Ramanujam, S. Renuka, B. Poornesha and A.N. Shylesha
ICAR-National Bureau of Agricultural Insect Resources, H.A. Farm Post, Bellary Road, Bengaluru – 560 024, India.
ABSTRACT
The six promising isolates of B. bassiana (NBAII-Bb-5a, 7, 14, 19, 23 and 45) were examined for Environmental Scanning Electron Microscopy studies (ESEM) and two B. bassiana isolates (NBAII Bb-5a and Bb-45) were used for Transmission Electron Microscopy (TEM) studies for their endophytic colonization in maize leaf tissues. All the six isolates showed the conidial germination and penetration after 5 days of treatment in maize leaf tissues through Environmental Scanning Electron Microscopy (ESEM) studies. Conidia of Bb-5a and Bb-45 isolates were observed inside the parenchymatic cells through Transmission Electron Microscopy (TEM) studies. This indicates the endophytic establishment of B. bassiana in maize leaf tissues.
Keywords: Beauveria bassiana, Endophyte, Maize, Environmental Scanning
Electron Microscope, Transmission Electron Microscope.
INTRODUCTION
Beauveria bassiana (Balsamo) Vuillemin (Ascomycota: Hypocreales) is the most extensively researched entomopathogenic fungus for biological control of a wide range of insect pests. Endophytes are the microorganisms (bacteria or fungi) which live within the plant system has immense diversity and varied roles in controlling plant pathogens and insects (Saikkonen et al., 2006; Arnold and Lutzoni 2007). B. bassiana has also been established as an endophyte by artificial inoculation in certain crops like, maize (Vakili, 1990), tomato (Leckie, 2002), cotton (Jones, 1994), opium poppy (Quesada-Moraga et al., 2006), cocoa (Posada and Vega, 2005), date palm (Gomez-Vidal et al., 2006), coffee (Posada and Vega, 2006), banana (Akello et al., 2008), jute (Biswas et al., 2012) and sorghum (Reddy et al., 2009 and Tefera and Vidal 2009). It has also been used as a biocontrol agent for management of insect pests like European corn borer (Ostrinia nubilalis) in corn (Bing and Lewis 1991, 1992) and banana weevil (Cosmopolites sordidus) in banana (Akello et al., 2008). In India, six indigenous isolates of B. bassiana were established as endophytes in maize stem and leaf tissues through foliar application of the aqueous conidial suspension and it was confirmed through plating technique and PCR studies (Renuka et al., 2016). The current study was taken up to confirm the colonization of B. bassiana in maize leaf tissues through electron microscopic studies.
MATERIALS AND METHODS
Host plant and fungal inoculation
Seeds of maize variety Nithyashree (University of Agricultural Sciences, Bengaluru, Karnataka, India) were surface sterilized with 3% sodium hypochlorite for two minutes, then with 70% ethanol for two minutes followed by three times rinsing with sterile distilled water. The surface sterilized seeds were dried and then sown in plastic pots of 30cm diameter containing sterile sandy loam soil and watered regularly. The plants were maintained in the greenhouse at 25-28°C, 60–80% RH, and with a 12-h photoperiod. Six indigenous isolates of B. bassiana (NBAII Bb-5a, 7, 14, 19, 23 and 45), which were established as endophytes in maize leaf tissues (Renuka et al., 2016) were selected for this study. Conidia of each isolate were produced on rice and the conidial suspension (1×108 spores/ml) was prepared in sterile distilled water with 0.01% Tween 80. The conidial suspension was applied as foliar spray @ 5ml/seedling on 15 days old maize seedlings using an atomizer. Untreated control plants were sprayed with sterile water containing 0.01% Tween 80.
Microscopic Studies
Sample collection for ESEM
The leaf samples for Environmental Scanning Electron Microscope (ESEM) were collected at 48 and 120 hrs after treatment for observation on conidial attachment and germination on maize leaf surface. The leaf samples from the treated plants were collected randomly, cut into 3mm2 size bits and were placed on the aluminum stub for ESEM observation (Pathan et al., 2008). The specimens were observed on ESEM (Quanta-200 SEM) at Department of Microbiology and Cell Biology, Indian Institute of Science, Bengaluru, India and the images were taken at magnification range of 2000x – 4000x (20Kilo-volts).
Sample preparation for TEM
The leaf samples for Transmission Electron Microscope (TEM) were collected from Bb-5a, Bb-45 treated and un-treated control plants after 20 days of treatment for observation of B. bassiana colonization in maize leaf tissues. The leaf samples were cut into small pieces (approximately 2 x 6mm) for TEM studies and the samples were processed as per the protocol of Wagner and Lewis (2000) with some modifications. Leaf samples were initially fixed in phosphate buffer (pH 7.2) containing 2.5% glutaraldehyde and 2% paraformaldehyde for 2 hrs at 22 to 24°C. Later, the fixative was decanted and the samples were re-fixed in a fresh fixative solution for 24 hrs at 4°C. Then, the samples were rinsed three times with phosphate buffer (10 ml) and post-fixed in 1% Osmium tetroxide (OsO4) for 2 hrs. The samples were washed twice in distilled water for 30 minutes, dehydrated with an acetone series (30, 50, 70, 80, 90 and 100 %) and embedded in epoxy resin (SPURR media). The blocks were placed in an oven at 60°C for overnight. Ultra thin sections of about 60 nm of the resin blocks were made with a glass knife on Ultra microtome (Leica Ultra cut UCT-GA-D/E-1/00). Sections were placed on copper grids, stained with saturated aqueous urenyl acetate, counter stained with Reynolds lead citrate (LC) and then observed under TEM (Model: Hitachi, H-7500) at RUSKA Lab, College of Veterinary Sciences, Rajendranagar, Hyderabad, Telangana, India. Images were taken at an 80-kV accelerating voltage.
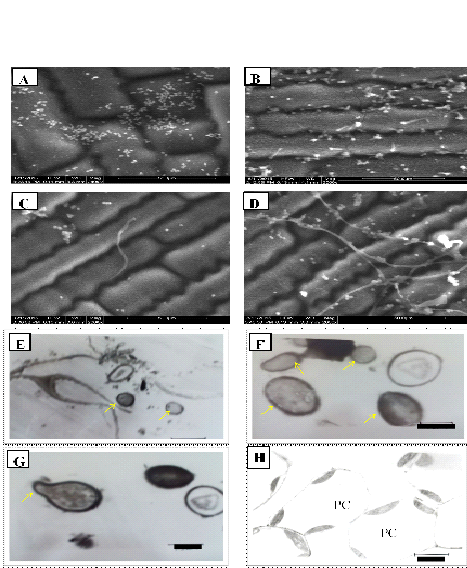
Fig. 1.ESEM Images ofBb-5a isolate:A. Conidialattachment of B. bassiana on maize leaf surface; B. Conidial Germination on leaf surface; C. Penetration of germ tube into the epidermal cells; D.Hyphal ramification on leaf surface.
TEM Images: E. Conidia of B. bassiana(Bb-5a isolate) inside parenchymatic cells Bar, 3.3 µm; F. Conidia of Bb-45isolateinside parenchymatic cells Bar, 1.0 µm; G. Conidia of Bb-45isolate showing germ tubeinside the parenchymatic cells Bar, 1.3µm; H.Parenchymatic cells of untreated leaf devoid of hyphae and conidia of B. bassiana Bar, 5.6 µm.
RESULTS AND DISCUSSION
In ESEM study, the conidial attachment of B. bassiana on the leaf surface was observed after 48 hours of treatment. No conidial germination was observed on leaf surface at 48hours after treatment (Fig. 1A). The conidial germination and penetration of the germ tube was observed after 120 hrs of inoculation. Among the numerous conidia observed on the leaf surface, only few showed germination (Fig. 1B). Germ tubes were gradually elongated and penetrated into the epidermal cell layer (Fig 1 C). The germ tubes of a few germinated conidia showed gradual elongation into hyphae and spread across the leaf surface (Fig. 1D). Nicholson and Epstein, 1991 reported that attachment of conidia on to the host plant cuticle is essential for germination, penetration and colonization. Several fungal pathogens of insects produce dry conidia which adhere to the hydrophobic outer surface of their host for germination and colonization. The germination of the conidia on host surface exhibits the formation of short hyphal growth followed by penetration or extensive mycelial growth and branching on the host surface. Wagner and Lewis (2000) reported that conidia attached to the leaf surface absorb moisture over a 24 to 48hrs period for the development of germ tube. Gautam et al., 2016 reported B. bassiana conidial germination, development and penetration of hyphae on cauliflower leaf surface. Quesada-Moraga et al., (2006) observed B. bassiana conidial attachment and penetration on the leaf surface of opium poppy after 24, 48, 72 and 144 hrs of treatment. In the present study the germination of the conidia was observed after 4 days of the treatment on the leaf surface of maize. This result indicates that, each strain of the fungus has its own adaptable characteristics.
In TEM studies, conidia of Bb-5a and Bb-45 isolates were observed inside the parenchymatic cells (Fig. 1E and F). Bb-45 isolate also showed conidial germination inside the parenchymatic cells (Fig. 1G). No B. bassiana conidia were observed in parenchymatic cells of untreated control leaf tissues (Fig. 1H). B. bassiana showed colonization in the parenchyma and in the vascular tissue of data palm petioles (Gomez-Vidal et al., 2006). Wagner and Lewis (2000) observed the hyphae and conidia of B. bassiana in the parenchyma tissue and xylem vessels in corn through TEM. In this study, conidia or hyphae of B. bassiana were not observed in the xylem vessels of maize leaf tissues.
The present study confirms the endophytic establishment of indigenous isolates of B. bassiana in maize leaf tissues and these isolates can be further field tested as biocontrol agents for management of stem borer in maize.
ACKNOWLEDGMENTS
The authors are thankful to the Director, National Bureau of Agricultural Insect Resources (NBAIR), Bengaluru, India for providing facilities for undertaking the work. Authors are also thankful to Dr. M. Krishna Reddy. and K. Balasubramanian, Indian Institute of Horticultural Research (IIHR), Bengaluru, India, for processing of samples for TEM studies.
REFERENCES
- Saikkonen, K., Lehtonen, P., Helander, M., Koricheva, J., Faeth, S.H.,. Model systems in ecology: dissecting the endophyte grass literature. Trends in Plant Science, 2006; 11: 428–433.
- Arnold, A.E., Lutzoni, F. Diversity and host range of foliar fungal endophytes:tropical leaves biodiversity hotspots? Ecology., 2007; 88: 541–549.
- Vakili, N. G. Biocontrol of stalk rot in corn. In: Proceedings of the Forty-fourth Annual Corn and Sorghum Industry Research Conference, December 6–7, 1989; Chicago, IL. American Seed Trade Association, Washington, DC, 1990; 87–105.
- Leckie, B. M. Effects of Beauveria bassiana mycelia and metabolites incorporated into synthetic diet and fed to larval Helicoverpa zea, and detection of endophytic Beauveria bassiana in tomato plants using PCR and ITS. M.S. thesis, (Entomology), 2002, Department of Entomology, The University of Tennessee, Tennesse, USA.
- Jones, K.D. Aspects of the biology and biological control of the European corn borer in North Carolina. Ph.D. Thesis, North Carolina State University, Raleigh, North Carolina, 1994.
- Quesada-Moraga, E., Landa, B. B., Munoz-Ledesma, J., Jimenez-Diaz, R. M., Santiago-Alvarez, C. Endophytic colonization of opium poppy Papaver somniferum by an entomopathogenic Beauveria bassiana strain. Mycopathology., 2006; 161: 323–329.
- Posada, F., Vega, F.E. Establishment of the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) as an endophyte in cocoa seedlings (Theobroma cacao). Mycologia. 2005; 97: 1195-1200.
- Gómez-Vidal, S., Lopez-Llorca, L. V., Jansson, H. B., Salinas, J. Endophytic colonization of date palm (Phoenix dactylifera L.) leaves by entomopathogenic fungi. Micron., 2006; 37: 624–632.
- Posada, F., Vega, F.E. Inoculation and colonization of coffee seedlings (Coffea arabica L.) with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycoscience., 2006; 47:284–289.
- Akello, J., Dubois, T., Coyne, D., Kyamanywa, S. Effect of endophytic Beauveria bassiana on populations of the banana weevil, Cosmopolites sordidus, and their damage in tissue-cultured banana plants. Entomol. Exp. Appl., 2008; 129: 157–165.
- Biswas, C., Dey, P., Satpathy, S., Satya, P. Establishment of the fungal entomopathogen Beauveria bassiana as a season long endophyte in jute (Corchorus olitorius) and its rapid detection using SCAR marker. Biocontrol. 2012; 57:565–571.
- Reddy, N. P., Ali Khan, A. P., Devi, U. K., Sharma, H. C. Reineke, A.. Treatment of millet crop plant (Sorghum bicolor) with the entomopathogenic fungus (Beauveria bassiana) to combat infestation by the stem borer, Chilo partellus Swinhoe (Lepidoptera: Pyralidae). J. Asia-Pacific Entomol., 2009; 12: 221–226.
- Tefera, T., Vidal, S. Effect of inoculation method and plant growth medium on endophytic colonization of sorghum by the entomopathogenic fungus Beauveria bassiana. BioControl., 2009; 54: 663-669.
- Bing, L.A, Lewis, L.C. Suppression of Ostrinia nubilalis (Hubner) (Lepidoptera: Pyralidae) by endophytic Beauveria bassiana (Balsamo) Vuillemin. Environ. Entomol., 1991; 20: 1207–1211.
- Bing, L.A, Lewis, L.C. Endophytic Beauveria bassiana (Balsamo) Vuillemin in corn: the influence of plant growth stages and Ostrinia nubilalis (Lepidoptera: Pyralidae). Biocontrol Sci. Techn., 1992; 2: 39–47.
- Renuka, S., Ramanujam, B., Poornesha, B. Endophytic ability of different isolates of entomopathogenic fungi Beauveria bassiana (Balsamo) Vuillemin in stem and leaf tissues of maize (Zea mays L.). Indian J. Microbiol., 2016; 56: 126–133.
- Pathan, A. K., Bond, J., Gaskin, R. E. Sample preparation for scanning electron microscopy of plant surfaces—Horses for courses. Micron., 2008; 39: 1049–1061.
- Wagner, B. L., Lewis, L. C. Colonization of corn, Zea mays, by the entomopathogenic fungus Beauveria bassiana. Appl. Environ. Microbiol., 2000; 66: 3468–3473.
- Nicholson, R. L., Epstein, L. Adhesion of fungi to the plant surface: prerequisite for pathogenesis, p. 3–23. In G. T. Cole and H. C. Hoch (ed.). The fungal spore and disease initiation in plants and animals. 1991, Plenum Press, New York, N.Y.
- Gautham, S., Mohankumar, S., Kennedy, J.S. Induced host plant resistance in cauliflower by Beauveria bassiana. J. Entomol. Zool. Stud., 2016; 4: 476-482.