ISSN: 0973-7510
E-ISSN: 2581-690X
Rice is an important crop, widely affected by blast; a devastating disease incited by Pyricularia oryzae. Rice blast caused by P. oryzae Cave continues to be a major constraint in rice production. Since, the existing chemical control measures being costly and may favor development of resistance in pathogens, the potential alternative methods have been explored in the present studies. The biological method; an ecofriendly and economic approach seems to be an alternative to chemotherapy in managing virulent strains like P. oryzae causing blast of rice. Biocontrol agents Trichoderma viride, T. harzianum, Pseudomonas fluorescens and botanicals; neem oil and neem oil + neem leaf extract (Azadirachta indica) were evaluated for their efficacy against blast in Pusa Basmati 1121; a high yielding variety of rice. Carbendazim was used for standard check fungicides for comparison. The result concludes that T. viride is best bio-control (24.53%) which was followed by T. harzianum (23.08%), neem oil (26.20%) and neem oil + neem leaf extract (24.15%) inhibit the blast respectively while P. fluorescens inhibit the blast (21.99%) as compared to treated and untreated control (18.57% and 34.15%, respectively).
Rice; Pyricularia oryzae; biocontrol; botanicals; Trichoderma viride; T. harzianum; Pseudomonas fluorescens.
About the production and consumption, 90 per cent or more of the world’s rice is grown and consumed in Asia, known as rice bowl of the world. Among the Asian countries, India is one of the leading producers of rice (Tony Cisse, 2005).
Rice is widely affected by P. oryzae in almost all the rice-growing areas of the world and is the most destructive fungal disease of rice causing yield loss up to 90 per cent (Mehrotra, 2003; Gupta and Kapoor, 2002) but normally can lead to 30% yield loss annually (Talbot, 2003). This disease was first reported in India in 1913 caused by an ascomycetes fungus, M. grisea Barr (anamorph P. grisea Sacc, synonym P. oryzae Cav.) (Padmanabhan, 1965). M. grisea is a hemibiotrophic pathogen, initially grow biotrophically and then switch to necrotrophic growth, killing the infected tissues (Perfect and Green, 2001; Munch et al., 2008). M. oryzae, however, invades foliar tissues biotrophically and necrotrophically simultaneously (Kankanala et al., 2007). The fungus is able to infect all plant parts except root. Symptoms can be either lesions or spots; the shape, color and size vary depending on varietal resistance, environmental conditions and the age of the lesions (Ou 1985). P. oryzae can survive on seeds and can easily move over other parts of plant if proper safety checks are not in place.
The use of bio-control agents and botanicals, for plant protection, has assumed greater importance in recent years all over the world due to environmental pollution and health insecurity associated with the promiscuous use of factitious fungicides. Use of natural compounds as economically accessible methods of disease control is receiving increased attention due to their nontoxicity and biodegradability (Sivamani, E. and Gnanamanickam, S. S. (1988); Zarandi et al., 2009; Sukanya et al., 2011; Hajano et al., 2012; Bhattacharji and Dey, 2014 and Ali and Nadarajah, 2014). With this aim of controlling the rice blast by using biological control methods the present investigation was undertaken as a field trail experiments against the disease in order to find out suitable biological control for soil borne pathogens.
The present investigation was conducted on “Evaluating efficacy of bio-control agents and botanicals against blast of paddy (P. oryzae)” during 2015-16 in the Research field of C.S. Azad Univ. of Agri & Tech., Kanpur (Uttar Pradesh), India. The details of materials used, experimental procedures followed and techniques adopted are described as follows-
Experimental site
The experiment was conducted in Laboratory and the Research field of C.S. Azad Univ. of Agri & Tech. during kharif season in the year 2014-2015.
The nursery
Seeds of rice cultivar Pusa basmati-1121 were soaked in water for 24 hours and incubated for 48 hours to hasten early germination. Pre-germinated seeds were uniformly broadcast @60g per square meter in the nursery on 15th may 2014.
Preparation of the experimental field
The selected field area was well prepared and plot marked as per the lay out plan of the experiment. The selected field was dug up, cleaned and the soil was pulverized after which the total area was divided in to sub-plots. Maximum Relative Humidity (%) during the crop period (July- October 2015) was in the range of 71.43-89.85. Minimum Relative Humidity (%) during the crop period (July- October 2015) was in the range of 34.29-64.24. Maximum temperature was 37.82oC. Minimum temperature was 20.79oC and rainfall 00.01-16.94 mm.
Microbial cultures
Pyricularia oryzae was isolated and maintained on potato dextrose agar (PDA) (potato, 250 g; dextrose, 20 g; agar, 15 g; and distilled water, 1000 ml; pH 7.0) medium. The most effective rhizobacterial isolate which was isolated from rice rhizospheric soil that showed the highest antifungal activity against rice blast pathogen, P. oryzae in dual culture was selected and it was identified as P. fluorescens used for further studies. P. fluorescens was grown on KB broth in a shaking bath at 28±2oC for 24 h. The bacterial culture was centrifuged at 5000 × g for 10 min, the supernatant discarded and the bacterial pellet suspended in 0.1M phosphate buffer (pH 6.8). The cell density was adjusted to 1×107 cfu/ml with the same buffer by measuring the absorbance at 420 nm and used as inoculum.
Method of application
Time of the application of the Trichoderma is also important. Trichoderma can’t tolerate heavy pressure. Therefore, it may be used strictly as a preventive measure, it can’t cure infection. Trichoderma is least effective against the systematic disease than against more superficial one. It cannot control the existing disease. A combination of a chemical treatment with Trichoderma will be highly effective. A single strain of Trichoderma spp. may not be sufficient to be effective under all conditions and agents are effective against the disease.
Count the colony forming units of T. viride and T. harzianum
One gram of bio agents powder respectively Trichoderma and Pseudomonas fluorescens was weighed and the volume was made up to 10 ml with sterilized distilled water, shaken well (1:10) inside laminar flow hood. Out of this suspension 1 ml was taken out and transferred to 9 ml of sterilized distilled water in a test tube (1:100). Serial dilution were made similarly by transferring 1 ml of each suspension to the subsequent tubes to get 10-7and10-8 dilution respectively. 1 ml of each suspension (10-7&10-8) was transferred to sterilized petri plates. 15 ml of each medium such as PDA for Trichoderma and Kings B Agar for P. fluorescens was poured into plates. The plates were incubated in an inverted position at 25 ± 2°C. After 3 days, average numbers of colonies were counted per plates of both bio agents. Colonies were observed per plate and the number of colony forming unit (c.f.u) present in 1 g was calculated by the formula (Aneja, 2004).
Use of Neem Botanical
Preparation of neem leaf extracts
The collected plant leaves were chopped after cleaning in running tap water three times to remove soil materials. The dried leaves of each plant species were made into powder separately using a sterilized mortar and pestle and then sieved with one millimeter sieve. The extracts were filtered through cheese cloth. The powder of neem leaf extracts was packed in water proof plastic bags and labeled appropriately as described by Akinbode & Ikotun and stored at 4ÚC until used. Crude plant extracts were obtained by infusing 50 g of plant material in 100 ml SDW to give 50% w/v in a 500 ml conical flask and the mixture was incubated at 25ÚC – 28ÚC for 20 hours. The infusion was filtered separately through sterile double-layered cheese cloth into a sterile 400 ml beaker and the resulting stock solution was collected and stored at 25ÚC – 28ÚC until used.
Application of spray solution
Plant extracts and chemicals were sprayed as solution into the experimental plots as per treatments. Spraying was done for 3 times with 10 days interval at 65, 75 and 85 DAT respectively. Adequate precautions were taken to avoid drifting of spray materials from one plot to the neighboring ones.
Assessment of the disease incidence
Each plot was visited for recording the incidence. The disease incidence was recorded in the three growth stage of the plant namely flowering stage, milking stage and maturity stage. Assessment of the disease severity in the field five plants from each unit plot were randomly selected and tagged for grading the severity of diseases. Disease severity of leaf blast (Pyricularia oryzae) of rice was recorded by Singh (2000) used a 0-9 scale as follow: 0 = no lesion observed; 1 = 1% leaf area covered; 3 = 10% leaf area covered; 5 = 25% leaf area covered; 7 = 50% leaf area covered and 9 = more than 50% leaf area covered. Disease severity of leaf blast (Pyricularia oryzae) of rice was recorded by Singh (2000) used a 0-9 scale as follow: 0 = no lesion observed; 1 = 1% leaf area covered; 3 = 10% leaf area covered; 5 = 25% leaf area covered; 7 = 50% leaf area covered and 9 = more than 50% leaf area covered by using the disease rating scale of 0-9 developed by International Rice Research Institute (IRRI. 1996) and then converting into percent disease by using the formulas.
Disease severity % = (Sum of diseases grades) × (No. of infected tillers/hill) / (No. of tillers) × (Maximum disease grades) × (No. of tillers asses)
Measurement of Shoot and Root weight
After the assessment of disease, 60 days after transplanting plants were uprooted carefully from the field. The root region was cut separated from the plants and washed thoroughly to remove adhered soil particles with much care and the fresh shoot weight, dry shoot weight, fresh root weight and dry root weight of the plants of each treatment were measured in gram.
Experimental design and statistical analysis
The experiment was done following Randomized Complete Block Design (RCBD) with three replications. The experimental field was primarily divided into 7 blocks. Each block was further divided into 3 plots. Total number of plots was 21.
Effects of biotic and botanicals inducers on rice crop
Number of tillers of paddy at 60 DAT
T0 |
Untreated control |
T1 |
Pseudomonas fluorescens @ 8g/kg (ST) & 0.2% (FS) |
T2 |
Trichoderma harzianum @ 8g/kg (ST) & 10g/l (FS) |
T3 |
Trichoderma viride @ 8g/kg (ST) & 10g/l (FS) |
T4 |
Neem oil @ 1 ml/kg (ST) & 0.5 ml/l (FS) |
T5 |
Neem oil @1 ml/kg (ST) & Neem leaf extract @ 20ml/l (FS) |
T6 |
Carbandazim 50 WP @ 2g/kg (ST) & 0.2% (FS) (Treated control) |
Among the bio-agents and botanicals used, the maximum number of tillers were recorded in T1-P. fluorescens (46.43) as compared to treated and untreated control (38.46 and 25.93, respectively). The second best treatment was T2- T. harzianum (19.53), which was followed by T3–T. viride (44.13), T4 – neem oil (42.66) and T5 – neem oil + neem leaf extract (40.50) as compared to T0 – untreated control (36.86). Among the treatments most effective was T1-Pseudomonas fluorescens (46.43) (Table 1 & Fig 3).
Table (1):
Fresh and dry shoot weight from different treatments and disease severity at 90 DAT.
Treatments |
Shoot length |
No. of tiller |
Fresh shoot wt. (g) |
Dry shoot wt. (g) |
Fresh root weight (g) |
Dry root weight (g) |
Disease severity at |
Yield (q/ha) |
|---|---|---|---|---|---|---|---|---|
T0 |
62.23 |
36.86 |
120.4 |
25.66 |
26.46 |
5.8 |
34.15 |
35.43 |
T1 |
74.7 |
46.43 |
126.16 |
30.63 |
32.2 |
8.53 |
21.9 |
38.22 |
T2 |
72.23 |
42.83 |
124.76 |
29.26 |
30.46 |
7.63 |
23.08 |
37.72 |
T3 |
70.16 |
44.13 |
124.43 |
28.5 |
29.06 |
7.33 |
24.53 |
37.26 |
T4 |
69.56 |
42.66 |
122.33 |
26.96 |
28.6 |
7.3 |
24.2 |
36.81 |
T5 |
67.93 |
40.5 |
123.4 |
27.8 |
28.03 |
6.7 |
24.15 |
36.15 |
T6 |
66 |
38.46 |
121.8 |
26.53 |
27.46 |
6.5 |
18.57 |
39.9 |
S. Ed. (+) |
2.43 |
0.566 |
0.252 |
2.566 |
2.556 |
4.126 |
0.47 |
2.566 |
CD(P=0.05) |
2.976 |
1.335 |
0.553 |
1.268 |
1.268 |
0.523 |
3.014 |
0.636 |
Shoot length (cm) of paddy at 90 DAT
Among the bio-agents and botanicals used the maximum shoot length (cm) was recorded in T1-P. fluorescens (74.70) as compared to treated and untreated control (66.00 and 62.23, respectively). The second best treatment T2- T. harzianum (62.23), which was followed by T3– T. viride (70.16), T4– neem oil (69.56) and T5– neem oil + neem leaf extract (67.93) as compared to T0-control (62.23). Among the treatments most effective was T1-P. fluorescens (74.70). Similar findings were reported by Meera and Balabaskar (2012); Karpagavalli (2001); and Shyamala and Sivakumaar (2012) under field condition all the treatments tested in this study gave satisfactory result against blast of paddy (P. oryzae). Malleswari and Bagyanarayana (2013) and Sivakumar et al., 2012 suggested that growth promotion by bio-agents might be due to direct involvement of some plant hormones such as auxins, cytokinins etc. (Table 1, Fig 3)

Fig. 1. Measurement of plant height in research field
Fresh shoot weight and dry shoot weight (g) of paddy as affected by different treatments at 60 DAT
The result presented in table 1.1 and in fig. 4.4 depicted shows that all the treatments were significant and fresh shoot weight (g) and dry weight as compared to control. Among the bio-agents and botanicals used the maximum fresh shoot weight (g) and dry shoot weight was recorded in T1-P. fluorescens 126.16 and 30.63 respectively as compared to control (120.40 and 25.66 respectively). The second best treatment was T2- T. harzianum (19.53, 29.26), which was followed by T3–T. viride (124.73, 28.50), T4–neem oil (122.33, 26.90) and T5–neem oil+neem leaf extract (123.40, 27.80) as compared to T0 control (120.40 and 25.66). Maximum fresh shoot weight in P. fluorescens may be due to activation of plant growth permoting indole acitic acid (IAA) of an array of host defense mechanism including induced activity of enzymes accompanied by a significant increase in the growth of P. fluorescens has also been reported to enhance the crop growth and yield in rice (Pathak et al., 2004; Arshad and Frankenberger, 1991 and Khalimi et al., 2012) (Table 1 & Fig 2, 4)

Fig. 2. Measurement of fresh Shoot and root weight
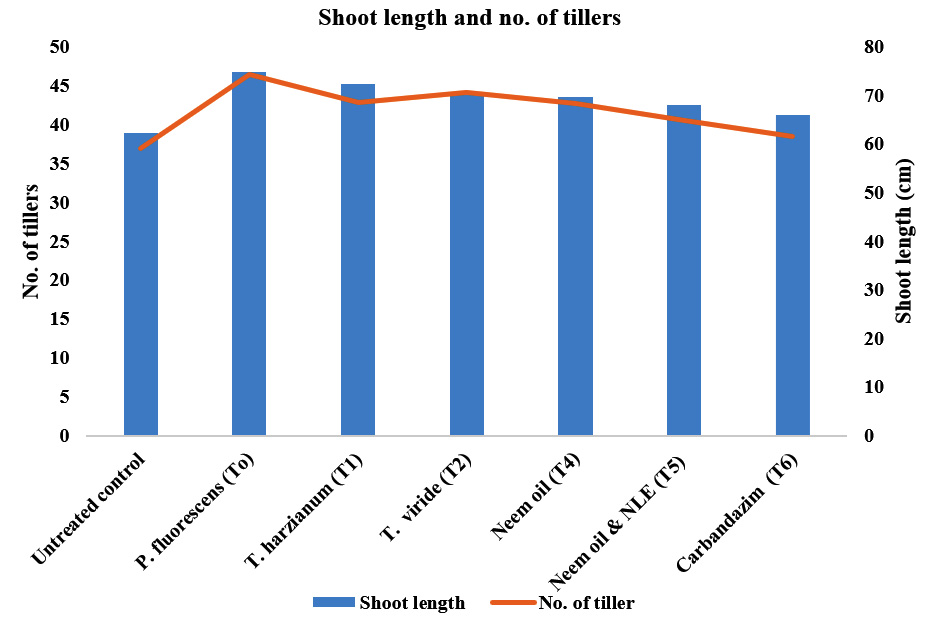
Fig. 3. Shoot length and No. of tillers from different treatments
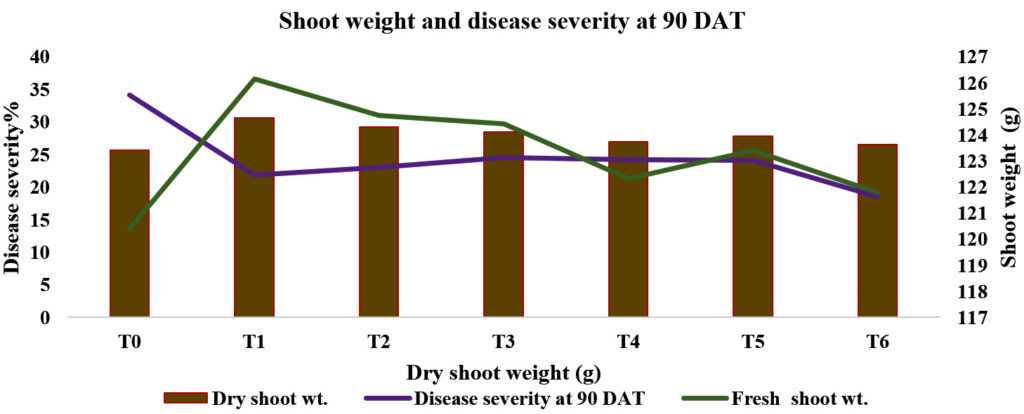
Fig. 4. Fresh and dry shoot weight from different treatments and disease severity at 90 DAT
Fresh root weight and dry root weight (g) of paddy as affected by different treatments at 60 DAT
Among the bio-agents and botanicals used the maximum fresh root weight (g) was recorded T1-P. fluorescens (32.20, 8.53) as compared to treated control (26.46, and 5.80 respectively). The second best treatment wasT2- T. harzianum (19.53, 19.53), which was followed by T3– T. viride (29.06, 7.33), T4 – neem oil (28.60, 7.30) and T5 – neem oil + neem leaf extract (28.03, 6.70) as compared to T0 – control (26.46, 5.80) (Table 1 & Fig 2, 5).
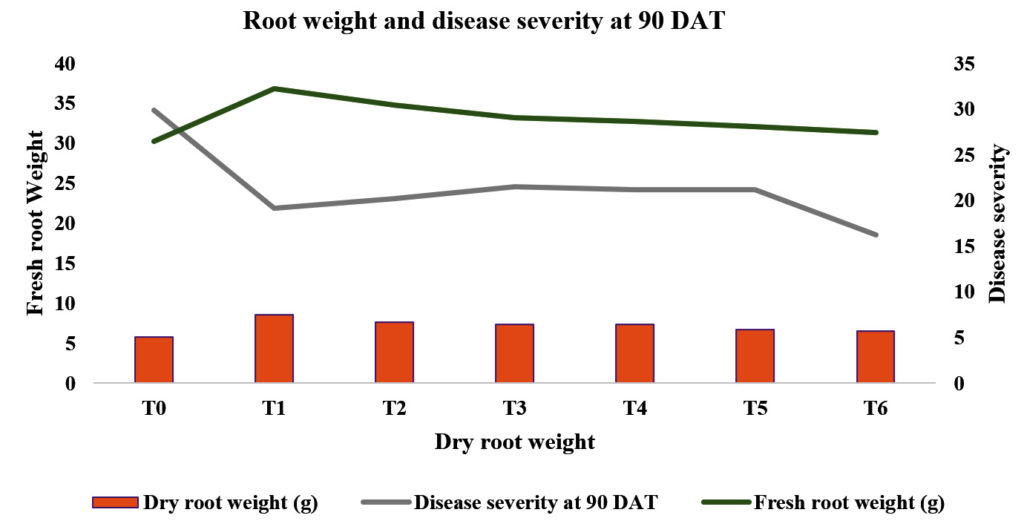
Fig. 5. Fresh and dry root weight from different treatments and disease severity at 90 DAT
Disease severity per cent at 90 DAT
It is clearly shown from the Table No. 1 that among the bio-agents and botanicals used the minimum disease severity per cent was in T1_P. fluorescens (21.99%) as compared to treated and untreated control (18.57% and 34.15 %, respectively). The second best treatment was T2- T. harzianum (23.08%), which was followed by T3–T. viride (24.53%), T4–neem oil (26.20%) and T5–neem oil + neem leaf extract (24.15%) as compared to T0-control (34.15 %) (Table 1 & Fig 6).
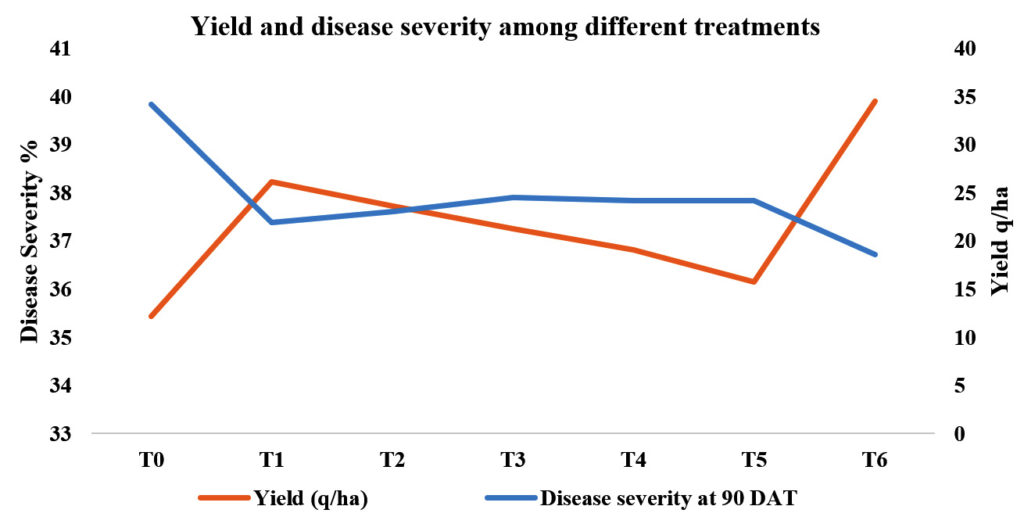
Fig. 6. Yield from different treatments and disease severity at 90 DAT
Yield of paddy as influenced by different treatments
Among the bio-agents and botanicals used yield was recorded in T1-P. fluorescens (38.22) as compared to treated and untreated control (39.90 and 35.43, respectively). The second best treatment was T2-T. harzianum (19.53), which was followed by T3– T. viride (37.26), T4 – neem oil (36.81) and T5–neem oil+neem leaf extract (36.15) as compared to T0-untreated control (35.43). Among the treatments most effective was T1-P. fluorescens (38.22). Similar findings were reported by Islam and Faruq, 2012; Razu and Hossain, 2015; Jha and Subramanian, 2013; Sharma, 2013; Ramezanpour, 2010 and Khorshidi et al., 2011 and they evaluated the efficacy of biocontrol agents used against blast of paddy incidence and promoting plant growth of paddy in field conditions.
Growers, in general still rely on the use of factitious fungicides for the management of plant diseases. However, the misuse of these chemicals may cause serious environmental and health problems. Microbial antagonists can be used as bio-pesticides to provide effective and safe means to manage plant diseases. Several microorganisms have been caught and proven having antagonistic properties against plant pathogenic fungi and other plant pathogens. Our findings showed that Pseudomonas fluorescens, Trichoderma viride and T. harzianum suppressed the growth of P. oryzae and proved its potential as bio-control agents. More studies are therefore needed to confirm the current findings and to determine the most effective formulation against P. oryzae.
- Akinbode, O. A. and Ikotun, T. Evaluation of some bio-agents and botanicals in Vitro in control of Colletotrichum destructivum. African J. of Biotec., 2008; 7(7): 868-872.
- Ali, H. and Nadarajah, K. Evaluating the efficacy of Trichoderma spp and Bacillus substilis as biocontrol agents against Magnaporthe grisea in rice. Australian J. Crop. Sci., 2014; 8(9): 1324-1335.
- Aneja, K. R. Experiment in Microbiology, Plant Pathology and Biotechnology. New Age International (P) Limited, Publishers. New Delhi. 2004; pp 147-156.
- Arshad, M. and Frankenberger, W. T. Microbial production of plant hormones. In: The Rhizosphere and plant growth. (Eds., Keister, D.L. and Cregan, P.B.), Kluwer Academic Publisher, Dordrecht. The Netherlands, 1991; pp. 327-334.
- Bhattacharji, R. and Dey, U. An overview of fungal and bacterial biopesticides to control plant pathogens/diseases. African J. Micro. Res., 2014; 8(17): 1749-1762. (th, tv, pf,)
- Gupta, V.K. and Kapoor, A.S. Fungal diseases of rice. Diseases of field crops. Edi. Indus Publishing Company, New Delhi. 2002; pp 79-85.
- Hajano, A.J., Lodhi, M., Mumtaz, A., Pathan, M., Ali, K. and Shah, G.S. In vitro evaluation of fungicides, plant extracts and bio-control agents against rice blast pathogen Magnaporthe oryzae Couch. Pak. J. Bot., 2012; 44(5): 1775-1778.
- IRRI. Standard evaluation system for rice. 4th ed. IRRI, Manila, Phillipine. 1996; 52 p.
- Islam, M. T. and Faruq, A. N. Effect of some medicinal plant extracts on damping-off disease of winter vegetable. World Appl. Sci. J., 2012; 17(11): 1498-1503.
- Jha, Y. and Subramanian, R. B. (2013). Root associated bacteria from the rice antagonizes the growth of Magnaporthe grisea. J. Pl. Pathol. Microb., 4(2): 164.
- Kankanala, P., Czymmek, K., Valent, B. Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell, 2007; 19: 706–724.
- Karpagavalli, S.; Marimuthu, T.; Jayaraj, J. and Ramabadran, R. An integrated approach to control rice blast through nutrients and biocontrol agent. Research on Crops. 2001; 2(2):197-202.
- Khalimi, K.; Suprapta, D. N. and Nitta, Y. Effect of Pantoea agglomerans on growth promotion and yield of rice. Agric. Sci. Res. J., 2012; 2(5): 240-249.
- Khorshidi, Y. R.; Ardakani, M. R.; Ramezanpour, M.R.; Khavazi, K. and Zargari, K. Response of yield and yield components of rice (Oryza sativa L.) to Pseudomonas flouresence and Azospirillum lipoferum under different nitrogen levels. American-Eurasian J. Agric. & Environ. Sci., 2011; 10(3): 387-395.
- Malleswari, D. and Bagyanarayana, G. In vitro screening of rhizobacteria isolated from the rhizosphere of medicinal and aromatic plants for multiple plant growth promoting activities. J. Microbiol. Biotech. Res., 2013; 3(1):84-91.
- Meera, T. and Balabaskar, P. Isolation and characterization of Pseudomonas fluorescens from rice field. Intern. J. Food, Agri. and Veterinary Sci. 2012; 2(1):113-120.
- Mehrotra, R. S. Plant Pathology. Tata MC Grow Hill Pub. Co. Ltd., New Delhi. 2003; 591 p.
- Munch, S., Lingner, U., Floss, D.S., Liudwig, N., Sauer, N., Deising H.B. The hemibiotrophic lifestyle of Colletotrichum sp. J. Plant Physiol 2008; 165: 41–51.
- Ou, S. H. Rice Diseases. Commonwealth Mycological Institute, Kew, Survey, England., 1985; 380 p.
- Padamnabhan, S.Y. Estimating losses from rice blast in India. The rice blast disease. The Johns Hopkins press, Baltimore, Maryland. 1965; pp 203-221.
- Pathak, A.; Sharma, A.; Johri, B. N. and Sharma, A. K. Pseudomonas strain GRP3 induces systemic resistance to sheath blight in rice. Intern. Rice Res., 2004; 29(1): 35-36.
- Perfect, S.E. and Green, J.R. Infection structures of biotrophic and hemibiotrophic fungal plant pathogens. Mol. Plant Pathol., 2001; 2: 101–108.
- Ramezanpour, M. R. Genetic diversity and efficiency of Indole acetic acid production by the isolates of Pseudomonads fluorescent from rhizosphere of rice (Oryza sativa L.). American-Eurasian J. Agric. & Environ. Sci., 2010; 7(1): 103-109.
- Razu, M. A. U. and Hossain, I. Eco-friendly management of rice diseases. Int. J. Appl. Sci. Biotechnol., 2015; 3(1): 80-88.
- Sharma, G. D. Characterization of plant growth promoting traits of diazotrophic bacteria and their inoculating effects on growth and yield of rice crop. Global research analysis, 2013; 2(4): 2277- 8160.
- Shyamala, L. and Sivakumaar, P.K. Integrated control of blast disease of rice using the antagonistic rhizobacteria Pseudomonas fluorescens and resistance inducing chemical salicylic acid. Intern. J. of Res. in Pure and Applied Microb., 2012; 2(4): 59-63.
- Singh, R.S. Introduction to principles of plant pathology, Third edition, Assessment of disease incidence and loss, Oxford & IBM publishing Co. Pvt. Ltd, New Delhi. 328 p. 2000.
- Sivakumar, T., Shankar, T., Vijayabaskar, P., and Ramasubramanian, V. Plant growth promoting activity of nickel tolerant Bacillus cereus TS1. Journal of Agricultural Technology, 2012 8(6): 2101-2113.
- Sivamani, E. and Gnanamanickam, S. S. Biological control of Fusarium oxysporum f.sp. cubense in banana by inoculation with Pseudomonas fluroscens. Plant Soil, 1988; 107 (2): 3-9.
- Sukanya, S.L., Yamin, D. and Fathima, S. K. Eco-friendly management of Pyricularia oryzae – The causal agent of blast of paddy. Current Botany, 2011; 2(8): 46-49.
- Talbot, N.J. On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2003; 57: 177–202.
- Tony Cisse, K. Techniques for organic paddy cultivation. Indigenous Agriculture News, 2005; 4: 1-4.
- Zarandi, M. E., Bonjar, G.H.S., Dehkaei, F. P., Moosavi, S.A.A., Farokhi, P. R. and Aghighi, S. Biological Control of Rice Blast (Magnaporthe oryzae) by use of Streptomyces sindeneusis isolate 263 in Greenhouse. American Journal of Applied Sciences 2009; 6(1): 194-199.
© The Author(s) 2017. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


