ISSN: 0973-7510
E-ISSN: 2581-690X
A urinary tract infection (UTI) is an infectious disease that infects humans in the presence of microorganisms, often not treated with broad-spectrum antibiotics because they contribute to developing resistant microorganisms. Therefore, this study aimed to investigate the antibacterial efficacy of medicinal plants used to treat infection in the urinary tract. One hundred samples were collected from patients with UTIs, ranging in age from 10–60 years. Outpatients and inpatients with UTIs live in Al-Najaf Center and attend treatment at Al-Hakeem Hospital and Al-Sader Teaching Hospital. Ten microliters of urine samples were inoculated on selective media to isolate and identify pathogenic bacteria, presumptive identification was performed using the Viteck-2 system. Eight antibiotics were used for showed antibiotic susceptibility: cefixime (5 μg), streptomycin (25 μg), amoxicillin (30 μg), erythromycin (10 μg), ciprofloxacin (10 μg), azithromycin (15 μg), gentamicin (10 μg), and nitrofurantoin (50 μg). Two species of plants were used to determine antibacterial activity: Castanea crenata and Saussurea costus. Of 100 urine samples, 80 tested positive for bacteriuria. Escherichia coli was the most prevalent bacterium in the urine 50 (62.5%), followed by Klebsiella pneumonia 10 (12.5%), Proteus vulgaris seven (8.75%), Pseudomonas aeruginosa six (7.5), Staphylococcus aureus four (5%), and Streptococcus pyogenes three (3.75%). Some bacteria with Gram staining comprising E. coli, Klebsiella spp., Pseudomonas spp., and Proteus spp. are resistant to many antibiotics. C. crenata and S. costus inhibited the growth of pathogenic bacteria isolated from the urine samples. Testing and determining the antimicrobial activities of medicinal plants will help pharmaceutical companies develop modifiers or precursors for synthesizing new therapeutic alternative drugs to treat infectious diseases caused by pathogens.
Urinary Tract Infections, Uropathogenic Bacteria, Medicinal Plant, Antibacterial Activity
In the past, urine was always considered a sterile liquid, and scientists have done many experiments to predict and confirm urinary tract infections (UTIs). In many cases, urine is not sterile; it contains urinary microbiota.1
UTIs are common in humans. Annually, approximately 150 million people worldwide are affected by UTIs, especially women. Around 40–50% of women suffer a UTI infection at least once.2 In some continents, such as Africa and Asia, a high incidence of tract infections in pregnant women has been recorded.3 Environmental and lifestyle factors play major roles in the prevalence of UTIs. Older adults have multiple diseases, and treatment and administration programs may increase the risk of UTIs. In particular, using catheters significantly increases the incidence of tract infections, particularly those caused by causative agents, such as gram-negative bacteria.4 In severe cases, the infection leads to uremia and bacteremia as it reaches the kidneys and invades the bloodstream.5 A UTI is an inflammatory disorder of the urinary tract that is affected by the existence and growth of microorganisms throughout the urinary tract. This is caused by bacteria moving from the gastrointestinal region to the urethra, multiplying and causing disease. Invasion of bacterial pathogens in the epithelium lining of the urinary tract from the small calyx to the prostatic urethra causes UTI. Bacterial growth in the urinary tract can be benign or severe.6 UTIs can be acquired from communities or hospitals. Community-acquired infections occur in an individual’s lifetime in a community or hospital setting with admission occurring <48 h. Community-acquired are the second most common type of microbial infections in communities. Nosocomial infections of the urinary tract occur 48 h after hospital admission, if the patient was not hospitalized at the time of admission or within 3 d after discharge.7 The most common type of UTIs is the uncomplicated kind, which mostly occurs due to the lack of functional or anatomical irregularities within the urinary tract. The other type is a complicated infection that occurs in an abnormal urinary tract, which increases susceptibility to infection.8 However, the causative organisms can be easily identified. Gram-negative bacteria, particularly Escherichia coli, are primarily responsible for spreading infections, they are the most prevalent causative agents of UTIs.9 The causative agents of UTIs are complex, and are affected by a variety of factors, including genetic inheritance, intestinal population, vaginal biological processes, behavioral factors, uropathogenic virulence features, and host-barrier factors.10,11 The most common pathogenic bacteria isolated from UTIs are E. coli, other bacterial species that occur include K. pneumoniae, Staphylococcus aureus, Proteus spp., Pseudomonas aeruginosa, Enterococcus spp., and Enterobacter spp.12 Females are more likely to get infected due to several reasons, including the short urethra, age, sexual activity, lack of prostate secretion, pregnancy, and the possibility of cross-contamination with the microflora of the fecal tract.13 UTIs are often not treated with broad-spectrum antibiotics due to the development of a resistant microorganism by the inappropriate usage of antibacterial agents.14 Different regimens have been used to treat UTIs. Trimethoprim and nitrofurantoin are currently used as first-line treatments. Second-line antibiotics such as quinolones are recommended for patients with prostatitis.15 Recent research has found that herbal medicines play a significant role in treating UTIs. Many plant compounds contain different functional groups in their structures, and their antimicrobial activities are attributed to several mechanisms.16 A recurrent UTI (rUTI) is defined as two or more lower tract infections within 6 months or 3 or more infections within 12 months.17 Although powerful antibiotics are available, resistant strains of microorganisms are constantly emerging, necessitating constant research and development of new drugs. For centuries, plants have gained attention worldwide as medicines and treatments for various ailments.18 Botanical therapies are the normal choice for long-standing treatment and traditional medicine for rUTIs, particularly with a synergistic antibiotic style, as they affect antimicrobial agents and decrease various signs and adverse effects. Furthermore, patients who have suffered from UTIs for many years can be preventatively treated with accurate medication.19 Also, several researchers have established the efficacies of therapeutic plants in healing and avoiding numerous health situations.20-22 The aim of this study is to characterize and identify the common causative agents of UTIs and highlight some of the medicinal plants used as alternative drugs for their prevention and treatment.
Source of samples
A random group of 100 samples was collected from registered patients ranging in age from 10–60 years, suffering from recurrent or complicated UTI, who were symptomatic and treated with antibiotics and plant extracts from October 2021 to January 2022. Urine samples were collected midstream for bacterial culture before treatment. A colony count ≥105 CFU/mL is considered significant for UTIs. Among inpatients with UTIs, those living in the Al-Najaf Center and receiving treatment at Al-Hakeem Hospital and Al-Sader Teaching Hospital were included in this study.
Sample collection and transportation
One hundred milliliters of fresh urine samples taken from midstream during urination were used for microscopic examination and inoculation of the culture medium. Urine samples were stored in sterile labeled containers containing transport culture media to prevent contamination. The urine samples were centrifuged at 1500/rpm for 5 min. Subsequently, a droplet of sediment was collected after centrifugation, placed on a slide, and covered with a cover slip. Finally, the slides were examined under a microscope at a magnification lens (40×) to recognize red blood cells, pus cells, epithelial cells, molds, crystals, and yeast cells.23 Three red blood cells per high-power field in males and females were considered an indicator of a positive UTI. Positive samples were subjected to urine culture.24
Isolation and identification of bacteria
Isolation and identification were performed at the Microbiology Laboratory of the Faculty of Science, University of Kufa. From each patient, 10 µL of urine were inoculated and streaked onto brain heart infusion (HiMedia) agar petri dishes and incubated for 1–2 d at 37°C. After incubation, the cells were subcultured on MacConkey (HiMedia), mannitol salt (HiMedia), and Salmonella-Shigella agar (HiMedia). Then, the isolates were preserved in 40% glycerol at -20°C. Bacterial counts were determined 103–105 CFU/mL. The bacteria were initially identified according to colony morphology and coloration, and bacterial isolates were identified by biochemical test standards containing triple sugar iron (TSI) agar for sugar fermentation, catalase, oxidase, Simmons’ citrate agar, motility, and Gram staining for gram-positive and -negative bacteria. Presumptive identification of the bacteria was performed using the Viteck-2 system.
Antibiotic susceptibility testing
The Clinical and Laboratory Standards Institute25 guidelines were used for antibiotic susceptibility testing. Plates containing Muller–Hinton Agar (MHA) were prepared according to the manufacturer’s instructions (38 g/L) for testing antibiotic resistance using the Kirby-Bauer disk diffusion method.26 A drop was taken from expected and isolated bacteria grown in nutrient broth for 24 h, then spread using the spreading method on MHA, after which antibiotic discs were placed into the culture media using sterile forceps, four discs per plate, transferred to the refrigerator for 3–4 h, and finally incubated for 24 h to determine the inhibition zones for different antibiotics.
Antibiotic discs
The following antibiotic discs were used: streptomycin (S, 25 g), ciprofloxacin (CIP, 10 g), amoxicillin (A, 30 g), azithromycin (AZM, 15 g), erythromycin (E, 10 g), cefixime (CFM, 5 g), gentamicin (CN, 10 g), and nitrofurantoin (NIF, 50 g).
Plant extract preparation
This study used two plant species: Castanea crenata and Saussurea costus. Extracts of these plants were prepared at the Plant Laboratory, Faculty of Science, University of Kufa to test the susceptibility of bacteria isolated from UTI. We crushed 10 g of the plant material with an electric mixer until it turned into a powder and dissolved it in an organic solvent, such as acetone. The blended material was shaken for 30 min on a rotating shaker and centrifuged at 5,000 rpm for 15 min.27
Of 100 urine samples, 80 tested positive for bacteriuria. Microscopic examination of red blood cells and leukocytes, were quantified using urine culture as described previously.
UTIs were noted at the highest level between 20–40 years old. Herein, we found that the prevalence of UTI was significantly higher in females than in males (Tables 1, 2). E. coli was the most prevalent bacterium in the urine 50 (62.5%), followed by K. pneumonia 10 (12.5%), P. vulgaris 7 (8.75%), P. aeruginosa 6 (7.5), S. aureus 4 (5%), and S. pyogenes 3 (3.75%). Analysis of the results indicated that E. coli was the dominant pathogen isolated from both sexes; however, it occurred more frequently in females than in males.
Table (1):
Numbers of different age groups by gender susceptible to urinary tract infection
Age |
Gender |
Total No. |
|---|---|---|
10-20 |
Male |
3 |
10-20 |
Female |
8 |
20-30 |
Male |
8 |
20-30 |
Female |
11 |
30-40 |
Male |
9 |
30-40 |
Female |
13 |
40-50 |
Male |
5 |
40-50 |
Female |
10 |
50-60 |
Male |
4 |
50-60 |
Female |
9 |
10-60 |
Gendered |
80 |
Table (2):
Different age groups that gave positive and negative for urinary tract infection
Age |
Positive UTI |
Negative UTI |
Total |
|---|---|---|---|
10-20 |
11 |
4 |
15 |
20-30 |
19 |
3 |
22 |
30-40 |
22 |
4 |
26 |
40-50 |
15 |
5 |
20 |
50-60 |
13 |
4 |
17 |
10-60 |
80 |
20 |
100 |
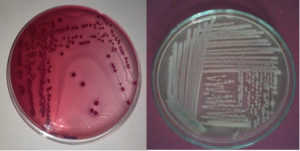
The pathogenic bacteria were identified based on the emergence of different bacterial colonies. The colonies had different colors, sizes, and textures (Figure 1). Diagnosis included a study of the shape of the colony, microscopic examination, and biochemical tests (Table 3).
Table (3):
The distribution and biochemical tests of pathogenic bacteria causing positive UTI
Bacteria |
Isolates number |
Gram stain |
TSI |
Catalase |
Oxidase |
Simmon citrate |
Motility |
|---|---|---|---|---|---|---|---|
E.coli |
50 |
G-ve |
A/A, G |
+ |
– |
– |
Motile |
Proteus vulgaris |
7 |
G-ve |
A/A, G, H2S |
+ |
– |
+ |
Motile |
Klebsiella pneumonia |
10 |
G-ve |
A/A, G |
+ |
– |
+ |
Non-Motile |
Staphylococcus aureus |
4 |
G+ve |
A/A |
+ |
– |
+ |
Non-Motile |
Pseudomonas aeruginosa |
6 |
G-ve |
K/K |
+ |
+ |
+ |
Motile |
Streptococcus pyogenes |
3 |
G+ve |
K/K |
– |
– |
– |
Non-Motile |
(G-ve) Gram-negative, (G+ve) Gram-positive, A: Acid, K: Alkaline, G: Gas
Figure 1. Primary identified of bacterial strains, left E. coli on selective media MacConkey agar.
Streptococcus pyogenes on Brain Heart Infusion agar
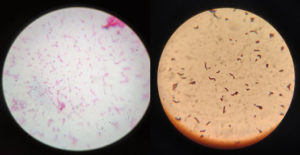
Examination of the pathogenic bacteria isolated from urine samples revealed gram-positive cocci identified as presumptive S. aureus and S. pyogenes and gram-negative bacilli identified as presumptive Enterobacteriaceae spp. (Figure 2).
Figure 2. Microscopic examination at 100x oil immersion revealed Gram Staining of isolated bacteria, left identified as Streptococcus pyogenes and right identified as E. coli
We used the biochemical tests, including triple sugar iron agar, catalase, oxidase, Simmons’ citrate agar, Gram staining, and motility to verify the bacterial type (Table 3).
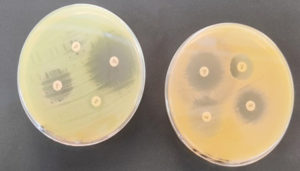
Antibiotic susceptibility tests are usually used to examine the effectiveness of antibiotics in inhibiting the growth of disease-causing microorganisms and to determine which antibiotics are most effective in treating the disease. This study revealed that gram-negative bacteria, comprising E. coli, Klebsiella spp., Pseudomonas spp., and Proteus spp., are resistant to many medications, including the most accessible antibiotics. These bacteria have the intrinsic ability to gain resistance which is inherited by their progeny. Multidrug resistance (MDR) is a natural phenomenon when bacterial isolates resist at least three or more antimicrobial categories. In this study, we used eight antibiotics: S (25 μg), A (30 μg), CIP (10 μg), E (10 μg), CFM (5 μg), AZM (15 μg), CN (10 μg), and NIF (50 μg). The highest resistance patterns were observed in gram-negative bacteria (E. coli, Klebsiella spp., Pseudomonas spp., and Proteus spp.). In contrast, all the isolates were sensitive to two antibiotics, NIF and CN (Table 4) (Figure 3).
Table (4):
Numbered and percentages of pathogenic bacteria isolated from people suffering from urinary tract infections revealed resistance and sensitivity to antibiotics
| Pathogen N (%) | ANTIBIOTICS N | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIP | S | AMP | E | AZM | CFM | CN | NIF | |||||||||
| Sensitive | Resistance | Sensitive | Resistance | Sensitive | Resistance | Sensitive | Resistance | Sensitive | Resistance | Sensitive | Resistance | Sensitive | Resistance | Sensitive | Resistance | |
| E. coli 50 (62.5) |
30 | 20 | 10 | 40 | 45 | 5 | 8 | 42 | 15 | 35 | 0 | 50 | 40 | 10 | 3 | 47 |
| Proteus vulgaris 7 (8.75) |
4 | 3 | 2 | 5 | 1 | 6 | 0 | 7 | 1 | 6 | 1 | 6 | 5 | 2 | 2 | 5 |
| Klebsiella pneumonia 10 (12.5) |
9 | 1 | 2 | 8 | 2 | 8 | 3 | 7 | 1 | 9 | 2 | 8 | 8 | 2 | 1 | 9 |
| Staphylococcus aureus 4 (5) |
4 | 0 | 4 | 0 | 1 | 3 | 3 | 1 | 2 | 2 | 1 | 3 | 2 | 2 | 2 | 2 |
| Pseudomonas aeruginosa 6 (7.5) |
6 | 0 | 1 | 5 | 0 | 6 | 1 | 5 | 0 | 6 | 1 | 5 | 6 | 0 | 0 | 6 |
| Streptococcus pyogenes 3 (3.75) |
3 | 0 | 2 | 1 | 0 | 3 | 3 | 0 | 2 | 1 | 1 | 2 | 2 | 1 | 0 | 3 |
Figure 3. Antibiotic susceptibility tests revealed diameter inhibition of E. coli (left) and S. aureus (right)
This study revealed that C. crenata and S. costus inhibit the growth of pathogenic bacteria isolated from urine samples. The medicinal plants inhibited the growth of E. coli and K. pneumonia ranging from 11–15 and 10–12 mm in diameter at 102 mm (Figure 4), respectively. Generally, these medicinal plants have biological activity, can fight causative agents, and are used as alternative or complementary treatments for UTIs. This was evidenced by its minimal bactericidal concentration, tested in parallel with modern antibiotics. Consequently, fully exploited traditional medicinal plants can overcome the health consequences of the drug-resistant bacteria that cause UTIs (Table 5).
Table (5):
Diameters inhibition zone of plant extract against pathogenic bacteria
Bacterial Species |
1×102 |
1×103 |
1×104 |
1×105 |
1×106 |
|---|---|---|---|---|---|
E. coli 50 (62.5) |
Sensitive |
Resistance |
Resistance |
Resistance |
Resistance |
Proteus vulgaris 7 (8.75) |
Sensitive |
Resistance |
Resistance |
Resistance |
Resistance |
Klebsiella pneumonia 10 (12.5) |
Sensitive |
Resistance |
Resistance |
Resistance |
Resistance |
Staphylococcus aureus 4 (5) |
Sensitive |
Resistance |
Resistance |
Resistance |
Resistance |
Pseudomonas aeruginosa 6 (7.5) |
Sensitive |
Resistance |
Resistance |
Resistance |
Resistance |
Streptococcus pyogenes 3 (3.75) |
Sensitive |
Resistance |
Resistance |
Resistance |
Resistance |
A UTI is an infectious disease that affects individuals of all ages and is a prominent cause of morbidity. Young females and females characterized by sexual activity are the most affected, whereas the other groups are at risk, including older patients and those receiving genitourinary and catheter assistance. UTIs are serious community health concerns affecting millions. The differences in the causes of bacterial tract infections are largely related to diverse lifestyles, poor healthcare systems, ignorance, inadequate water supply, and geographical differences. Therefore, the most common bacterium regarded as causative agents of UTIs are E. coli, which has become resistant to many antibiotics, such as penicillin, ampicillin, and trimethoprim. Recently, an increase in the rate of drug resistance to commonly used antimicrobial agents has been observed in gram-positive and -negative bacteria28 to the excessive and incorrect use of antibiotics. E. coli is the most common causative agent of UTIs in females. The distribution of species and their resistance to antibiotics varies with time and location.29 This study aimed to identify urinary pathogenic microorganisms and their sensitivity and resistance mechanisms, to compare them with treatment with plant extracts. Of the 100 urine samples, 80 were significantly positive for UTIs, and the prevalence was higher in females than males. This result agrees with Saha et al., who showed that of the 100 patients tested, only 74 were positive for UTI, and 73.57% of them were females infected with UTI between 26–36 years old.30 Microscopic and biochemical characterizations confirmed the main bacterial strains identified based on colony morphology. The results obtained from microscopic examination indicated the prevalence of gram-positive and -negative strains. In this study, E. coli represented the most prevalent bacterium among UTI patients: 50 (62.5%), followed by K. pneumonia 10 (12.5%), P. vulgaris 7 (8.75%), P. aeruginosa 6 (7.5), S. aureus 4 (5%), and S. pyogenes 3 (3.75%). Similarly, the most frequently described bacterial pathogen in UTIs, according to Johansen et al.31 and Kauer et al.32 was E. coli (31%) and (71.7%), respectively. Additionally, Odoki et al.33 noted that the most prevalent uropathogenic bacteria were E. coli (41.9%), followed by S. aureus, K. pneumoniae, K. oxytoca, P. mirabilis, E. faecalis, and P. vulgaris. One of the main problems in treating tract infections is antibiotic resistance caused by these microorganisms. Antibiotic resistance increased over time. Antibiotic resistance rates differ across countries.34 The resistance mechanisms established by MDR bacteria are transmitted from one strain to another via efflux, hypermutability, and plasmid encoding. Though UTIs are caused by MRSA, extended-spectrum beta-lactamase-producing organisms, enterococci resistant to vancomycin, and carbapenem-resistant organisms increase morbidity and mortality.35 In this study, the isolates were tested for eight different antibiotics, and the uropathogens showed good sensitivity to CN and NIF. Therefore, these antibiotics can be used in UTI treatment. While, good susceptibility profiles for NIF have been described, they have a limited spectrum, which has been studied by several researchers with >70% sustained sensitivity <80%.36-38 NIF is effective against E. coli isolates with low resistance owing to its low levels of use and access. NIF is relatively expensive compared to other antibiotics, and can be regarded as an alternative in the experimental treatment of UTIs. However, this medication is not recommended for high-risk patients with UTIs or those with systematic participation.39 This offers an interesting profile when selecting an experimental treatment for UTI management to obtain high referral rates. This study showed that this antibiotic has always had a susceptibility >50% in all bacterial isolates. Additionally, the study revealed the resistance of E. coli to beta-lactam antibiotics like ampicillin. One of the virulence factors found in E. coli is beta-lactamase, which dissolves the four-atom ring in this antibiotic. The Enterobacteriaceae family carries the resistance genes to beta-lactam antibiotics on their plasmid, including TEM, CTX-M, OXA, and SHV. The bla-TEM enzyme, responsible for approximately 90% of the resistance to ampicillin in E. coli, is produced by gram-negative bacteria. These enzymes also exist in K. pneumoniae, which produces a different enzyme responsible for approximately 20% of ampicillin resistance.11 The resistance of different bacterial species to diverse antibiotics has increased worldwide as a community health hazard. The emergence of new antibiotic resistance mechanisms and decreased efficacy for treating common infections have led to a failure in the response of microbes to typical treatment, resulting in extended illness, increased healthcare spending, and risks of mortality. Nearly all infections exhibit high levels of MDR to increased morbidity and mortality.40 The genetic structure of E. coli used for the production of MDR strains is suitable for the inactivation of antibiotics by reducing permeability and pump efflux. Therefore, searching for secondary metabolites with antimicrobial activity in medicinal plants remains a timely concern. Thus, using medicinal plants can overcome the social, economic, and health impacts caused by MDR bacteria such as MRSA, E. coli, and K. pneumoniae.41 Medicinal plants are among the compounds that exhibit antibacterial activity against UTIs. Since ancient times, medicinal plants have been proven to treat and prevent several diseases successfully, and the general public and pharmaceutical companies have used them extensively.42 Our findings demonstrated that some generally available, but not commonly studied, medicinal plants in Iraq are very active against pathogenic bacteria isolated from patients with UTI, one of the most common diseases in developing countries. We studied the effect of two different plant extracts that have antibacterial activity against the tested pathogenic bacteria, observing that P. vulgaris was the most susceptible species amongst them to the extracts. This was further confirmed by the antimicrobial activity displayed by the tested medicinal plants against UTI inducing microbes. Previous studies concluded that plant-derived therapeutic agents can be used as alternative drugs to treat and prevent several diseases.43,44 The effects of antimicrobials against plant extracts are due to multiple mechanisms: direct killing, interference via adhesion to epithelial cells, biofilm formation, preventing their multiplication, returning to dysbiosis, improving host protection by enhancing natural barriers, and acting as antimicrobial agents. Immune regulators enhance the redox state in the body. Therefore, whole plant extracts are recommended as bioactive compounds that act synergistically. Recently, some researchers have listed useful plants for preventing and treating UTIs and summarized their beneficial potential.19 Herbal medicines significantly reduce antibiotic resistance in bacteria. Therefore, patients with urological disorders may benefit from this treatment. Herbal medicines can play a significant role in UTI treatment. Many antimicrobial plant compounds contain different functional groups in their organization. Their antimicrobial activities are due to multiple mechanisms.8 The most important aspect in future studies is to estimate the combined effective of medicinal plants with commercial antibiotics on UTI-inducing pathogens. A recent study compared the combined antimicrobial drug effect to the single antimicrobial drug effect with a 2–3 times larger area diameter (range 20–26 mm). Subsequently, by combining the previously ineffective antibiotics methicillin and E with plant extracts, the antibacterial activity against E. coli and Proteus spp. increased significantly.45 These bacterial strains were found to be resistant to the tested antibiotics such as CIP and A. However, all these pathogens were sensitive to the aqueous plant extracts of C. crenata and S. costus with clear inhibitory areas. This indicates that the extracts of these plants may contain one or more bioactive combinations that inhibit pathogen growth. The leaves of these plants can be used to formulate herbal medicines to treat UTIs as they are non-toxic.
We concluded that the most significant bacteria associated with UTIs were E. coli 50 (62.5%), followed by K. pneumonia 10 (12.5%), P. vulgaris 7 (8.75%), P. aeruginosa 6 (7.5), S. aureus 4 (5%), and S. pyogenes 3 (3.75%). Hence, testing and determining the antimicrobial activities of medicinal plants will help pharmaceutical companies develop modifiers or precursors for synthesizing new therapeutic alternatives to treat infectious diseases caused especially by multidrug-resistant bacteria.
ACKNOWLEDGMENTS
The authors would like to extend their sincere thanks to the University of Kufa and to the Faculty of Science, especially the Department of Biology, for their continued support in producing this article.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
Both authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Morand A, Cornu F, Dufour JC, Tsimaratos M, Lagier JC, Raoult D. Human Bacterial Repertoire of the Urinary Tract: a Potential Paradigm Shift. J Clin Microbiol. 2019;57(3).
Crossref - Qiao LD, Chen S, Yang Y, et al. Characteristics of urinary tract infection pathogens and their in vitro susceptibility to antimicrobial agents in China: data from a multicenter study. BMJ open. 2013;3(12):e004152.
Crossref - Belete MA, Saravanan M. A systematic review on drug resistant urinary tract infection among pregnant women in developing countries in Africa and Asia; 2005-2016. Infect Drug Resist. 2020;13:1465.
Crossref - Medina M, Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther Adv Urol. 2019;11:1756287219832172.
Crossref - Zhang L, Huang W, Zhang S, et al. Rapid Detection of Bacterial Pathogens and Antimicrobial Resistance Genes in Clinical Urine Samples With Urinary Tract Infection by Metagenomic Nanopore Sequencing. Front Microbiol. 2022;13:858777.
Crossref - Williams G, Craig JC. Long-term antibiotics for preventing recurrent urinary tract infection in children. Cochrane Database Syst Rev. 2019(4):CD001534.
Crossref - Iacovelli V, Gaziev G, Topazio L, Bove P, Vespasiani G, Agro EF. Nosocomial urinary tract infections: A review. Urologia. 2014;81(4):222-227.
- Bazzaz BSF, Fork SD, Ahmadi R, Khameneh B. Deep insights into urinary tract infections and effective natural remedies. Afr J Urol. 2021;27(1):6.
Crossref - Moges A, Genetu A, Mengistu G. Antibiotic sensitivities of common bacterial pathogens in urinary tract infections at Gondar Hospital, Ethiopia. East Afr Med J. 2002;79(3):140-142.
Crossref - Andreu A. Pathogenesis of urinary tract infections. Enfermedades Infecciosas y Microbiología Clinica. 2005;4:15-21.
Crossref - Thangavelu S, Dhandapani R, Arulprakasam A, et al. Isolation, Identification, Characterization, and Plasmid Profile of Urinary Tract Infectious Escherichia coli from Clinical Samples. Evid Based Complement Alternat Med. 2022;2022:7234586.
Crossref - Manges A, Natarajan P, Solberg OD, Dietrich PS, Riley LW. The changing prevalence of drug-resistant Escherichia coli clonal groups in a community: evidence for community outbreaks of urinary tract infections. Epidemiol Infect. 2006;134(2):425-431.
Crossref - Gessese YA, Damessa DL, Amare MM, et al. Urinary pathogenic bacterial profile, antibiogram of isolates and associated risk factors among pregnant women in Ambo town, Central Ethiopia: a cross-sectional study. Antimicrob Resist Infect Control. 2017;6(1):132.
Crossref - Chandrasekhar D, Dollychan A, Roy BM, Cholamughath S, Parambil JC. Prevalence and antibiotic utilization pattern of uropathogens causing community-acquired urinary tract infection in Kerala, India. J Basic Clin Physiol Pharmacol. 2018;29(6):671-677.
Crossref - Lane DR, Takhar SS. Diagnosis and management of urinary tract infection and pyelonephritis. Emergency Medicine Clinics of North America. 2011;29(3):539-552.
Crossref - Khameneh B, Iranshahy M, Soheili V, Bazzaz BSF. Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control. 2019;8(1):1-28.
Crossref - Kaabi SAG, Abdulrazaq RA, Rasool KH, Khassaf SA. Western herbal remedies for Urinary Tract infections. Archive of Urological Research. 2020;4(1):049-060.
Crossref - Sharma A, Chandraker S, Patel VK, RamtekeP. Antibacterial Activity of Medicinal Plants Against Pathogens causing Complicated Urinary Tract Infections. Indian J Pharm Sci. 2009;71(2):136-139.
Crossref - Tache AM, Dinu LD, Vamanu E. Novel Insights on Plant Extracts to Prevent and Treat Recurrent Urinary Tract Infections. Appl Sci. 2022;12(5):2635.
Crossref - Zalewska-Piatek BM, Piatek RJ. Alternative treatment approaches of urinary tract infections caused by uropathogenic Escherichia coli strains-Review. Acta Biochimica Polonica 2019;66(2):129-138.
Crossref - Terlizzi ME, Gribaudo G, Maffei ME. UroPathogenic Escherichia coli (UPEC) infections: virulence factors, bladder responses, antibiotic, and non-antibiotic antimicrobial strategies. Front Microbiol.2017;8:1566.
Crossref - Marouf RS, Mbarga JAM, Ermolaevdoi: AV, et al. Antibacterial Activity of Medicinal Plants against Uropathogenic Escherichia coli. J Pharm Bioallied Sci. 2022;14(1):1-12.
Crossref - Assefa A, Asrat D, Woldeamanuel Y, G/Hiwot Y, Abdella A, Melesse T. Bacterial profile and drug susceptibility pattern of urinary tract infection in pregnant women at Tikur Anbessa Specialized Hospital Addis Ababa, Ethiopia. Ethiop Med J. 2008;46(3):227-235.
- Cheesbrough M. District laboratory practice in tropical countries, part 2. Cambridge university press. 2005.
Crossref - Abbey TC, Deak E. What’s New from the CLSI Subcommittee on Antimicrobial Susceptibility Testing M100, 29th Edition. Clin Microbiol Newsl. 2019;41(23):203-209.
Crossref - Bauer A, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. American J Clin Pathol. 1966;45(4):493.
Crossref - Nair R, Kalariya T, Chanda S. Antibacterial activity of some selected Indian medicinal flora. Turk J Bio. 2005;29(1):41-47.
- Belete MA. Bacterial Profile and ESBL Screening of Urinary Tract Infection Among Asymptomatic and Symptomatic Pregnant Women Attending Antenatal Care of Northeastern Ethiopia Region. Infect Drug Resist. 2020;13:2579-2592.
Crossref - Kulkarni SR, Peerapur BV, Sailesh KS. Isolation and Antibiotic Susceptibility Pattern of Escherichia coli from Urinary Tract Infections in a Tertiary Care Hospital of North Eastern Karnataka. J Nat Science,doi: Biol Med. 2017;8(2):176-180.
Crossref - Saha S, Rahman MS, Hassan FMN, et al. Antimicrobial Resistance in Uropathogen Isolates from Patients with Urinary Tract Infections. Biomed Res Ther. 2015;2(5):11.
Crossref - Johansen TEB, Cek M, Naber KG, et al. Hospital acquired urinary tract infections in urology departments: pathogens, susceptibility and use of antibiotics: data from the PEP and PEAP-studies. Int J Antimicrob Agents. 2006;28(Suppl 1):91-107.
Crossref - Kaur R, Walia G, Mehta M. Prevalence of Urinary tract infections in children and their sensitivity to various antibiotics. J Acad Indus Res. 2012;1(4):161-163.
- Odoki M, Aliero AA, Tibyangye J, et al. Prevalence of Bacterial Urinary Tract Infections and Associated Factors among Patients Attending Hospitals in Bushenyi District, Uganda. Int J Microbiol. 2019;4246780.
Crossref - Kahan NR, Chinitz DP, Waitman DA, Dushnitzky D, Kahan E, Shapiro M. Empiric treatment of uncomplicated urinary tract infection with fluoroquinolones in older women in Israel: another lost treatment option? Ann Pharmacother. 2006;40(12):2223-2227.
Crossref - Gadisa E, Tadesse E. Antimicrobial activity of medicinal plants used for urinary tract infections in pastoralist community in Ethiopia. BMC Complement Med Ther.doi: 2021;21(1):74.
Crossref - Armando GP, Sara MB, Esther MT. Urinary Infections Acquired in Community: Epidemiology, Resistance to Antibiotics and Therapeutic Options. Kasmera. 2011;39(2):87-97.
- Pinto J, Carvajal P, Lopez Y, et al. Etiologic agents of urinary tract infections and their resistance to antibiotics in the pediatric population; Medellín, Colombia. Archivos de Medicina (Col), 2011;11(2):159-168
- Zuniga-Moya JC, Bejarano-Caseres S, Valenzuda-Cervantes H, et al. Antibiotic sensitivity profile of bacteria in urinary tract infections. Acta Medica Costarricense. 2016;58(4):146-154.
- Angoti G, Goudarzi H, Hajizadeh M, Tabatabaii Z. Bacteria isolated from urinary tract infection among patients and determination of the antibiotic susceptibility patterns of the gram negative bacteria in Iran. Novelty in Biomedicine. 2016;4(1):1-4.
- Tanwar J, Das S, Fatima Z, Hameed S. Multidrug Resistance: An Emerging Crisis. Interdiscip Perspect Infect Dis. 2014;541340.
Crossref - Gadisa E, Weldearegay G, Desta K, et al. Combined antibacterial effect of essential oils from three most commonly used Ethiopian traditional medicinal plants on multidrug resistant bacteria. BMC Complement Altern Med. 2019;19(1):24.
Crossref - Elamary RB, Albarakaty FM, Salem WM. Efficacy of Acacia nilotica aqueous extract in treating biofilm-forming and multidrug resistant uropathogens isolated from patients with UTI syndrome. Sci Rep. 2020;10(1):11125.
Crossref - WHO. WHO global report on traditional and complementary medicine 2019. World Health Organization. 2019.
- Rossiter SE, Fletcher MH, Wuest WM. Natural products as platforms to overcome antibiotic resistance. Chem Rev. 2017;117(19):12415-12474.
Crossref - Acharjee M, Zerin N, Ishma T, Mahmud MR. In-vitro anti-bacterial activity of medicinal plants against Urinary Tract Infection (UTI) causing bacteria along with their synergistic effects with commercially available antibiotics. New microbes and new infections. 2023;51:101076.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.