling better, without completing the full treatment course. This can lead to the disease returning (relapse) and increase the risk of spreading the infection to others in the community, and also resistance to drugs.25
Drug Side Effects and Compliance Problems
Nowadays, leprosy is treated using an MDT regimen recommended by the WHO, which includes Rifampicin, Dapsone, and Clofazimine. Although this combination is highly effective in eliminating the infection, it is associated with side effects that may interfere with patient compliance.24 Rifampicin can cause discoloration of urine, sweat, and tears, turning them reddish orange, along with symptoms such as nausea, vomiting, and a flu-like syndrome. More seriously, it can lead to liver toxicity.26 Dapsone is known to produce gastrointestinal discomfort, headaches, and skin rashes. In individuals with a deficiency of glucose-6-phosphate dehydrogenase (G6PD), it can cause haemolytic anaemia and, in some cases, methemoglobinemia.27
Clofazimine is a significant drug, but it is associated with pigmentation of the skin, ranging from reddish-brown to nearly black. It may also cause abdominal pain, diarrhoea, and skin dryness. Patients undergoing antileprosy therapy often face compliance problems due to the long duration of treatment, adverse effects, and the social stigma attached to the disease.28 Skin discoloration, systemic side effects, lack of awareness, poor counselling, and fear of discrimination discourage patients from continuing their medications. To address these types of issues, patient education and regular counselling are essential to help patients understand the importance of completing the full course of therapy.29 Early management of side effects and adjustments to treatment, when necessary, can improve patient tolerance. Promoting community awareness and reducing stigma play a significant role in encouraging patients to adhere to therapy and complete their treatment.30
Reactions and relapse
Leprosy can trigger severe immune reactions in the body, even while the patient is undergoing treatment or after completing it. These reactions can cause painful swelling, skin nodules, fever, and nerve damage, resulting in loss of sensation for the patient.31 If not treated in time, these reactions can lead to serious complications, such as permanent disabilities or deformities. Managing these reactions often requires additional medication, such as corticosteroids, for several months, which can lead to immune suppression and other side effects.32 Relapse, when the disease returns after treatment, is another problem. MDT is designed to reduce relapse rates, but relapse still happens, especially if the patient did not complete the full course or if the bacteria were resistant to one or more drugs. Distinguish between relapse and post-treatment reactions is difficult without proper tests and specialist doctors, which are not always available in rural and resource-poor areas.33
Recent advances and emerging drug candidates
Anti-leprosy drugs have been effective in eliminating leprosy. However, adverse reactions such as reactional episodes can still occur months or even years after completing treatment, making it a significant challenge due to their potential to cause nerve damage and disability.34
Corticosteroids are the main drugs used to treat these reactions, but they can cause significant side effects or toxic effects. To avoid this condition, careful dose adjustments and the addition of other drugs to treatments targeting different pathways are required. Over time, a range of alternative drugs has been identified to help manage reactional episodes, including the repurposing of drugs like thalidomide. Thalidomide treats ENL by suppressing TNF-α production and degrading its mRNA, thereby reducing inflammation, fever, and tissue damage. It also modulates T-cell responses towards an anti-inflammatory Th2 profile, lowers cytokines such as IL-6, IL-8, and IFN-γ, and inhibits angiogenesis.35 Overall, it effectively controls type 2 leprosy reactions by reducing painful nodules and nerve inflammation; however, it is highly teratogenic, causing severe birth defects such as limb malformations. A few other drugs, such as minocycline, apremilast, are also used.36,37 Some novel drug therapy treatments are also employed, including plasma exchange, intravenous immunoglobulins, and immunotherapy. However, most of these treatments still focus only on the symptoms and underlying processes of the reaction. To better manage reactional episodes, new approaches are required, and the use of biomarkers, such as genetic, tissue, and serological markers, is necessary to monitor and prevent the recurrence of reactional episodes. There is a necessity of discovering new therapeutic targets and drugs that can offer more effective and long-lasting solutions for managing leprosy reactional episodes.
Recent advances and emerging drug candidates
Currently, Leprosy is treated using a combination of dapsone, rifampicin, and clofazimine, known as multidrug therapy (MDT), which has been recommended by the WHO since 1981. However, over the past decade, the global number of new leprosy cases has remained steady, and instances of drug resistance have been reported in different regions, even though relapses are still uncommon among patients treated with MDT.38 One of the significant challenges in controlling leprosy is the inability to culture M. leprae in laboratory conditions, which limits the ability to perform antimicrobial resistance testing or evaluate the effectiveness of new drugs.39 Moreover, developing entirely new medications for leprosy is not financially appealing for pharmaceutical companies, given the limited commercial market. In this situation, an encouraging alternative is to explore the potential of existing approved drugs, a strategy known as drug repurposing. This approach involves identifying new therapeutic uses for existing drugs that are already approved for other conditions. Combining these repurposed drugs with existing first-line (MDT) or second-line medications could enhance their bactericidal and synergistic effects, offering a more powerful strategy against drug-resistant leprosy. Such combinations may bring us a step closer to the long-standing goal of a leprosy-free world. In this part of review, we will explore new possibilities for repurposing medications to strengthen the fight against drug resistance in leprosy treatment.40
Minocycline
In a retrospective cohort study conducted by Sivakumaran et al., the efficacy and safety of a monthly regimen of rifampicin, ofloxacin, and minocycline (mROM) in the treatment of leprosy were evaluated. Minocycline, a highly potent and lipophilic tetracycline-class antibiotic, was included in the regimen for its proven activity against M. leprae. It works by inhibiting bacterial protein synthesis through binding to the 30S ribosomal subunit, thereby preventing the addition of amino acids to the growing peptide chain.41 Among 29 patients treated with mROM, 26 (89.7%) successfully finished the treatment course. The combination was generally well tolerated, with most adverse effects being mild, and no cases required treatment discontinuation. The study concluded that minocycline, as part of the mROM regimen, is a safe and effective alternative for leprosy management, particularly in non-endemic, resource-rich settings, offering a fully bactericidal therapeutic option.42
Similarly, a clinical trial was conducted by Gelber et al. In this study, the efficacy of minocycline in treating lepromatous leprosy was evaluated. Patients received either a single 200 mg dose or 100 mg twice daily for up to 3 months. The single-dose regimen of minocycline did not significantly reduce M. leprae viability. Still, the twice-daily dose showed regular clearance of viable M. leprae from the dermis by a month’s course. An increase in side effects was observed with higher dosing frequencies, leading to the recommendation of a 100 mg twice daily regimen for managing lepromatous leprosy.43
A study conducted by Celestino et al. at a National Reference Center in Brazil compared the safety profiles of two leprosy treatment regimens: MDT/WHO (Rifampicin, Clofazimine, Dapsone) and ROM (Rifampicin, Ofloxacin, Minocycline). Among 433 patients treated between 2010 and 2021, adverse drug reactions were assessed using clinical and laboratory data. The minocycline regimen was associated with a 44% reduction in the risk of adverse reactions, although this was not statistically significant. Minocycline was responsible for only 1.9% of all adverse events reported; Minocycline adverse reactions included urticaria and scalp itching. These reactions appeared later, with a median onset of around 470 days, likely due to the monthly dosing schedule. The study concluded that the ROM regimen, containing minocycline, was associated with fewer and milder adverse reactions compared to the MDT/WHO regimen. It indicates a safer therapeutic option for treating leprosy.
Ofloxacin
Ofloxacin is a DNA gyrase inhibitor and a major class of antibiotics. There are a few studies conducted on anti-leprotic activity of ofloxacin. A study conducted by Sivakumaran et al. at the Hospital for Tropical Diseases, London, evaluated the efficacy and safety of a monthly regimen of rifampicin, ofloxacin, and minocycline (mROM) in the treatment of leprosy. It acts by inhibiting bacterial DNA gyrase. Among 29 patients treated with mROM, 26 (89.7%) successfully completed the treatment course. The regimen was well tolerated by 29 patients, with adverse effects being mild and no cases leading to discontinuation. The study concluded that ofloxacin, major key component of the mROM regimen, contributes to a safe, effective, and fully bactericidal alternative for managing leprosy in non-endemic, resource-rich settings. Recently, research by Ahuja et al. at Purulia, West Bengal, investigated the prevalence of ofloxacin resistance among newly diagnosed multibacillary leprosy patients. In this study, clinical samples were obtained from patients with new MB cases, and molecular diagnostic methods, including polymerase chain reaction (PCR) and DNA sequencing, were utilized to identify mutations in the gyrA gene, which are known markers of ofloxacin resistance. The findings revealed a significant proportion of cases with primary resistance to ofloxacin, with mutations frequently detected at critical positions within the gyrA gene of bacteria.
The identification of ofloxacin resistance in newly diagnosed MB cases is a serious concern. This is an indicator of the potential circulation of drug-resistant Mycobacterium leprae strains within the local population, which could compromise the efficacy of second-line treatment protocols, especially for cases resistant to rifampicin and dapsone. The occurrence of primary resistance in these patients, despite no prior exposure to ofloxacin and other fluoroquinolones, suggests community-level transmission of resistant strains, representing a concerning development for leprosy control efforts.44
Similarly, Chhabra et al. conducted a study to assess the frequency and resistance patterns of ofloxacin in Mycobacterium leprae isolates obtained from various regions in India.45 Ofloxacin is an essential second line drug, especially for the treatment of rifampicin-resistant leprosy cases. In the study, clinical samples from multibacillary leprosy patients were examined by using polymerase chain reaction (PCR) and DNA sequencing to detect mutations in the gyrA gene, which is responsible for mediating resistance to fluoroquinolones. The findings showed a high rate of ofloxacin resistance, with frequent mutations identified at key positions, particularly at codons 91 and 94 of the gyrA gene. These mutations are directly linked with reduced susceptibility to ofloxacin in leprosy patients. The findings highlighted the increasing problem of both primary and acquired resistance to ofloxacin and pointed to the need for regular drug-resistance monitoring.46
Another study conducted by Priyanto et al. at the National Referral Hospital, Jakarta, Indonesia, aimed to improve the treatment of lepromatous leprosy by adding ofloxacin to the standard multidrug therapy (MDT) regimen. Lepromatous leprosy, being a severe and highly infectious form of the disease, often demands extended treatment. This study evaluated the effect of including ofloxacin, a fluoroquinolone antibiotic known for its antibacterial action against M. leprae in combination with the MDT regimen. The objective was to assess whether ofloxacin could enhance bacterial clearance, enhance clinical outcomes, and potentially reduce treatment duration. Clinical and bacteriological evaluations of patients on this combined MDT regimen were part of these evaluations. The findings showed that adding ofloxacin to the regimen resulted in a quicker drop in the bacterial index and positive clinical outcomes with no notable side effects. Ofloxacin may be a valuable supplement to current treatment regimens, according to the study, especially when leprosy who have a high bacterial load and extensive disease involvement.47
Moxifloxacin
Moxifloxacin, a broad-spectrum antibiotic that is a derivative of fluoroquinolones, kills bacteria by preventing their DNA replication. Research was conducted by Pardillo et al. to evaluate the bactericidal activity of moxifloxacin in the treatment of leprosy. The fourth-generation fluoroquinolone called doxifloxacin had showed significant action against M. leprae in earlier preclinical experiments, particularly in mouse footpad models used to assess bacterial multiplication, but nothing was known about how well it worked in human clinical trials. Moxifloxacin was used in this study to treat multibacillary leprosy patients. Before and after therapy, bacterial viability was assessed using molecular methods and the mouse footpad method. The findings showed that moxifloxacin significantly decreases M. leprae viability, with a 99% reduction in viable bacteria observed after just five days of treatment. This research provided evidence of the strong bactericidal effect of moxifloxacin in human leprosy, supporting its possible use in alternative or second-line treatment regimens, particularly in cases where resistance or intolerance to standard drugs occurs.48
Similarly, a study by Paredes et al. reported a case series from the United States evaluating a novel MDT regimen consisting of monthly rifampin, moxifloxacin, and minocycline (RMM) for the treatment of Hansen’s disease. This approach was used particularly in patients who required alternative therapy due to intolerance or limitations with standard regimens. Moxifloxacin, known for its vigorous bactericidal activity against Mycobacterium leprae, is a key component of this combination. The study observed favourable clinical outcomes in patients, showing remarkable improvement in skin lesions and decline in bacterial index. This regimen was well-tolerated without any significant adverse effects. These findings highlighted the potential of moxifloxacin as an effective agent in modified multidrug therapy (MDT) protocols for leprosy. It is an essential option in cases where standard treatment is not feasible.49
Another study by Pardillo et al. demonstrated that moxifloxacin had a rapid bactericidal effect on two patients with lepromatous leprosy. Both patients got moxifloxacin for the investigation of bactericidal activity, and the mouse footpad technique was used to determine the bacterial viability both before and after therapy. A significant decrease in M. leprae viability was noted after a few days of therapy. The bacterial index rapidly decreased in both cases, demonstrating moxifloxacin’s potent and quick bactericidal effect on leprosy patients. These results showed that moxifloxacin was efficient in rapidly lowering the bacterial burden and indicated that it may be used to treat leprosy, especially in situations when swift bacterial clearance is necessary.50
Thalidomide and its analogues
Thalidomide was initially used in the 1950’s as a non-sedative treatment for the management of morning ailment. However, it was pulled back in early 1960s due to its high teratogenic activity. The drug is a derivative of glutamic acid that comprises of a chiral centre and two amide ring and classified as an immunomodulatory agent that has antiangiogenic activity. Thalidomide is a racemic mixture of R and S isomers at physiologic pH. The S isomer is responsible for teratogenicity; the R isomer results in sedative property. Recent studies have found that currently thalidomide and its analogues are effective in the treatment of leprosy and leprosy reactions.
The research by Lockwood et al. focused on the established use of thalidomide in managing erythema nodosum leprosum (ENL), a common and severe inflammatory complication seen in multibacillary leprosy. The findings explained how thalidomide act by reducing the production of tumour necrosis factor alpha (TNF-α) and it is a key factor in the development of erythema nodosum leprosum. They also discussed the potential benefits of newer thalidomide analogues that may offer similar anti-inflammatory effects with fewer adverse effects. The results highlight the importance of thalidomide analogues in controlling leprosy reactions and emphasize the need for safer and more effective alternatives for long-term use in patients. Similarly, Tadesse et al. studied the effect of thalidomide on the expression of TNF-α mRNA and the production of TNF-α in cells from leprosy patients undergoing reversal reactions. Reversal reaction is a severe inflammatory episode that can lead to nerve damage during leprosy treatment. According to the study, thalidomide dramatically reduces the production of TNF-α protein and TNF-α mRNA in the patient’s cells. A decrease in the inflammatory response has been associated to a drop in TNF-α levels. By decreasing the generation of pro-inflammatory cytokines, thalidomide can help regulate reversal responses, reduce inflammation, and stop further nerve damage in leprosy patients.51
One more study was done by Hernandez et al. They studied the effect of thalidomide on the immune response caused by Mycobacterium leprae, focusing on the NF-κB signalling pathway. This pathway is known to regulate the release of inflammatory cytokines, which are responsible for the reactions seen in leprosy, particularly in conditions like erythema nodosum leprosum and reversal reactions. The study discovered that when M. leprae activated immune cells, thalidomide decreased the activation of the NF-κB pathway. Consequently, the production of pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β b was significantly decreased. Based on these results, the researcher’s teams clarified that thalidomide helps patients with severe leprosy symptoms avoid nerve and tissue damage and minimize inflammation because it is associated with all reactions. This research demonstrates that thalidomide is a useful immunomodulatory medication for controlling leprosy reaction states.52
New molecules in the pipeline
Another severe type of leprosy known as lepromatous leprosy is distinguished by a high bacterial load and weak cell mediated immune response. Even while multidrug treatment has been successful in reducing infection, nerve injury and inflammatory consequences still pose serious management challenges.53 New treatment approaches, such as the repurposing of already approved immunomodulatory medications, are being investigated to address these problems. Gary et al. applied a bioinformatic approach to identify potential immunomodulatory drugs for lepromatous leprosy. By studying gene expression profiles and immune-related pathways involved in the disease, they identified several drug candidates with the potential to modulate immune responses in patients affected by the disease.54 The study highlighted new molecules currently under investigation that could provide additional options for managing the complex immunological reactions associated with lepromatous leprosy. This approach opens new possibilities for improving treatment outcomes and reducing complications in these patients.
A study conducted by Jaiswal et al. aimed to identify potential shared therapeutic and vaccine targets against both species, utilizing subtractive genomics and reverse vaccinology. The genomes of M. leprae and M. lepromatosis were analysed in this work using a comparative bioinformatics technique. The objective was to find conserved, essential, non-host homologous proteins that may serve as broad-spectrum therapeutic targets. The focus is concentrating on surface-exposed and secretory proteins that are likely accessible targets for medications or immunological responses. The researchers screened away human homologs and non-essential proteins. According to their research, the two species share several high-priority targets that may be useful in the development of novel medications and vaccines effective against both types of leprosy. This dual targeting approach presents a potential direction for further study into inclusive and efficient therapies.55
In recent years, drug repurposing has emerged as a valuable approach to identify new therapeutic uses for pre-existing drugs, particularly for neglected diseases like leprosy.
A study done by Thangaraju et al. investigated the potential antileprotic activities of three antiviral agents, Tenofovir, Emtricitabine, and Lamivudine, collectively termed ‘TEL’. Through molecular docking studies, these drugs were evaluated for their binding affinity with phosphoglycerate mutase (gpm1), an essential enzyme in Mycobacterium leprae. Using BIOVIA Discovery Studio software, the enzyme structure (PDB ID: 4EO9) was first energy minimized to stabilize its conformation, reducing its energy from +14,264.5 kcal/mol to -17,588.1 kcal/mol. The docking results demonstrated that all three drugs could interact with the enzyme, with Tenofovir showing the highest binding affinity, indicated by a docking score of -37.7297 kcal/mol. These results suggest that Tenofovir may effectively inhibit the activity of key enzymes, and it may potentially interfere with the survival and metabolism of M. leprae. This study highlights the potential of incorporating Tenofovir and related molecules into a novel approach to leprosy treatment, and encourages further laboratory and clinical investigations to confirm their therapeutic potential. These types of approaches may significantly contribute to the development of new and effective treatment options aimed at achieving a future free from leprosy.56
Barreto et al. have examined the possibility of bedaquiline monotherapy in the treatment of multibacillary leprosy in a proof-of-concept phase 2 open label research carried out in Brazil. Primary use of bedaquiline in MDR TB first time. This drug acts by blocking the ATP synthase enzyme, which alters mycobacteria’s ability to produce ATP. Nine patients with untreated multibacillary leprosy who had at least six skin lesions at the time of enrolment were included in the research. In therapy, 200 mg of bedaquiline was administered orally daily for two weeks, followed by 100 mg three times a week for the next six weeks. Patients started the standard WHO combination medication after completing the 8-week monotherapy and were observed for 112 weeks. The primary objective was to evaluate the change in the growth of Mycobacterium leprae in mouse footpads, while secondary outcomes included assessments of safety, clinical response, and bacterial viability. The results showed a rapid bactericidal effect, with the odds of M. leprae growth dropping from 100% at baseline to no detectable growth by week 4 in all patients. Clinically, noticeable improvements in skin lesions were observed by week 7. In terms of safety, 7 out of 9 patients experienced mild adverse events, none of which were severe. The study concluded that bedaquiline monotherapy resulted in significant bacterial clearance and clinical improvement within a short period, suggesting its potential role as a valuable addition and alternative to current leprosy treatments. These encouraging findings support further research into the integration of bedaquiline into future multidrug or shortened regimens for multibacillary leprosy.57
Advances in drug discovery techniques
Mycobacterium leprae is a complex organism, and the primary difficulties in cultivating it in a laboratory setting have made the process of discovering novel antileprotic medications slow and challenging. New avenues for finding leprosy medicines have been made possible in recent years by developments in drug discovery methods (Figure 3). Molecular docking and in silico screening are two of them that have shown the most promise. To predict how different chemical compounds interact with specific biological targets, in silico screening employs computer-based techniques. This technology is faster and less expensive than standard laboratory techniques because it allows researchers to digitally screen vast chemical libraries against the three-dimensional structures of essential M. leprae proteins.
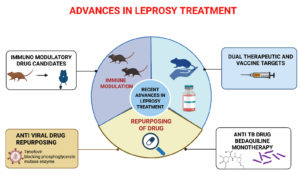
Figure 3. Recent advances in leprosy treatment involve four key strategies: immune modulation, drug repurposing, dual therapeutic and vaccine targets, and antiviral drug repositioning. Immune modulation studies using animal models aim to enhance host immune responses against Mycobacterium leprae. Drug repurposing, particularly with existing therapeutics, is being explored to improve treatment outcomes. Tenofovir, an antiviral drug, is being repurposed to block the phosphoglycerate mutase enzyme in M. leprae, demonstrating promising activity. Additionally, targeting dual-purpose molecules for both therapeutic and vaccine development offers a synergistic approach for long-term disease control and prevention
One crucial technique in in silico screening is molecular docking, which predicts the optimal orientation and binding affinity of small molecules within the active sites of target proteins. This method facilitates the selection of compounds for further experimental testing that exhibit the most promising interactions. These computational techniques limit the amount of chemicals that require in vitro assessment and the necessity for substantial biological culture; they are particularly significant in leprosy research. In addition to studying possible drug-protein interactions that might enhance the effectiveness of currently available medications, such as dapsone and rifampicin. These approaches have been utilized to identify inhibitors of key M. leprae enzymes, including dihydropteroate synthase (DHPS), DNA gyrase, and RNA polymerase.
Additionally, through computational screening of natural products, synthetic compounds and phytochemicals, new candidates with potential antileprotic properties have been identified. Modern computational methods enable scientists to rationally develop novel medicines and modify current ones to enhance their efficacy and reduce research time, particularly when it comes to drug-resistant strains of M. leprae. Considering all these factors, the application of in silico screening and molecular docking in antileprotic drug discovery represents a significant advancement, offering speedier, more efficient, and economically feasible methods to identify new therapeutic options for a neglected disease.
Novel drug delivery systems
Leprosy continues to affect many people globally, but India, Brazil, and Indonesia are the most affected countries. The introduction of MDT in the 1980s by the WHO, using dapsone, rifampicin, and clofazimine, has dramatically reduced the disease burden worldwide; however, the treatment still faces several challenges. One of the major problems is the poor water solubility of most antileprotic drugs, which affects their pharmacokinetic parameters, including absorption and bioavailability. To overcome these, higher doses are required to achieve adequate blood levels, which increases the chances of side effects and can lead to irregular drug concentrations and promote drug-resistance. Rifampicin and clofazimine also face an additional challenge, such as degradation in the stomach and changes in drug behaviour depending on pH. Minocycline, although water-soluble, has poor absorption through the intestine. To address these issues, the role of drug delivery systems has become crucial in leprosy treatment. New formulations and delivery methods are being explored to enhance the absorption, distribution, and release of these drugs in the body. By improving the bioavailability and stability of antileprotic drugs, these systems can help reduce the required doses, lower side effects, and achieve better control over drug levels in the blood. New drug delivery makes treatment safer and helps in preventing the development of drug resistance, improving drug delivery. It is a crucial step towards making leprosy treatment more effective, patient-friendly, and reliable, especially in areas where long-term treatment adherence remains a challenge.
We aim to summarize various innovative drug formulations developed to enhance the treatment of leprosy, focusing on improving the bioavailability, solubility, and controlled release of key leprosy medications, including dapsone (DAP), rifampicin (RIF), clofazimine (CFZ), ofloxacin (OFL), and minocycline (MINO).
For Dapsone (DAP), Several strategies have been investigated to increase the release and solubility of dapsone. When compared to a physical combination, solid dispersion (SD) formulations had a roughly two-fold increase in drug release, and in the first ten minutes, they were 7.5 times more than pure DAP. Furthermore, 82% of the DAP was released over 24 hours when combined with clofazimine in polymeric nanoparticles. The solubility and controlled release of co-crystal and hydrogel formulations were also improved; hydrogels maintained release of up to 20% over 24 hours.58
For Rifampicin (RIF), the solubility and bioavailability of the drug have been tested using new formulations like solid lipid nanoparticles (SLNs) and hydrogel beads. The solid lipid nanoparticles formulation released only 70.12% of the drug over 9 days, whereas free RIF released over 90% in 24 hours. However, the SLNs showed significantly higher plasma concentrations with bioavailability that increased 8.16 times when compared to free Rifampicin. Other formulations, such as particulate hydrogel beads and chitosan-based nanoparticles, also showed improved drug release profiles and solubility.59
For clofazimine (CFZ) formulations, progress has also advanced significantly. Enzyme-mediated carriers and nanoporous silica particles enhanced permeability and solubility; in simulated stomach fluid, nanoporous silica increased the solubility of CFZ by 20 times. Additionally, polymeric nanoparticles improved the overall therapeutic efficacy of CFZ by delivering a regulated release of the medication for up to eight hours.60
In the case of ofloxacin, innovative formulations such as co-crystals and inclusion complexes improved the solubility of ofloxacin (OFL) by up to 3.7 times when compared to the antibiotic in its pure form. Additionally, nanoparticles and polymeric complexes offered regulated release and enhanced pharmacokinetics. For example, PEGylated nanoparticles released 96% of the medication over 36 hours, as opposed to the free drug’s fast release of less than 4 hours. Additionally, these formulations showed enhanced antibacterial efficacy.61
In case of minocycline, to enhance its release profile, minocycline (MINO) has been added to hydrogels, solid lipid nanoparticles, and nanoparticles. After a first burst of release, the hydrogel formulation demonstrated persistent drug release, reaching 100% release only 48 hours later. Specifically, the regulated and continuous release of nanoparticles demonstrated better release kinetics and antibacterial efficacy that was equivalent to that of the free medication.62
Immunomodulators and host-directed therapies
Although the frequency of leprosy has significantly decreased worldwide because of MDT introduced by WHO, there is still persistent transmission, medication resistance, and nerve injury, which necessitate the development of supplemental therapeutic approaches. To boost immune responses, improve treatment outcomes, and prevent long-term problems, immunomodulators and host-directed therapies are becoming increasingly attractive. The primary purpose of immunotherapy is to modulate the host immune response, thereby improving the control of Mycobacterium leprae infection and reducing disease-associated nerve damage. The host immune response plays a significant role in determining the clinical spectrum of leprosy, being substantial in tuberculoid leprosy and minimal in lepromatous forms. Adjuvant immunotherapies, such as the use of immune modulators or recombinant cytokines, have been studied for their ability to shift immune responses towards a more protective Th1 profile. Among these, BCG (Bacillus Calmette-Guerin) immunotherapy has been explored for its potential to enhance cell-mediated immunity against M. leprae. Studies have shown that BCG vaccination, either alone or in combination with killed M. leprae vaccines, can boost Th1-type responses, improve bacterial clearance, and reduce nerve involvement and reactional episodes. These treatments thus hold promise in not only minimizing immune-mediated complications but also in enhancing the overall efficacy of leprosy management.
LepVax
LepVax is a vaccine developed by Duthie et al. aimed at providing both pre-exposure and post-exposure prophylaxis against Mycobacterium leprae infection. The vaccine is composed of a fusion protein (LEP-F1) incorporating three M. leprae specific antigens, combined with the adjuvant GLA-SE to promote a strong Th1-type immune response. In this study, LepVax was tested in nine-banded armadillo and mouse models of leprosy. The findings showed that while post-exposure immunization successfully postponed disease development and reduced nerve damage, pre-exposure vaccination dramatically decreased bacterial loads. LepVax prevented nerve involvement, a key cause of leprosy-related impairment, in the armadillo model in addition to limiting bacterial growth. LepVax was advanced into Phase 1 clinical trials to assess its safety and immunogenicity in humans after the study demonstrated that it was a viable candidate for both therapeutic and preventive uses in leprosy.63
Action of statins against Mycobacteria
Lobato et al.’s study examined the effects of atorvastatin and simvastatin on Mycobacterium leprae and M. tuberculosis infections. M. leprae induces lipid droplet formation in host cells, relying on host cholesterol for survival. Although it lacks HMG-CoA reductase, inhibiting host cholesterol pathways with statins showed mycobactericidal activity within infected macrophages without causing toxicity. Statins alone did not kill M. tuberculosis in culture but enhanced bacterial killing when combined with rifampin, due to preventing cholesterol accumulation and inhibiting ESAT-6 mediated phagosomal escape. In mice, high-dose atorvastatin reduced M. leprae viability and inflammation, though such doses are impractical in humans. Overall, combining statins with rifampin enhanced mycobacterial clearance, reduced tissue inflammation, and may lower reactional episodes in leprosy. This suggests statins could be useful adjuncts to standard MDT, especially in treating MDR and XDR strains.
Future advancements in Leprosy therapy
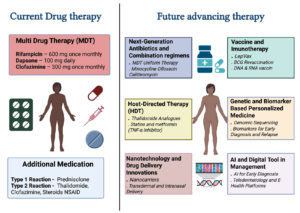
Future advancements in leprosy therapy aim to overcome the limitations of current multidrug treatment regimens and improve disease management. Next-generation antibiotics and combination regimens, such as MDT uniform therapy and the use of drugs like minocycline, ofloxacin, and clarithromycin, are being explored to enhance bactericidal activity and shorten treatment duration.64 Host-directed therapies (HDT) represent a promising approach, targeting the host immune response rather than the bacteria alone. This includes thalidomide analogues, statins, and metformin, which act as TNF-α inhibitors to reduce inflammation and nerve damage. Additionally, vaccine and immunotherapy advancements, such as LepVax, BCG revaccination, and novel DNA and RNA-based vaccines, are under development to enhance protective immunity and reduce transmission.65 Genetic and biomarker-based personalized medicine is another emerging area, focusing on genomic sequencing and identification of biomarkers for early diagnosis and tailored treatment strategies.66 Figure 4 shows these future advancements, including nanotechnology applications in drug delivery through innovations such as nanocarriers for transdermal and intranasal delivery, potentially improving drug absorption and patient compliance. Furthermore, integrating AI and digital tools into leprosy management can aid in early diagnosis, teledermatology, and e-health platforms to enhance patient monitoring and healthcare accessibility. Together, these advancements hold promise for more effective, personalized, and accessible leprosy care in the future. Future advancements in leprosy therapy (Table 2).
Table (2):
Clinical Trials and Regulatory Landscape
Section |
Details |
|---|---|
Recent and Ongoing Clinical Trials |
LepVax Vaccine studies: Safety and immunogenicity were validated in phase 1 studies conducted in the United States. In Brazil, a Phase 1b clinical trial is being conducted to assess the immunological response and safety in endemic areas.63 Single-Dose Rifampicin (SDR) Prophylaxis: The COLEP trial, conducted in Bangladesh, revealed that contacts who received single-dose rifampicin had a 57% lower incidence of leprosy. Its efficacy was validated by other tests performed in Nepal, Indonesia, and India. Immunomodulatory and host-directed therapies: Research is ongoing on recombinant cytokines, such as IFN-γ, immune checkpoint inhibitors, and adjuvant immunotherapies, which aim to enhance immunity and mitigate nerve damage. |
Fast-Tracking and Orphan Drug Status |
In the US and the EU, several leprosy therapies have been designated as Orphan Drugs. Market exclusivity, lower regulatory fees, tax breaks, and regulatory assistance are among the advantages. Drugs that address unmet medical needs, including LepVax and host-directed treatments, are eligible for priority review systems and fast-track approvals. |
Challenges in Clinical Trial Design |
Long Incubation Period: The design of preventative trials is complicated by leprosy’s prolonged incubation period, which can last for several years. Low Incidence in Some Regions: Multinational trials are required due to declining case numbers. Because of clinical heterogeneity, patients must be categorized into several illness categories (TT, BT, BB, BL, LL). Ethical and Social Barriers: Informed consent and patient recruitment are hampered by social issues and stigma. Absence of Standard Biomarkers: For effective therapy monitoring and early diagnosis, reliable biomarkers are essential. Nerve Damage Assessment: In endemic, resource-constrained locations, it might be difficult to conduct long-term, specialized monitoring of peripheral nerve function.5 |
Figure 4. Current leprosy treatment involves Multidrug Therapy (MDT) with rifampicin, dapsone, and clofazimine, along with additional medications for reaction management. Advancing therapies include next-generation antibiotics, host-directed therapies like thalidomide analogues and TNF-α inhibitors, and nanotechnology-based drug delivery systems. Vaccine developments such as LepVax and DNA/RNA vaccines, and genomic tools for personalized medicine are also emerging. AI-driven diagnostic tools and digital health platforms are being integrated to enhance early detection, treatment monitoring, and individualized care in leprosy management
In many endemic areas of the world, leprosy remains a serious public health problem while being overlooked in the global health landscape. Although the WHO’s MDT has made strides, there are still many obstacles to overcome, such as the lengthy course of treatment (minimum six months to one year), drug-resistance, and the possibility of nerve damage-related incapacity. New therapeutic approaches, such as immunomodulatory medicines, novel therapeutic compounds, and repurposed medications, hold promise for more affordable and efficient solutions. Advances in drug discovery techniques, such as high-throughput screening and genomic tools, are also paving the way for more targeted and efficient therapies. The development of new drug delivery systems promises to enhance drug efficacy while minimizing side effects by increasing drug absorption and bioavailability. Immunotherapy and vaccines (such as LepVax) are emerging as adjunctive treatments to bolster the immune response against disease, potentially shortening treatment duration and reducing relapse rates. However, the challenges in clinical trial design, including recruitment issues, stigma, and regulatory hurdles, continue to impede progress. The future of leprosy treatment depends on continued research and collaboration between the public and private health sectors to ensure that new drug therapies are not only practical but also accessible to the populations. The aim to eradicate leprosy can be achieved by sustained efforts in drug development, integration into national control programs, and overcoming socio-economic barriers that will enable us to control and ultimately eliminate leprosy as a public health threat.
ACKNOWLEDGMENTS
The authors extend their appreciation to Northern Border University, Saudi Arabia, for supporting this work through project number NBU-CRP-2025-2042.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Santacroce L, Del Prete R, Charitos IA, Bottalico L. Mycobacterium leprae: A historical study on the origins of leprosy and its social stigma. Infez Med. 2021;29(4):623-632.
Crossref - Bennett BH, Parker DL, Robson M. Leprosy: steps along the journey of eradication. Public Health Rep.2008;123(2):198-205.
Crossref - World Health Organization. Leprosy. https://www.who.int/news-room/fact-sheets/detail/leprosy, Accessed 03 July, 2025.
- Li YY, Shakya S, Long H, Shen LF, Kuang YQ. Factors Influencing Leprosy Incidence: A Comprehensive Analysis of Observations in Wenshan of China, Nepal, and Other Global Epidemic Areas. Front Public Health. 2021;9:666307.
Crossref - Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19(2):338-381.
Crossref - van Brakel WH, Sihombing B, Djarir H, et al. Disability in people affected by leprosy: the role of impairment, activity, social participation, stigma and discrimination. Global Health Action. 2012;5(1).
Crossref - Celestino IC, Antunes DE, Santos DF, Gimenes VL, de Souza FM, Goulart IMB. Adverse reactions induced by MDT/WHO (Rifampicin+ Clofazimine+ Dapsone) and ROM (Rifampicin+ Ofloxacin+ Minocycline) regimens used in the treatment of leprosy: a cohort study in a National Reference Center in Brazil. Front Pharmacol. 2024;15:1346169.
Crossref - Worobec SM. Current approaches and future directions in the treatment of leprosy. Res Rep Trop Med. 2012;3:79-91.
Crossref - Alrehaili J. Leprosy classification, clinical features, epidemiology, and host immunological responses: failure of eradication in 2023. Cureus. 2023;15(9):e44767.
Crossref - Williams DL, Spring L, Harris E, Roche P, Gillis TP. Dihydropteroate synthase of Mycobacterium leprae and dapsone resistance. Antimicrob Agents Chemother. 2000;44(6):1530-1537.
Crossref - Triglia T, Menting JGT, Wilson C, Cowman AF. Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc Natl Acad Sci USA. 1997;94(25):13944-13949.
Crossref - Holmes IB, Hilson GRF. The Effect Of Rifampicin And Dapsone On Experimental Mycobacterium Leprae Infections: Minimum Inhibitory Concentrations And Bactericidal Action. J Med Microbiol. 1972;5(2):251-261.
Crossref - Allen HB, Moschella SL. The Role of Rifampin in Leprosy: Leprosy Through a New Lens. JAMA Dermatol. 2017;153(3):261-262.
Crossref - Monama MZ, Olotu F, Bishop OT. Investigation of Multi-Subunit Mycobacterium tuberculosis DNA-Directed RNA Polymerase and Its Rifampicin Resistant Mutants. Int J Mol Sci. 2023;24(4):3313.
Crossref - Hu Y, Liu A, Ortega-Muro F, Alameda-Martin L, Mitchison D, Coates A. High-dose rifampicin kills persisters, shortens treatment duration, and reduces relapse rate in vitro and in vivo. Front Microbiol. 2015;6:641.
Crossref - Koval A, Bassanini I, Xu J, et al. Optimization of the clofazimine structure leads to a highly water-soluble C3-aminopyridinyl riminophenazine endowed with improved anti-Wnt and anti-cancer activity in vitro and in vivo. Eur J Med Chem. 2021;222:113562.
Crossref - Cholo MC, Mothiba MT, Fourie B, Anderson R. Mechanisms of action and therapeutic efficacies of the lipophilic antimycobacterial agents clofazimine and bedaquiline. J Antimicrob Chemother. 2017;72(2):338-353.
Crossref - The CRyPTIC Consortium. Genome-wide association studies of global Mycobacterium tuberculosis resistance to 13 antimicrobials in 10,228 genomes identify new resistance mechanisms. PLoS Biol. 2022;20(8):e3001755.
Crossref - Yano T, Kassovska-Bratinova S, Teh JS, et al. Reduction of Clofazimine by Mycobacterial Type 2 NADH:Quinone Oxidoreductase: A pathway for the generation of bactericidal levels of reactive oxygen species. J Biol Chem. 2011;286(12):10276-10287.
Crossref - De Bruyn EE, Steel HC, Van Rensburg CEJ, Anderson R. The riminophenazines, clofazimine and B669, inhibit potassium transport in gram-positive bacteria by a lysophospholipid-dependent mechanism. J Antimicrob Chemother. 1996;38(3):349-362.
Crossref - Williams DL, Hagino T, Sharma R, Scollard D. Primary multidrug-resistant leprosy, United States. Emerg Infect Dis. 2013;19(1):179-181.
Crossref - Li MC, Lu J, Lu Y, et al. rpoB Mutations and Effects on Rifampin Resistance in Mycobacterium tuberculosis. Infect Drug Resist. 2021;14:4119-4128.
Crossref - Kumar A, Girdhar A, Girdhar BK. Six months fixed duration multidrug therapy in paucibacillary leprosy: risk of relapse and disability in Agra PB cohort study. BMJ Open. 2012;2(4):e001403.
Crossref - Lazo-Porras M, Prutsky GJ, Barrionuevo P, et al. World Health Organization (WHO) antibiotic regimen against other regimens for the treatment of leprosy: a systematic review and meta-analysis. BMC Infect Dis. 2020;20(1):62.
Crossref - Ali MKS, Thorat DM, Subramanian M, Parthasarathy G, Selvaraj U, Prabhakar V. A study on trend of relapse in leprosy and factors influencing relapse. Indian J Lepr. 2005;77(2):105-115.
- Holdiness MR. A Review of the Redman Syndrome and Rifampicin Overdosage. Med Toxicol Adverse Drug Exp. 1989;4(6):444-451.
Crossref - Kannan G, Vasantha J, Rani NV, et al. Drug usage evaluation of dapsone. Indian J Pharm Sci. 2009;71(4):456-460.
Crossref - Murashov MD, LaLone V, Rzeczycki PM, et al. The Physicochemical Basis of Clofazimine-Induced Skin Pigmentation. J Invest Dermatol. 2018;138(3):697-703.
Crossref - Pepito VCF, Loreche AM, Samontina RED, Abdon SJA, Fuentes DNL, Saniel OP. Factors affecting treatment adherence among leprosy patients: Perceptions of healthcare providers. Heliyon. 2023;9(7):e17975.
Crossref - Stuart H. Reducing the stigma of mental illness. Global Mental Health. 2016;3:e17.
Crossref - Serrano-Coll H, Wan EL, Restrepo-Rivera L, Cardona-Castro N. Leprosy reactions: Unraveling immunological mechanisms underlying tissue damage in leprosy patients. Pathog Dis. 2024;82:ftae013.
Crossref - Yasir M, Goyal A, Sonthalia S. Corticosteroid adverse effects. StatPearls. 2018.
- Nery JAC, Sales AM, Hacker M, et al. Low rate of relapse after twelve-dose multidrug therapy for hansen’s disease: A 20-year cohort study in a brazilian reference center. PLoS Negl Trop Dis. 2021;15(5):e0009382.
Crossref - Teo SK, Resztak KE, Scheffler MA, et al. Thalidomide in the treatment of leprosy. Microbes Infect. 2002;4(11):1193-1202.
Crossref - Kopp KO, Greer ME, Glotfelty EJ, et al. A new generation of imids as treatments for neuroinflammatory and neurodegenerative disorders. Biomolecules. 2023;13(5):747.
Crossref - Narang T, Ashraf R, Kaushik A, Dogra S. Apremilast in multibacillary leprosy patients with chronic and recurrent erythema nodosum leprosum: a prospective single-centre pilot study. J Eur Acad Dermatol Venereol. 2021;35(12):e917-e919.
Crossref - Narang T, Sawatkar GU, Kumaran MS, Dogra S. Minocycline for Recurrent and/or Chronic Erythema Nodosum Leprosum. JAMA Dermatol. 2015;151(9):1026-1028.
Crossref - da-Silva Cruz RC, Buhrer-Sekula S, Penna MLF, Penna GO, Talhari S. Leprosy: current situation, clinical and laboratory aspects, treatment history and perspective of the uniform multidrug therapy for all patients. An. Bras Dermatol. 2017;92(6):761-773.
Crossref - Cambau E, Saunderson P, Matsuoka M, et al. Antimicrobial resistance in leprosy: results of the first prospective open survey conducted by a WHO surveillance network for the period 2009-15. 2018;24(12):1305-1310.
Crossref - Sharma M, Singh P. Repurposing Drugs to Combat Drug Resistance in Leprosy: A Review of Opportunities. Comb Chem High Throughput Screen. 2022;25(10):1578-1586.
Crossref - Martins AM, Marto JM, Johnson JL, Graber EM. A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics. 2021;10(7):757.
Crossref - Hellmann-Regen J, Clemens V, Grozinger M, et al. Effect of minocycline on depressive symptoms in patients with treatment-resistant depression: a randomized clinical trial. JAMA network Open. 2022;5(9):e2230367-e2230367.
Crossref - Gelber RH, Fukuda K, Byrd S, et al. A clinical trial of minocycline in lepromatous leprosy. BMJ. 1992;304(6819):91-92.
Crossref - Ahuja M, Singh I, Lavania M, et al. Ofloxacin resistance in multibacillary new leprosy cases from Purulia, West Bengal: a threat to effective secondary line treatment for rifampicin-resistant leprosy cases. J Glob Antimicrob Resist. 2022;30:282-285.
Crossref - Chhabra S, Narang T, Sahu S, et al. High frequency of ofloxacin resistance patterns of Mycobacterium leprae from India: an indication to revisit second line anti-leprosy treatment regimen. J Glob Antimicrob Resist. 2023;35:262-267.
Crossref - Singh SK, Kumar A, Nath G, Singh TB, Mishra MN. Resistance to anti leprosy drugs in multi-bacillary leprosy: A cross sectional study from a tertiary care centre in eastern Uttar Pradesh, India. Indian J Dermatol Venereol Leprol. 2018;84(3):275-279.
Crossref - Priyanto MH, Yunifananda MS, Menaldi SLS, Nelwan EJ, Marissa M. Optimizing treatment of lepromatous form of leprosy using ofloxacin on top of standard multi-drug therapy in National Referral Hospital, Jakarta, Indonesia. F1000Research. 2025;14:252.
Crossref - Pardillo FEF, Burgos J, Fajardo TT, et al. Powerful Bactericidal Activity of Moxifloxacin in Human Leprosy. Antimicrob Agents Chemother. 2008;52(9):3113-3117.
Crossref - Franco-Paredes C, Garcia-Creighton E, Henao-Martinez A, et al. Novel approaches in the treatment of Hansen’s disease (Leprosy): a case series of multidrug therapy of monthly rifampin, moxifloxacin, and minocycline (RMM) in the United States. Ther Adv Infect Dis. 2022;9:20499361221135885.
Crossref - Pardillo FEF, Burgos J, Fajardo TT, et al. Rapid killing of M. leprae by moxifloxacin in two patients with lepromatous leprosy. Leprosy Review. 2009;80(2):205-209.
Crossref - Tadesse A, Markos A, Elizabeth B, Wondwossen M, Abraham A, Shannon EJ. Effect of Thalidomide on the Expression of TNF-a m-RNA and Synthesis of TNF-a in Cells from Leprosy Patients with Reversal Reaction. Immunopharmacol Immunotoxicol. 2006;28(3):431-441.
Crossref - Hernandez MdO, Fulco TdO, Pinheiro RO, et al. Thalidomide modulates Mycobacterium leprae-induced NF-kB pathway and lower cytokine response. Eur J Pharmacol. 2011;670(1):272-279.
Crossref - Pitta IJR, Hacker MA, Vital RT, et al. Leprosy Reactions and Neuropathic Pain in Pure Neural Leprosy in a Reference Center in Rio de Janeiro – Brazil. Front Med (Lausanne). 2022;9:865485.
Crossref - Espitia GJ, Arenas NE, Guterrez-Castaneda LD, Guerrero MI. Bioinformatic approach for repurposing immunomodulatory drugs for lepromatous leprosy. Int J Mycobacteriol. 2023;12(4):388-393.
Crossref - Jaiswal AK, Tiwari S, Jamal SB, et al. Reverse vaccinology and subtractive genomics approaches for identifying common therapeutics against Mycobacterium leprae and Mycobacterium lepromatosis. J Venom Anim Toxins Incl Trop Dis. 2021;27:e20200027.
Crossref - Thangaraju P, Yella SST, Ramamurthy VA, Navabshan I, Mohamed TALH. A Perspective into “TEL”-Tenofovir, Emtricitabine and Lamivudine Antileprotic Activities by Drug Repurposing and Exploring the Possibility of Combination Chemotherapy with Drug Rescued Molecules for a Leprosy Free Mankind. Recent Adv Antiinfect Drug Discov. 2023;18(3):170-177.
Crossref - Barreto J, Sammarco Rosa P, Adams L, et al. Bedaquiline Monotherapy for Multibacillary Leprosy. N Engl J Med. 2024;391(23):2212-2218.
Crossref - Chaves LL, Vieira ACC, Ferreira D, Sarmento B, Reis S. Rational and precise development of amorphous polymeric systems with dapsone by response surface methodology. Int J Biol Macromol. 2015;81:662-671.
Crossref - Singh H, Jindal S, Singh M, Sharma G, Kaur IP. Nano-formulation of rifampicin with enhanced bioavailability: development, characterization and in vivo safety. Int J Pharm. 2015;485(1-2):138-151.
Crossref - da Rocha NP, Barbosa EJ, Barros de Araujo GL, Bou-Chacra NA. Innovative drug delivery systems for leprosy treatment. Indian J Dermatol Venereol Leprol. 2022;88(3):1-6.
Crossref - Suresh A, Gonde S, Mondal PK, Sahoo J, Chopra D. Improving solubility and intrinsic dissolution rate of ofloxacin API through salt formation via mechanochemical synthesis with diphenic acid. Journal of Molecular Structure. 2020;1221:128806.
Crossref - Chen Q, Shah KN, Zhang F, et al. Minocycline and Silver Dual-Loaded Polyphosphoester-Based Nanoparticles for Treatment of Resistant Pseudomonas aeruginosa. Mol Pharm. 2019;16(4):1606-1619.
Crossref - Duthie MS, Pena MT, Ebenezer GJ, et al. LepVax, a defined subunit vaccine that provides effective pre-exposure and post-exposure prophylaxis of M. leprae infection. npj Vaccines. 2018;3(1):12.
Crossref - Terreni M, Taccani M, Pregnolato M. New antibiotics for multidrug-resistant bacterial strains: latest research developments and future perspectives. Molecules (Basel, Switzerland). 2021;26(9):2671.
Crossref - Ayodele S, Kumar P, van Eyk A, Choonara YE. Advances in immunomodulatory strategies for host-directed therapies in combating tuberculosis. Biomed Pharmacother. 2023;162:114588.
Crossref - Zalli D, Mai Z, Ferati E, Ramaj A, Bregu R, Pranjol MZI. Advancing Precision Medicine. In: Rezaei, N. (eds) Handbook of Cancer and Immunology. Springer, Cham. 2023:1-31.
Crossref
© The Author(s) 2025. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.