ISSN: 0973-7510
E-ISSN: 2581-690X
The present study tested the remediation potential of Eichhornia crassipes (water hyacinth), for the removal of chromium (Cr) and Zinc (Zn) and Nickel (Ni). Fresh and young plants of equal size were grown in hydroponic medium and supplemented with 300, 600, 1200 and 2400µg/L of Cr and 600, 1200, 2400 and 6000 µg/L of Zn and 300, 600, 1200 and 2400 µg/L of Ni individually for 15 days. The bioaccumulation pattern was reported high in Zn culture. Metal toxicity in the floating macrophyte showed a significant reduction (P <0.001) on phytomass, chlorophyll, NO3-N and PO4-P uptake inhibition in comparison to control. The rate and amount of Cr uptake were minimum as compared to Zn and Ni. The rate of uptake increased with concentration and decreased with increasing time duration. The uptake and accumulation of Cr in the root were always higher than that of shoot except between 2 h to 72 h period at an initial concentration of test metal. The lowest and the highest tolerance indices in Eichhornia crassipes were recorded for Cr and Zn respectively. Bioconcentration factor (BCF) for Zn, Ni, and Cr were 14.6, 12.5 and 10.2 respectively, indicates that Eichorrnia crassipes can be a moderate accumulator of heavy metals and the ubiquitous weed could be used to clean aquatic bodies threatened with pollutants.
BCF, Eichhornia crassipes, Phytoremediation.
Macrophytes are a distinct feature of an aquatic ecosystem and play important roles in wetland biogeochemistry through their active and passive transport of elements. Through their action as the nutrient reservoir1, active uptake of elements into plant tissue may promote immobilization in plant tissues, as reported in wetlands constructed for wastewater treatment2 and in the use of wetland plants in remediation technology. Wetlands are often used as contaminants storage basins, and there are many cases in which wetland plants perform as a pollutant hyperaccumulators for removal of contaminants, including metals. Macrophytes are hyperaccumulators of heavy metals and capable of improving water quality by accumulating heavy metals with their efficient root system. Phytoremediation is an attractive economic cleanup method for moderately contaminated areas. Heavy metal removal from the industrial and domestic polluted stream via bio-absorption is marked beneficial because of releasing negligible secondary pollutants in comparison to conventional physicochemical water treatments plants. Technological advancements and industrial progress have widely disrupted the aquatic ecosystems by various pollutants damaging the ecosystem balance and water quality. Heavy metals penetrate from an aquatic medium into a biological system through water–plant–human or water–plant–animal–human biological network3 herefore finding a solution to overcome the problem of toxicity tolerance in an aquatic body is necessary for an ecosystem and its components. Several reports are describing the effects of heavy metals on water and hydrophytes and their properties, their enzymatic activity, and nutrition pattern4. A negative connection was revealed between heavy metal concentration in growth medium and plants’ submerged organs and green biomass5. Low molecular weight proteins e.g. metallothioneins and phytochelatins seem to work against metal toxicity and other physiological processes to protect plant cells from environmental strain6. Plants work as a machine serving the function of both “accumulation” and “exclusion”. Accumulator plants can survive despite gathering contaminants in their shoot system. They biodegrade or biotransform the contaminants into inactive substances in their tissues. The excluders confine the contaminant uptake into their biomass as stored content. Zinc (Zn) and nickel (Ni) are categorized as essential trace elements needed for the normal growth and healthy metabolism of plants and may produce toxicity if the concentration limit is exceeded7 Whereas, other metals e.g. chromium (Cr) and cadmium are nonessential and pose extreme toxicity to plants even at low supply8.
The suitability of aquatic macrophytes for heavy metal removal has been investigated and reviewed extensively9, 10. Bioaccumulative and persistent nature of heavy metals signifies them as one of the major water pollutants11,12. Implementing phytoremediation technology in environmental remediation is an economical energy saving technology. Macrophytes represent the base of the aquatic food chain they consume metals from water and sediment and subsequently releasing them on senescence and decomposition13, 14, 15. Metal suspension in soil and water are major environmental and human threats. Living plants represent a mechanical system working under the solar energy, consuming certain elements from the biosphere. Phytofiltration of heavy metals and their retention by aquatic vegetation represent the green technology where extensiveness of the root structure consumes the toxic metals from polluted water via evapotranspiration over an extended period.
Eichhornia crassipes, has well-established root, considered as the suitable option for phytoremediation in the aquatic ecosystems for heavy metals and wastewater remediation than terrestrial plants as their rapid growth and significant biomass processing supports higher pollution uptake and better purification method due to direct contact with the water column. t is evident that there is a great scope to explore the potentialities of aquatic plants for heavy metal remediation from the metal contaminated wastewater. Therefore, the present investigation was carried out to study the phytoremediation of Cr, Zn and Ni by Eichhornia crassipes.
Experimental Procedures
E. crassipes plants were collected from a local pond outside the city of Patna, from the Indian subcontinent and washed with tap water to remove attached impurities and insect larval growth on test plant. The plants were grown in cement tanks with tap water under natural sunlight for one week to provide them the natural environment, and then the plants of the same size were selected for the further experiment. A stock solution (1000 µg/L) each was prepared in distilled water with analytical grade K2Cr2O7, ZnCl2, and NiCl2.6H2O that was later, diluted as required. The test metals (Cr, Zn, and Ni) were introduced into the experimental trays at various concentrations (Zn: 600, 1200, 2400 and 6000 g/Land Cr: 300, 600, 1200 and 2400 µg/L, Ni: 300, 600, 1200 and 2400 µg/L). Growth was measured concerning dry weight (dw) at the end of the experiment. Chlorophyll a was estimated using methods of Broody & Broody16. NO3-N uptake was calculated by phenol-di sulphonic acid method19 and PO4–P uptake was estimated by phospho-molybdenum blue color method20. Metal uptake was calculated by the method of Martin21 with the help of an Atomic Absorption Spectrophotometer (Perkin–Elmer 2380). A control experiment without plant was also planned simultaneously with the experimental batch. All experiments were performed in triplicates. The test durations were 2 hours, 3, 5, 10 and 15 days. All the trays were exposed to enough sunlight. Everyday water was added to maintain the same level of water in each tray, to compensate the loss of water through plant transpiration, sampling, and evaporation. At the end of 15th day, plants were harvested. They were separated into roots and shoot and were analyzed for metal accumulation. The specific objective of the study was to examine the uptake of Zn and Cr and Ni at various concentrations for 15 days by Eichhornia crassipes, the effect of metals on chlorophyll, plant biomass, nitrate and phosphate uptake. Also, the metals remained in the solution were measured to assess the removal potential of water hyacinth. The bioconcentration factor (BCF) works as an indicator of the plant ability to accumulate the metal concerning the concentration of metal in the experimental solution. It is calculated as the ratio of the trace element concentration in the plant tissues during harvesting to the concentration of the element in the external environment and is dimensionless6. The BCF was calculated as follows,
BCF= Metal concentration in plant tissue / Metal concentration in external solution.
Metal accumulation
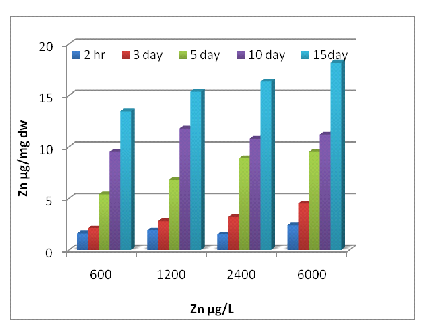
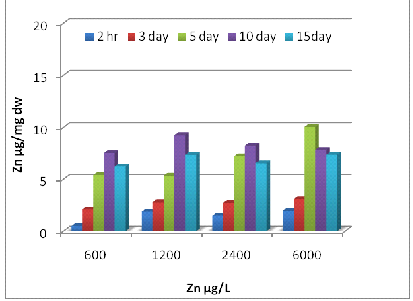
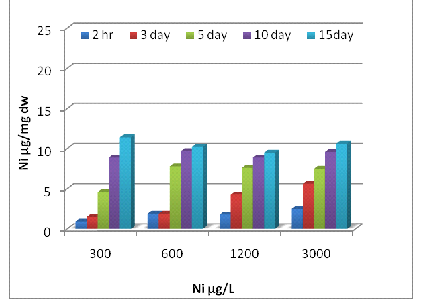
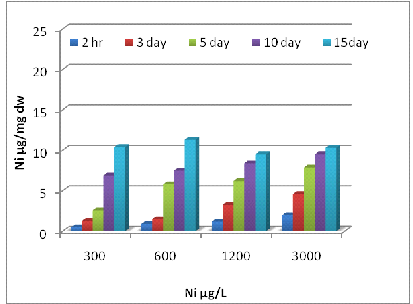
Uptake of metals by Eichhornia crassipes was dependent on time and concentration (Fig 1, 2 and 3). Metal uptake and accumulation in the test plants were estimated in roots and shoots separately. At the beginning of the growth phase, absorption was rapid in all the metals supply, and there was a gradual reduction in uptake rate with increasing time and concentration. For Zn, significant differences (P<0.001) between control and treated plants were found at all metal supply (Fig 1A). The minimum uptake of Zn was 1.6 µg/mg d.w. of Eichhornia crassipes at 600 µg/L at a 2h time interval of which 41% was concentrated by the shoot and almost 60% by the root. The uptake amount increased about fivefold after 15 days at the above concentration of test metal (Fig-1 A). There was a less absorption of metal via shoot system when compared with the root, but at the end of the experiment, shoot concentration increased up to 57 % at the initial concentration of test metal. In the beginning rate of metal, uptake was quite high, and it gradually declined. The amount and rate of uptake in root increased with increasing test metal concentration, and a maximum amount of Zn uptake was 18.2 µg mg-1 d.w. at 6000µg/L test metal level after 15 days of treatment. A significant difference (P <0.001) in Cr accumulation with the passage of time at all concentrations except for 0 and 0.5 µg/L. Plants treated with 1200 µg/L of Cr on the 10th day, accumulated the highest level of metal in shoots (15.4 µg/mg; Fig 2A) and roots (14.3 µg/mg; Fig 2B). Plants treated with 2400 µg/L of Zn on day five accumulated the highest level of metal in shoot region of the plant (10.5 µg/mg; Fig 1A).The ratio of root and shoot Ni uptake varied with time and concentration. At the first two concentration of test metal, the percentage of root uptake was high in most of the time duration except longer term shoot uptake was either high or equal to the root uptake. At 1200 µg/L concentration of test metal between 2 h to days duration root uptake was low but at 7 and 15 days equilibrium exists between roots and shoot uptake. The rate and amount of Ni uptake were comparatively lower than that of Zn. The minimum amount of Ni uptake was 2.5 µg/mg d.w. Ni at 3000 µg/L of test metal after 2 hours and the maximum amount of Ni uptake was 11.4 µg Ni mg-1 d.w. at 300 µg/L of test metal after 15 days. The rate of uptake of Ni was higher at shorter time duration, and it gradually declined with time and concentration (Fig-2B). Cr uptake and accumulation was always greater in the roots than that of shoot except between 2 hr to 72 hr duration at an initial concentration of test metal. After two hr 54 % to 59 % uptake of Cr was done by the root, and after 15 days 49 to 60 % uptake of Cr was done by the shoot.
1(A)
1(B)
Fig. 1- Zn uptake by Eichhornia crassipes [Root (A) and Shoot (B)]
2(A)
2 (B)
Fig. 2- Ni uptake by Eichhornia crassipes [Root (A) and Shoot (B)]
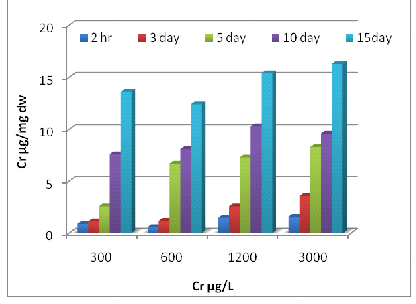
3(A)
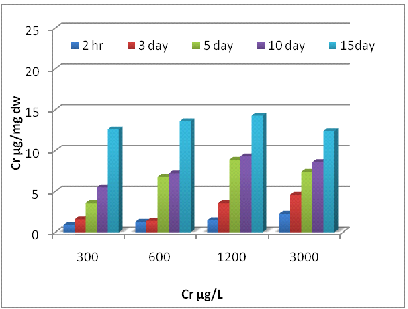
3 (B)
Fig. 3- Cr uptake by Eichhornia crassipes [Root (A) and Shoot (B)]
Root and shoot absorption in Eichhornia crassipes
Floating aquatic plants have a well-designed root system, provides them growth substrates from metal contaminants through rhizofiltration mechanism, adsorption, or precipitation onto plant roots or absorption into the roots of contaminants available in root zone solution. Metal uptake through root systems of aquatic macrophytes and subsequent release of metal during decomposition of plant material represent a cycle between plant biology and metals in aquatic ecosystems. Such a mechanism could have a substantial change in intensity of metal toxicity in aquatic systems and therefore could exert some restrictions on the toxicity of these metals to sensitive organisms.
There are studies on estimation of the metal concentration of macrophytes growing in natural and metal-enriched water bodies [22], [23], [24], [25]. Several reports are on metal uptake and their toxicity to macrophytes [26], [27], [28], [29] [30]. There are studies on shoot vs. root phosphorus storage in Pistia stratiotes [31] and Eichhornia crassipes [32], [31]. The uptake of P by leaves or root of aquatic plants was investigated by several authors [33], [34], [35], [36]. Roots of aquatic plants help absorb nitrogen as an essential nutrient and translocate it to shoots and leaves [37]. Sutton & Blackburn [26] reported that when plants that had accumulated high levels of copper were placed into water containing no copper, showed a decrease in the metal concentration in the plants; evidently, demonstrated the potential role of hydrophytes in metal cycling.
Metals residual in the experimental basins
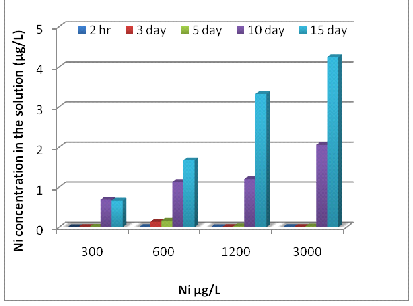
The amounts of remaining dissolved metals in the remaining metal solutions were shown in Fig 2. They were significantly decreased (P<0.001) when the exposure times were increased. The concentrations of dissolved Zn in the solutions at 600, 1200, 2400 and 6000 μg/L were 0.82, 2.42, 5.06 and 6.29 μg/L, respectively on day 15 (Fig 2 A). The concentration of Ni in the culture solution at 300, 600, 1200 and 3000 μg/L were 0.67, 1.67, 3.324, and 4.25 μg/L respectively on the 15th day of the exposure (Fig 2 B). The concentrations of dissolved Cr in the solutions at 300, 600, 1200 and 3000 μg/L were 0.004, 0.006, 0.097 and 0.176 μg/L, respectively on day15 (Fig 2 C).
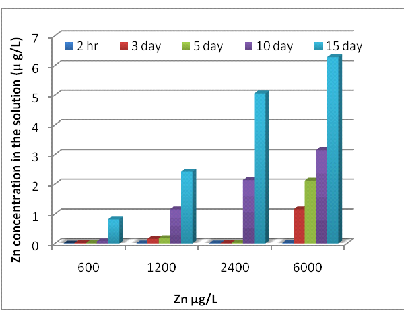
2(A)
2(B)
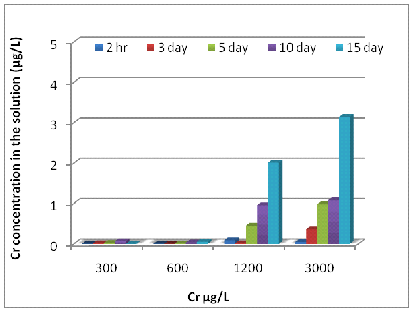
2 (C)
Fig. 4- Zn (A), Ni (B) and Cr (C) concentrations in the experimental solution after 15 days.
Bioconcentrationof metal in Eichhornia crassipes
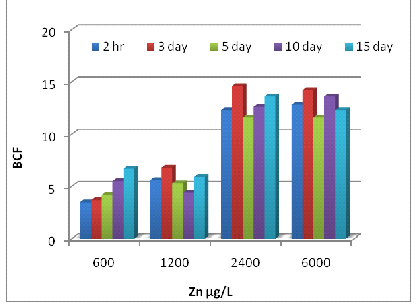
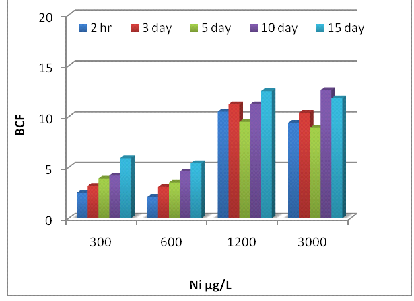
The BCF values for Zn, Ni, and Cr at different concentrations and exposure times were shown in Fig 3 (A, B, and C). In general, the BCF values for Zn, Ni, and Cr increased with the passage of time (p<0.001). The BCF values for Zn significantly increased (p<0.001) with increased Zn dilutions in the test solution at each exposure time and then decreased when the Zn concentration was over 2400 μg/L (Fig 3A). The maximum BCF of 14.6 was obtained in plants treated with 2400 μg/L of Zn on day 3. The BCF values for Ni and Cr decreased (p<0.001) with Ni and Cr concentrations in the basin solutions at each exposure time and the maximum BCF of 12.6 was found in plants treated with 3000 μg/L of Ni on day 10 (Fig 3B). The comparison of maximum BCF of Eichhornia exposed to Cr was 10.2 (3000 μg/L) on day 15 (Fig 3C), over 2400 μg/L (Fig 3A). The maximum BCF of 14.6 was obtained in plants treated with 2400 μg/L of Zn on day 3. The BCF values for Ni and Cr decreased (p<0.001) with Ni and Cr concentrations in the basin solutions at each exposure time and the maximum BCF of 12.6 was found in plants treated with 3000 μg/L of Ni on day 10 (Fig 3B). The comparison of maximum BCF of Eichhornia exposed to Cr was 10.2 (3000 μg/L) on day 15 (Fig 3C).
3(A)
3(B)

3(C)
Fig. 5– The bioconcentration factor (BCF) values of Zn (A), Ni (B) and Cr (C) in E. crassipes at different metal concentrations and exposure times.
Table (1):
Effect of Zn, Ni, and Cr on Biomass (dw), chlorophyll a and NO3-N and PO4-P uptake of shoot and root of Eichhornia crassipes after 15 days exposure time.
Metal concentration µg /L |
DW mg/g |
Chlorophyll µg /mg dw |
NO3-N µg /mg dw |
PO4-P µg /mg dw |
|---|---|---|---|---|
Shoot |
||||
Control |
226±13 |
3.2±0.11 |
120±4 |
90±6 |
Zn |
||||
600 |
165±9 |
0.91±0.03 |
72±3 |
46±2 |
1200 |
139±8 |
0.55±0.05 |
63±4 |
36±2 |
2400 |
90±8 |
0.46± 0.03 |
38±3 |
22±2 |
6000 |
56±5 |
0.32± 0.06 |
30±2 |
14±1 |
Ni |
||||
300 |
182±8 |
0.35± 0.03 |
86±9 |
54±5 |
600 |
158±8 |
0.21± 0.02 |
64±3 |
34±5 |
1200 |
93±5 |
0.61± 0.01 |
39±3 |
22±2 |
3000 |
39±4 |
0.11± 0.01 |
29±3 |
16±2 |
Cr |
||||
300 |
183±10 |
0.22± 0.02 |
66±5 |
44±5 |
600 |
146±14 |
0.15± 0.01 |
32±2 |
21±1 |
1200 |
83±6 |
0.11± 0.01 |
18±1 |
12±1 |
3000 |
28±2 |
0.07± 0.01 |
11±1 |
7±1 |
Root |
||||
Control |
126 ± 10 |
– |
122±6 |
89±4 |
Zn |
||||
600 |
91±4 |
– |
66±6 |
49±3 |
1200 |
82±3 |
– |
55±3 |
38±3 |
2400 |
42±3 |
– |
30±2 |
22±2 |
6000 |
32±2 |
– |
16±2 |
16±2 |
Ni |
– |
|||
300 |
91±5 |
– |
83±6 |
55±3 |
600 |
68±5 |
– |
55±5 |
35±3 |
1200 |
42±1 |
– |
37±2 |
24±2 |
3000 |
24±2 |
– |
13±2 |
11±2 |
Cr |
– |
|||
300 |
99±7 |
– |
63±6 |
41±3 |
600 |
68±2 |
– |
47±7 |
24±2 |
1200 |
38±3 |
– |
22±3 |
20±2 |
3000 |
21±1 |
– |
13±1 |
9±1 |
Table (2):
Two way ANOVA to test for uptake of metals in shoot and root of the species between time (hours), concentration and interaction between hours and concentration.
| Shoot Metals |
F- values | ||
|---|---|---|---|
| Time | concentration | Time* Concentration |
|
| Zn | 541* | 690* | 30* |
| Cr | 519* | 94* | 8* |
| Ni | 771* | 386* | 42* |
| Root | |||
| Zn | 523* | 902* | 47* |
| Cr | 1328* | 176* | 18* |
| Ni | 905* | 419* | 64* |
*All the F values are significant at p < 0.001, at time 15, concentration 3 and time and concentration 45
Plants are the primary producers occupying the autotrophic level of the ecosystem and have the ability to accumulate essential elements from the abiotic medium. Matagi et al.,38 have extensively reviewed on the heavy metal removal mechanisms in wetlands. Denny23, 24 noted that the main route of heavy metal uptake in wetland plants was from the roots in the case of emergent and surface-floating plants like water hyacinth. In locating the sites of mineral uptake in plants, Arisz39 found that ions enter the plants in passive mode, mostly by substitution of cations, occupying place in the cell wall. Denny 24 concluded that plants used heavy metals by absorption and translocation, in their metabolism to some degree and released by excretion. Sharpe and Denny40 and Welsh41 reported, however, that maximum uptake of metals by plant tissues is by absorption to anionic sites located in the cell walls and the metals do not penetrate the living plant. This acknowledges why wetland plants can have a very high magnitude of heavy metal concentration in their tissues compared to their surrounding environment42, 43. Physiology of root pressure and transpiration from the aerial parts of the plants, primarily control pathway of cell sap mixed with metal, from the root to the shoot called translocation44. Accumulation of some metals in roots may be due to some physiological obstacle preventing metal transportation to the stem and leaf part, while others can readily transported in the plants. In the present study, although Cr, Ni and Zn translocation to the plant aerial parts occurred and continued to go on during the whole experiment, the process of sorption was slower than by roots. Translocation of trace elements from roots to shoots could be a limiting factor for the bioconcentration of elements in shoots45. Water hyacinth successfully removes a remarkable quantity of heavy metals (Cd, Co, Cr, Cu, Mn, Ni, Pb, and Zn) from water bodies especially at low concentrations46. Usually, macrophytes root concentrates metals 10 (or more) times higher in root than in shoot. Soltan and Rashed46 while studying with water hyacinth and heavy metals (Cd, Co, Cr, Cu, Mn, Ni, Pb and Zn) observed that water hyacinth accumulated higher concentrations of heavy metals in the roots in comparison to aerial parts.
Growth and morphological changes are often the first and most evident reactions of plants to heavy metal stress47. In plants treated with Zn, macrophyte growth increased in (600 and 1200 µg/L) treatments but decreased in (2400 and 600 µg/L) concentrations. The addition of Zn at low concentration had a favorable effect on the growth of water hyacinth, which may be attributed to the fact that the Zn concentration could encourage plants’ growth and plants utilize Zn as an essential element for their growth48. Delgado et al.,49 found that in long-term experiment (24 days), water hyacinth treated in (9mg/L) of Zn resulted in 30% reduction in weight. Schat et al.,50 reported that Zn toxicity was first visible in the form of reduced root growth. As soon as the saturation state was reached, it seemed a little difficult for plants to absorb Cd or Zn further. Still, the concentration decreased with the metal exposure time.
Metals are required by the plants in different phases of their growth and development. Degradation of heavy metals does not follow the same pattern like organic pollutants51. When the metal deposition in plant tissues reach above optimal levels, they start damaging cell structure by phytotoxicity. E. crassipes in the aquatic system is an efficient cosmopolitan, metal hyperaccumulator because its extensive root system that favors the selective metal uptake. A linear trend was observed in the metal bioabsorption by E. crassipes, indicating more uptake of Zn in comparison to Cr (Fig-1). The root zone technology in floating weed has significant performance in phytoremediation. E. crassipes has submerged, and the extensive root set that can absorb greatest concentrations of heavy metals because of greater root surface area52 and thereby higher heavy metal adsorption capacity of the roots compared to the shoots. Studies revealed the role of cumulative ecosystem effects, involving not only macrophytes but also sediments and another biota necessary to provide a complete picture of the effects of heavy metals on aquatic ecosystems. Metal uptake comprises various factors surrounding the ecosystem such as the availability and amount of contaminants, plants ability to interrupt the process of metal uptake, bioabsorption of metals in the shoot system via root zone, an interaction between metals in the aqueous medium and sediment and biota. Many studies have reported the usage of floating aquatic macrophytes (FAM) design vegetated with Eichhornia53, Pistia54 and Lemna55 as an appropriate heavy metal accumulator. It has been reported that metal accumulating plant species can concentrate heavy metals in proportion to 100 or 1000 times compared to non-accumulator plants. Microorganisms bacteria and fungi associated with the rhizosphere of the plants helps mobilization of metal ions and enhances the bioavailability of the same3.
Phytotoxicity at high degree limits the plant growth and causes various physiological anomalies48. Toxicity of heavy metals is thought to be related with ions and not with their concentration48. There was a reduction in metal treated plant biomass particularly in a high dose of Zn, Ni, and Cr (Table-1). Heavy metal translocation was negligible in the old age plants in the leaves of eelgrass while pectic compounds from the cell wall material thought to play a significant role in the absorption of ions56. E. crassipes population showed a high reduction in biomass when compared to control at a concentration of (1200 µg/L) of Zn and (300 µg/L) of Cr solution. The reason behind a reduction in biomass could be root deformity causing reduced transport of nutrients and water to the shoots. The results obtained demonstrate that the ion exchange is the mechanism solely responsible for the ions uptake. The photosynthetic plastid is one of the most studied biological characteristics used to determine the physiological disorders as a result of metal phytotoxicity. It is reported that some metals decreased chlorophyll content in many plant species58. E. crassipes plant exposed to the metal culture of Zn, Ni and Cr for long duration affected the chlorophyll biosynthesis and thereby resulted in visible symptoms of chlorosis, petiolar chlorosis and necrosis leading to plant decay in the experimental tray (Table-1). Metal toxicity degrades the chlorophyll synthesizing substance and hinders the chlorophyllase activity by Cr metal59.
Among the various forms of nitrogen NO3–N and NH4+ are mostly utilizatized by plants60. The increase metal concentration reflected the inhibition of NO3–N uptake by leaf and root tissues. Nitrate is an essential component of chlorophyll molecule61 gradual decrease in N uptake may be due to binding of metals with the enzyme, which finally resulted in damage to catalytic functions62.The decaying organic matter of the aquatic macrophytes enters detritivores food chains63 and the heavy metals bound in the biomass may be released to higher trophic levels. P was found to decrease heavy metal uptake but in the presence of excess Fe phosphorus inhibition was reported64. Bioconcentration factor (BCF) serves the purpose of evaluating plant potential in the accumulation of metals, this value was calculated on dry weight basis. In aquatic stream, accumulation of metals by macrophytes are under the influence of metal availability in water and sediments65. The surrounding metal concentration in water is the major key factor affecting the metal uptake efficiency66. In general, the BCF values for metals increased with the exposure time (P<0.001). In general, the metal concentration in water is in direct proportion to the amount of metal accumulation in plants tissues, whereas the BCF value goes inversely67. In the present study BCF values decreased when Zn concentration increased, Ni and Cr followed the same pattern except at (1200 µg/L) Ni concentration was high. A report from Jain et al.,48 showed the BCF values for floating species; Azolla pinnata and Lemna minor growing with Pb and Zn gradually decreased with increasing metal concentration in the test solution. A study in the floating macrophytes, Pistia and Salvinia showed BCF of Zn 15.1 and 11.666 respectively. Zhu et al.,45 found that the BCFs of water hyacinth were very high for Cd, Cu, Cr and Se at externally supplied low concentration, and found to decrease as the external supply increased. In the present study, BCF values for Zn were a little higher than those of Cr for the same duration in most cases, indicating that the accumulation potential of Zn by water hyacinth was slightly greater than that of Ni and Cr. The maximum BCF values for Zn, Ni and Cr were 14.6, 12.5 and 10.2, respectively, indicating that E. crassipes is an average accumulator of Zn, Cr and Ni66. Brix et al.,67 found that the BCF values for Cd and Zn in the aboveground parts of eelgrass (Zostera marina) were only 0.62 and 78, respectively. Overall, the floating aquatic macrophytes exhibited better bioconcentration factor for metals was in the following order: Zn>Ni>Cr. This uptake behavior is explained by Zn and Ni as they belong to essential nutrients, unlike the Cr, which can be toxic for photosynthetic activity and production and chlorophyll synthesis. In another study, water hyacinth accumulated higher concentrations of Cu, Ni and Zn in the roots Highest accumulation in root tissues (from 24.75 to 660.0 mg/kg dry wt.) was recorded for Zn, while Cu accumulation was in the range of 18.75 & 115.0 mg/kg dry wt. On the other side, the least accumulation of 5.65 to 16.0 mg/kg dry wt. was observed for Ni; these values showed that the affinity of water hyacinth in Zn accumulation is more than that for Cu and Ni. In shoot tissues, also Zn recorded the highest level of collection (9.26 – 112.5 mg/kg dry wt.) followed by Cu (2.5 – 19.0 mg/kg dry wt.) then by Ni (0.5 – 2.2 mg/kg dry wt.). This demonstrated that Zn is more mobile from roots to shoots than Cu and Ni68. Lu et al.,70 reported that the accumulation of metals in the roots and shoots of water hyacinth had been shown in many field studies in which water hyacinth was used as a biological monitor for metal pollution. Stratford et al.,71 found that the metals’ accumulations in water hyacinth were mono-dimensional only in the culture solution, exhibiting the order of accumulation in leaves < stems < roots. This study also demonstrated a pattern of metal uptake similar to that of Stratford et al.,71.
Metal uptake in the rooted emergent plants is entirely dependent on the roots while in surface floating plants select their leaves for metal uptake.
Free-floating rooted plants have the mechanism of metal uptake involving both roots and leaves. In Submerged macrophytes, metal uptake occurs through roots and leaves growing under the water column. These submerged plants have some potential for the extraction of metals from sediments as well as water.
Toxic metal contaminated aquatic bodies, ground waters are becoming a threat to the economy and public health safety. Monitoring of the surface water quality is, therefore, required to evaluate the condition of surface water of the water bodies all over the world. The use of plants to remove these pollutants would provide an efficient, low cost, in situ cleanup technology, which can readily scavenge toxic metals from the site leaving it intact for normal ecosystem functioning and development. The bioaccumulative and rhizofiltration capacity of Eichhornia, an aquatic weed, helps infiltration of the metallic contaminants from the water systems. Maintenance of proper density of the weed in the water body can be managed by controlled harvesting followed by disposal to regulate the heavy metal contamination in the lake ecosystem without introducing any foreign chemical substance. The phytotoxicity of Eichhornia was noticeable in the presence of metals (Zn, Ni and Cr) Culture and can be ideal for the heavy metal pollutant monitoring agent in eco-technology.
- W. E. Odum, “Comparative ecology of tidal freshwater and salt marshes” Annual Review Ecological Systematics, 1988; 19: pp. 147 – 176.
- R.H. Kadlec and R.I. Knight, Treatment wetlands. Boca Raton, FL: CRC Press, 1996.
- B.V.Tangahu, S.R. Abdullah, H. Basri, M. Idris, N. Anuar and M. Mukhlisin, “A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation”, International Journal of Chemical Engineering (doi.org/10.1155/2011/939161), 2011.
- G. T.W. Wittmann, “Toxic chemicals. In. Metal pollution in the aquatic environment”, In U, FÜrstnerr & GTW, Wittmann (ed.), Springer verslag, Berlin Germany, 1979; pp.3-68.
- A. Zayed, S. Gowthaman and N. Terry, “Phytoaccumulation of trace elements by wetland plants: I Duckweed”, Journal Environmental Quality, 1998; 27: pp. 715-721.
- A.R. Memon and P. Schroder, “Implications of metal accumulation mechanisms to phytoremediation”, Environmental Science and Pollution Research, 2009; 16(2): pp. 162-175.
- Z. Rengel, Heavy metals as essential nutrients, Heavy Metal Stress in Plants. From Biomolecules to Ecosystems, In: M.N.V. Prasad (Ed.), 2nd edn. Springer, New York, 2004; pp. 271–285.
- A.M. Zayed and N. Terry, Chromium in the environment: Factors affecting biological remediation, Plant Soil, 2003; 249: 139–156.
- S. Cheng, “Heavy metal pollution in China: Origin, pattern and control”, Environmental Science & Pollution Research, 2003; 10(3): pp. 192-198.
- P.K. Rai, “Heavy metal phytoremediation from aquatic ecosystems with special references to macrophytes”, Crtical Reviews in Environmental Science & Technology, 2009; 39(9): pp. 697-753.
- J.S. Chang, I.H. Yoon, K. W.Kim, “Heavy metal and arsenic accumulating fern species as potential ecological indicators in As-contaminated abandoned mines,” Ecological Indicators, 2009; 9: pp. 1275–1279.
- S.K. Yadav, A.A. Juwarkar, G.P. Kumar, P.R. Thawale, S.K. Singh and T. Chakrabarti, “Bioaccumulation and phyto-translocation of arsenic, chromium and zinc by Jatropha curcas L.: Impact of dairy sludge and bio fertilizer”, Bioresource Technology, 2009; 100: pp. 4616-4622.
- H. Brix and H. Schierup, “The use of aquatic macrophytes in water pollution control”, Ambio, 1989; 18: pp.100-107.
- Shardendu, N. Salhani, S.F. Boulyga and E. Stengel, “Phytoremediation of selenium by two helophyte species in subsurface flow constructed wetland”, Chemosphere, 2003; 50(8): pp. 967-973.
- Azaizeh, N. Salhani, Z. Sebesvari, S. Shardendu and H. Emons, “Phytoremediation of Selenium using subsurface-flow constructed wetland”, International Journal Phytoremediation, 2006; 8: pp. 187-198.
- S. S. Brody and M. Brody, “Absorption properties of aggregated (dimeric) chlorophyll”, Biochemistry Biophysics Acta, 1961; 54: pp. 495-505.
- O.H. Lowry, N.J. Rosenbrough, A.L. Farr and R.J. Randall, “Protein measurement with the folin-phenol reagent”, Journal Biological Chemistry, 1951; 193: pp. 265-275.
- M. DuBois, K.A. Gilles, J.K. Hamilton, P.A. Rebers, PA and F. Smith, “Colorimetric Method for Determination of Sugars and Related Substances”, Analytical Chemistry, 1956; 28(3): pp. 350-356.
- D.J. Nicholas A. Nason, “Determination of nitrate and nitrite”, In Methods in Enzymology, SP Colowick & NO Kaplan (ed.), vol. 3, Academic Press, New York 1957.
- American Public Health Association (APHA), Standard methods for the examination of water and wastewater, 16th ed, Washington DC, USA, 1999.
- J.H. Martin, “Bioaccumulation of heavy metals by littoral and pelagic marine organisms”, US Environment Protection Agency 600/3-77-038, 1979.
- F.S. Adams, H, Cole, and L.B. Massie, “Element constitution of selected vascular plants from Pennsylvania: submerged and floating leaved species and rooted emergent species”, Environmental Pollution, 1973; 5: pp. 117—147.
- R.P. Welsh and P. Denny, “The uptake of lead and copper by submerged aquatic macrophytes in two English lakes”, Journal Ecology, 1980; 68: pp. 443-455.
- Denny P (1987) Mineral cycling by wetland plants – a review. ( Arch Hydrobiol 27,1-25.
- E. Pip, “Cadmium, copper and lead in aquatic macrophytes in Shoal Lake (Manitoba-Ontario)”, Hydrobiology, 1990; 208: pp. 253-260.
- D.L. Sutton, R.D. Blackburn and W.C. Barlowe, “Response of aquatic plants to combinations of endothal and copper”, Weed Science, 1971; 19, pp. 643-651.
- R. A. Stanely, “The toxicity of heavy metals and salts to euroasian watermill foil (Myriophyllum spictum L.)” Archive Environmental Contamination Toxicology, 1974; 2: pp. 331-341.
- M. J. Behan, T.B. Kinraid and W.I. Selser, “Lead accumulation in aquatic plants from metallic sources including shot”, Journal Wildlife Management, 1979; 43: pp. 240–244.
- R. Peter, H. Welsh and P. Denny, “Translocation of lead and copper in two submerged aquatic angiosperm species”, Journal Experimental Botany, 1979; 30: pp. 339-345.
- S.H. Kay, W.T. Haller and L.A. Garrard, “Effects of heavy metals on water hyacinth (Eichhornia crassipes)”, Aquatic Toxicology, 1984; 5: pp. 117-128.
- M. Agami, and K.R. Reddy, “Competition for space between Eichhornia crassipes (Mart.) solms and Pistia stratiotes L. cultured in nutrient enriched water”, Aquatic Botany, 1990; 38: pp. 195-205.
- C.S. Tucker and T.A. DeBusk, “Seasonal growth of Eichhornia crassipes (Mart.) Solm: Relationship to protein, fiber and available carbohydrate content”, Aquatic Botany, 1983; 11: pp. 137-141.
- C.P. McRoy and R.J. Barsdate, “Phosphate absorption in eelgrass”, Limnology Oceanography, 1970; 15: pp. 6-13.
- M. Bristow and M. Whitecomb, “The role of roots in the nutrition of aquatic vascular plants”, American Journal Botany, 1971; 58: pp. 8-13.
- C.P. McRoy, R.J. Barsdate and M. Nebert, “Phosphorus cycling in an eelgrass (Z. marina L.) ecosystem”, Limnology Oceanography, 1972; 17, pp. 58–67.
- J.A. Demarte, and R.T. Hartman, “Studies on absorption of 32-P, 59-Fe and 45-Ca by water- milfoil (Myriophyllum exalbescens Fernald)” Ecology, 1974; 55: pp. 188-194.
- D. W. Toetz, “Uptake and translocation of ammonia by freshwater hydrophytes”, Ecology, 1974; 55: pp. 199-201.
- S.V. Matagi, D. Swai and R. Mugabe, “A review of heavy metal removal mechanisms in wetlands”, Afr J Trop Hydrobiol, 1998; 8: 23-25.
- W. H. Arisz, “Symplasm theory of salt uptake into and ( transports in parenchymatic tissues”, In:Recent Advances in ( Botany, vol 11, pp 1125-28. University of Toronto. 1961.
- V.Sharpe and P. Denny, Electron microscope studies on the absorption and localization of lead in the leaf tissue ( of Potamogeton pectinatus L. Journal Experimental Botany, 1976 27: 1135-62.
- H. Welsh, “The uptake of cations by Vallisneria leaves”, Acta Botanica Neerl, 1961; 10: 341-93.
- E.L. Edroma, Copper pollution in Rwenzori National Park, Uganda. Applied Ecology, 1974; 11: 1043-56.
- B.H. Oke and A.S. Juwarkor, “Removal of heavy metals ( from domestic wastewater using constructed wetland”, 5thInternational Conference on Wetland Systems for Water Pollution Control, Vienna, 1996; September 15-19.
- M.M. Lasat, “Phytoextraction of metals from contaminated soil: a review of plant/soil/metal interaction and assessment of pertinent agronomic issues”, J Hazardous Substance Research, 2000; 2: 1-23.
- Y.I. Zhu, A.M. Zayed, J-H. Qian, M. Souza and N, Terry, “Phytoremediation of trace elements by wetland plants”: II. Water hyacinth. J Environ Qual, 1999; 28: 339-44.
- M.E. Soltan and M.N. Rashed “Laboratory study on the( survival of water hyacinth under several conditions of heavy metal concentrations”, Advance Environmental Research, 2003; 7: 321-34.
- J. Hagemeyer, “Ecophysiology of plant growth under heavy metal stress”. In: Heavy Metal Stress in Plants (Edited by Prasad MNV and Hagemeyer J) Springer-Verlag, Berlin, Heidelberg: 1999; pp 157-81.
- S.K. Jain, P. Vasudevan and N. Jha, Azolla pinnata R. Br. and Lemna minor L. for removal of lead and zinc from polluted water. Water Research, 1990; 24(2): 177-83.
- M. Delgado, M. Bigeriego and E. Guardiola, “Uptake of zinc, chromium and cadmium by water hyacinth”, Water Research, 1993; 27: 269-72.
- H. Schat, R. Vooijs and E. Kuiper, “Identical major gene loci for heavy metal tolerances that have independently evolved in different local populations and sub species of Silene vulgaris”, Evolution, 1996; 50: 1888-95.
- H.A. Schroder, “Pollution by Industria metals. In trace elements and nutrition some positive and negative aspects”, Faber Faber Lonson, 1973; pp. 119-128.
- G. W. Smith, S.S. Hayasaka and G. W. Thayer, “Root surface area measurements of Zostera marina and Halodule wrightii” Botanical, 1979; 22: 347-358.
- M.A. Maine, N.L. Sune and S.C. Lagger, “Chromium bioaccumulation: Comparison of the capacity of two floating macrophytes”, Water Research, 2004; 35: pp. 2629-2634.
- Lu. Qin, Lhe. Zhenli, Lhe, Donald, A. Graetz, J. Peter, Stofella, Xiaoe and Yang, “Uptake and distribution of metals by Water Lettuce (Pistia stratiotes L.)”’ Environmental Science Pollution Research, 2011; 18: pp.978-986.
- R. Jhon, P. Ahmad, K. Gadgil and S. Sharma, “Effect of Cd and Pb on growth, biochemical parameters and uptake in Lemna polyrhiza L.”, Plant Soil Environment, 2008; 54: pp. 262-270.
- M. Maeda, M. Koshikawa, K.K. Nisizawa and Takano, “Cell wall constituents, especially pectic substance of a marine phanerogram Zostera marina”, Botanical Magazine, Tokyo, 1966; 79: pp. 422-426.
- I. Schneider and J. Rubio, “Sorption of heavy metals onto plant biomass of freshwater macrophytes”, Environmental Science Technology, 1999; 33: pp. 2213-2217.
- A. Fargosova, “Trace metal interaction expressed through photosynthetic pigment content in Sinapsis alba seedlings”, Rost. Vyr, 2000; 46: pp. 337-342.
- S, Jana and A, Bhattacharjee, “Effect of combinations of heavy metal pollutants on Cuscuta reflexa”, Water Air Soil Pollution, 1988; 42(3-4): pp. 303-310.
- L.P. Solomonson and M.J. Barber “Assimilatory nitrate reductase: functional properties and regulation”, Plant Molecular Biology, 1990; 45, pp. 225-253.
- M. Sela, J. Garty and E.Tel-Or, “The accumulation and the effect of heavy metals on the water fern Azolla filiculoides”, New Phytologist, 1989; 112, pp. 7-12.
- P.B. Nicholas, J.D. Couch and S.H. Al-Hamdani, “Selected physiological responses of Salvinia minima 004. Nitrogen uptake by arctic soil microbes and plants in relation to soil nitrogen supply”, Ecology, 2000; 85(4): pp. 955-962.
- T. Fenchel, “Aspects of the decomposition of seagrasses”. In: P. McRoy and C. Helfferich (Eds.), Seagrass Ecosystem, a Scientific Perspective’, Marine Science 4.International workshop, Leiden, The Netherlands. Marcel Dekker, New York,1973: pp. 123-145.
- M. Singh and S.S. Dahiya, ” Effect of calcium carbonate and iron on the availability and uptake of iron, manganese, phosphorus and calcium in Pea (Pisum sativum L.)” Plant Soil, 1976; pp. 511-520.
- Chaney RL, Malik M, Li YM, Brown SL, Angle JS and Baker AJM. Phytoremediation of soil metals. Curr Opin Biotechnol, 1997; 8(3), 279-84.
- P.J. Sanches Filho, L.V. Nunes, N.N Da Rosa, G.R Betemps and R.S. Pereira, “Comparison among native oating aquatic macrophytes for bioconcentration of heavy metals”, Ecotoxicol. Environ. Contam., V. 10, n. 1, 2015, 1-6, 2015 (doi: 10.5132/eec.2015.01.01)
- H, Brix H, J.E. Lyngby and H.H. Schierup, Eelgrass (Zostera marina L.) as an indicator organism of trace metals in the Limfjord, Denmark. Mar Environ Res, 1983; 8: 165-81.
- Doaa M. Hammad, Cu, Ni and Zn Phytoremediation and Translocation by Water Hyacinth Plant at Different Aquatic Environments. Australian Journal of Basic and Applied Sciences, 2011; 5(11): 11-22.
- Lu. X., M. Kruatrachue, P. Pokethitiyook and K. Homyok, “Removal of cadmium and zinc by water hyacinth, Eichhornia crassipes”, Science Asia, 2004; 30: 93-103.
- H.K. Stratford, T.H. William and A. Leon, “Effects of heavy metals on water hyacinths (Eichhornia crassipes)”, Aquatic Toxicology., 1984; 5(2): 117-28.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


