ISSN: 0973-7510
E-ISSN: 2581-690X
The present study aimed to investigate the biological effects of different concentrations of Ulva lactuca aqueous extract (ULAE) on the growth parameters and biochemical characteristics of Zea mays. The ULAE was applied by foliar spray or directly added to the growth medium of maize plant cultivated in hydroponic system. The growth parameters (shoot length, root length, fresh weight, dry weight and seedling length), minerals, biochemical constituents (chlorophyll content, the activity of ribulose bisphosphate carboxylase (Rubisco), and the detection of Rubisco activase (rca1) gene in seedlings of Zea mays hybrid line (M10) were determined. ULAE was characterized by high abundance of calcium (3255.86 ppm) followed by potassium (287.9 ppm). Among the different treatments, 0.5% and 1% concentration of ULAE as foliar spray exhibited significant effects on growth parameters and biochemical constitutions of the Zea mays seedlings. However, at the higher concentration of ULAE (5%), inhibitory effects were observed. Moreover, as a foliar spray, ULAE at 0.5% and 1% concentration showed a significant effect on seedling length.
Ulva lactuca; Rubisco; Photosynthetic Pigments; RCA1
Concerns have been raised over the harmful effects of chemical fertilizers on humans and environment directly or indirectly (Dubey, 2010). Consequently, farmers began to shift from conventional chemical-based farming systems to organic, alternative, or low-input sustainable agriculture. Seaweed extracts (SWE) as foliar sprays for several crops have gained significant importance as they contain growth promoting hormones (IAA and IBA), cytokinins, minerals (Fe, Cu, Zn, Co, Mo, Mn, Ni), vitamins, and amino acids (Sivasankari, 2006; Abdel Khalik et al., 2013). Seaweed extracts have been suggested to enhance the growth and yield of the crop plants, increase plant tolerance to environmental stresses, increase nutrient uptake from soil and enhance antioxidant properties (Rathore, 2009; Eissa et al., 2017). Ulva lactuca, also known as sea lettuce, is one of the most abundant green macro algae worldwide (Lahaye and Robic, 2007). Despite its wide geographical distribution, it is poorly utilized (Ray and Lahaye, 2007) and only a small part of its biomass is used as food or animal feed because of its nutritional constituents (vitamins, oligo elements, minerals, and dietary fibers) (Pengzhan et al., 2003); organic crop fertilizer (Mulbry et al., 2005), effluent biofilter (Msuya and Neori, 2002) and more recently, as plant protectant (Cluzet et al., 2004).
Sridhar, (2011) reported an enhanced seed germination and protein profile when the seeds of five plants were treated with 1.0% SWE of Ulva lactuca and Sargassum wightii. The earlier study revealed that SWE of Ulva reticulata could also be used as a foliar spray at low concentration (2%) to maximize the growth and yield of Vigna mungo and also increase the number of stomata in the leaf (Ganapathy and Sivakumar, 2013). Maize (Zea mays), a C4 plant, is one of the most important cereal crop grown worldwide, serving as an essential source of food, livestock feed, and fuel (Osman et al., 2015; Abdel Latif and Osman, 2017). In maize, the C4 photosynthetic pathway is followed, which is characterized by several biochemical and anatomical modifications that allow plants with this photosynthetic pathway to concentrate CO2 at the site of ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco; EC 4.1.1.39). The enzyme of primary CO2 fixation in this pathway catalyzes the photosynthetic carbon metabolism by combining CO2 with ribulose 1,5-bisphosphate. Various studies have shown that the activity of Rubisco is regulated by an enzyme, Rubisco activase (RCA) (Portis, 2003; Osman et al., 2013).
RCA, a soluble chloroplast ATPase, is associated with a variety of cellular activities. RCA catalyzes the activation of Rubisco in vivo by the ATP-dependent removal of inhibitory sugar phosphates (Portis et al., 2008). A significant reduction (Mate et al., 1993; Hammond et al., 1998) or absence of RCA (Portis, 1992) considerably impairs the photosynthetic process in higher plants. The presence of RCA in various plants provides evidence that activation of Rubisco in vivo requires a specific chloroplast protein in order to occur at physiological levels of CO2 and RuBP (Salvucci et al., 1985; Abouseadaa et al., 2015). By modulating the activation level of Rubisco in response to light intensity, RCA exerts an important regulatory control on photosynthesis. Furthermore, various studies have reported different numbers of RCA polypeptides in maize, from one to three; the molecular masses of these polypeptides are approximately 41, 43, and/or 45 to 46 kD, respectively (Crafts-Brandner and Salvucci, 2002; Vargas-Suárez et al., 2004; Ristic et al., 2009). The present study aimed to evaluate the biological effects of ULAE on growth parameters (shoot length, root length, fresh weight, dry weight, and seedling length), minerals, biochemical constituents (chlorophyll content, the activity of ribulose bisphosphate carboxylase (Rubisco)), and Rubisco activase (rca1) gene in seedlings of Zea mays. The SWE obtained from Ulva lactuca was applied as a foliar spray or directly added to the growth medium of maize plant cultivated in a hydroponic system.
Collection of Seaweed Materials
Marine algae Ulva lactuca was collected manually from Abu-Qir coast, Alexandria, Egypt during October 2015. The algae was then washed thoroughly with filtered seawater many times to remove epiphytes and sands, then was brought to the laboratory and washed thoroughly in tap water 3 to 4 times to remove excess salt.
Preparation of Seaweed extract
The washed and cleaned seaweed was shade-air dried for 2–4 days followed by oven drying for 12 h at 60°C. The oven dried seaweed was hand crushed and finely powdered with mixer-grinder. Of the dried material, 100 g was extracted with 1000 ml of distilled water for 24h. The contents were then filtered through a double-layered muslin cloth. The filtrate thus obtained was considered as 100% seaweed aqueous extract. A 10% extract concentration was prepared using double distilled water and stored at 4°C.
Plant material and experimental design
Zea mays grains (M10) were procured from the Crop Institute, Agricultural Research Center, Giza, Egypt. The grains having the uniform size, shape, color and weight were selected for the study. Ten grains were arranged in 18-cm diameter Petri dishes on two layers of filter paper (Whatman number 1) under normal laboratory conditions at 20–23°C day/14–16°C night. Afterward, 15 mL of distilled water was then added. Before sowing, the grains were surface sterilized by soaking for 2 min in 4% sodium hypochlorite, then, rinsed four times with double distilled water. Seven–day old seedlings were transferred to the growth units which consisted of 4 polyethylene tubes (5 cm diameter, 51 cm length). Each tube had an out and inlet in order to circulate the nutrient solution (Fig 1).

Fig. 1. Polyethylene tubes used in the study as hydroponic growth units
The capacity of each tube was 1500 mL with 31 pores (8 mm) distributed in two alternation lines, where the seedlings should be settled. The micropipettes plastic tips were used to support the seedlings during growth and when they were harvested. An air pump terminal with a flow rate of 200 ml/min was used to aerate and circulate the solution. The nutrient solution was completely renewed every three days. The experiment was performed under normal laboratory conditions (20 ±2°C temperature, 75 ±2% relative humidity, and 14/10h light/dark photoperiod).
The experimental design
Four treatment groups were designed for the application of ULAE in the growth medium of maize plant cultivated in the hydroponic system. The first group of seedlings was grown in medium containing 50% of half-strength Hoagland’s solution and 50% of ULAE (50% U + 50% H). The second group of seedlings was grown in a medium containing 25% of half-strength Hoagland’s solution and 75% of ULAE (75% U + 25% H). Whereas, the third group of seedlings was grown in 100% half-strength Hoagland’s solution and were not treated with ULAE (control) and seedlings of the fourth group were grown in ULAE alone (10%) served as a positive control. In a parallel experiment, four foliar applications consisting of three different ULAE concentrations (0.5%, 1%, and 5%) and one control treatment (no spray) were given to seedlings grown in the hydroponic system with half-strength Hoagland’s solution. About 50 ml of different concentrations of the extract or water (for the control treatment) was given at 3 days intervals up to 14 days. After 14 days, the homogenous seedlings were carefully collected from each treatment, and then gently blotted with filter paper. The growth parameters such as root length, shoot length, fresh and dry weight, and seedling length were recorded. The biochemical parameters including chlorophyll a, b, and total chlorophyll content, carotenoid and Rubisco were also analyzed. Other samples were dried at 65oC till constant weight to determine the dry weight. The experiment was carried out in triplicate.
Determination of some growth parameters
Root length and shoot height were measured by laying the plants (control and treated) on a moist black cloth with a millimeter scale fixed alongside the plants and the whole set up photographed. The photos were then projected on a rigid screen and the length of roots and height of the shoots were measured from the image. The exact length of the organ in millimeter was then determined by reference to the image of the millimeter scale. Leaf area was determined by tracing outlines of leaves on paper and measuring the enclosed area with a planimeter.
Determination of minerals
The extraction method of Humphires (1956) was used. Oven dried samples of seedlings were finally ground and assayed for mineral ion content by the wet digestion method. One ml of conc.H2SO4 was added to about 200mg of powdered samples in boiling tube. After complete charring one ml of 50% sulphuric – 30%perchloric mixture (1:1 by volume) was added. The tube was placed on a hot plate till the sample became colorless. After cooling the solution was filtered then diluted to volume. Aliquots of this solution were taken for determination of different elements. Minerals were determined using an atomic absorption spectroscopy ( Perkin-Elmer,2380) and expressed on basis of dry weight.
Similarly, dried seaweeds were hand crushed and powdered with coffee-grinder. This milled material (100 g) of each sample was subjected to acid digestion and analyzed by atomic absorption spectrophotometry for mineral analysis of sodium, potassium, calcium, magnesium, manganese, ferrous, zinc, calcium, cadmium and cupper (by colorimetry) following procedures from the Association of Official Analytical Chemists (AOAC 1990).
Determination of total protein
Protein content was estimated according to the method described by Hartree, (1972).
Measurement of Photosynthetic Pigments. Photosynthetic pigments, chlorophyll a, chlorophyll b and carotenoids were extracted and determined from new and fully expanded young leaves according to the method of William and Bloom, (1985).
Analyses of photosynthetic pigments
Leaf tissue was added to a pre-chilled mortar in an ice bath and were ground with pestle in Chlorophyll extraction from harvested leaves was done using 4ml of N,N-dimethyl formamide (DMF) for 24h at 4°C. The samples were subsequently kept in a deep freezer for further spectrophotometric analysis. Chlorophyll was quantitatively determined using spectrophotometry at wave lengths of 646.8 and 663.8nm (Porra, 1991). Total caroteneoids content was calculated according to Moran, (1982) and related to leaf weight.
Chl. a = 12.00 ·Abs.663.8-3.11·Abs.646.8
Chl. b = 20.78 ·Abs.646.8-4.88·Abs.663.8
Chl. a+b = 17.67 ·Abs.646.8+7.12·Abs.663.8
Total caroteneoids = 1000 Abs.470 –0.89 (Chl. a)-52.02(Chl. b)/ 245
The contents were expressed as mg Chl or carotenoids g”1 fresh weight (FW).
Protein Extraction and Relative Amount of Rubisco Enzyme Estimation
The 2nd leaf of ten plants was used for the extraction of protein and subsequent enzyme activity and protein amount measurements. The leaves were coarsely ground in liquid N2 with a mortar and pestle. Then, 600 mg of this coarsely ground leaf tissue was homogenized in 6 mL of buffer containing of 0.1 M (Tris [hydroxymethyl] aminomethane)-HCl (Tris-HCl, pH 7.5), 5 mM ethylene glycol-bis (²-aminoethyl ether)-tetraacetic acid (EGTA), 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 ¼M pepstatin, 2 mM dithiothreitol (DTT), 5 mM MgCl2, 10 mM NaHCO3, 1.5% (w/v) polyvinylpolypyrrolidone, and 5 ¼g/mL leupeptin. Soluble and insoluble fractions were separated using centrifugation at 17,608 g at 48°C for 15 min. Then, 2.5 mL of protein-containing supernatant was subsequently desalted on a Sephadex G25 column (Pharmacia PD–10, Orsay, France) in darkness at 48°C with elution buffer composed of 0.1 M Tris-HCl (pH 7.5) and 2 mM DTT. The desalted protein extract was immediately used for the determination of enzyme activities. Proteins present in the remaining supernatant (about 1 mL) were then precipitated according to Leitao et al., (2007). After centrifugation (17608 g, 48°C, 10 min), the pellets were resuspended in 200 ¼l of extraction buffer. Relative amounts (%) of Rubisco (SSU and LSU) were measured using HPLC analysis (C18 column) according to Leitao et al., (2007).
Protein concentrations in both desalted and precipitated extracts were determined using the method described by Hartree, (1972).
Rubisco activase 1 gene (rca1) detection technique
Total DNA was extracted from new fully expanded young leaves using GeneJET Genomic DNA purification kit following the manufacturer’s protocol (Thermo- Scientific).
PCR Amplification of rca1
The PCR was carried out in 25 µL reaction tubes containing 50 ng of template DNA, 1 pmol of each primer, and 1X PCR Master mix. The reaction mixtures were subjected to the following cycling steps: 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 30s, annealing at 52°C for 30 s and extension at 72°C for 30s. The thermal profile ended with a final extension at 72°C for 10 min (Abdel Khalik and Osman, 2017). The PCR product was stored at 4°C. The PCR products were analyzed using 0.45, 0.6 and 0.75% agarose gel electrophoresis (SeaKem LE agarose, Cambrex, gels for genomic and amplified DNA) and visualized under UV fluorescence after staining with ethidium bromide and the images were acquired using Gel Doc XR+ Imaging system (Bio-Rad Laboratories Inc., Germany). The following primer set was used for the PCR amplification of rca1, forward primer, 5’-CGAATGGCTCATTAAAACAG-3’ and reverse primer 5’-CCAACTACGAGCTTTTTAAC -3’. Gene specificity of primers was confirmed throughout BLAST searches in public databases.
Statistical Analysis
The quantitative data were analyzed by one-way analysis of variance (ANOVA) and Student’s t-test using COSTAT 2.00 statistical analysis software manufactured by CoHort Software Company (Zar, 1984). The differences in the treatment means were compared using the Least Significant Differences (LSD) test to evaluate the significant differences of the data at 0.05 probability level.
Determination of mineral composition of Ulva lactuca
The essential minerals such Calcium (Ca), Potassium (K), Zinc (Zn), Magnesium (Mg), and Iron (Fe) are present in significant amounts in U. lactuca (Table 1).
Table (1):
Minerals composition of U. lactuca
Element |
K |
Cu |
Cd |
Ca |
Mg |
Fe |
Mn |
Zn |
Na |
|---|---|---|---|---|---|---|---|---|---|
Concentration (ppm) |
287.9 |
5.8 |
0.8667 |
3255.86 |
90.9 |
71.17 |
17.63 |
79.8 |
27.06 |
Among these elements, Ca (3255.86 ppm) is the most abundant element in the U. lactuca seaweed, followed by Potassium (287.9 ppm). The other elements are Mg (90.89 ppm), Zn (79.79 ppm) and Fe (71.17 ppm). The high mineral content of U. lactuca seaweed is due to the consumption of the nutritive elements in the medium, where the algae grows.
Growth parameters
Shoot and root length
The shoot length of seedlings grown in Hoagland solution (control treatment) was greater (21.9 cm) as compared to seedlings grown in 100% ULAE (17.7 cm). A mixture of 50% H + 50% U did not affect the shoot length; however, the maximum shoot length (24.8 cm) was found in seedlings treated with 25% H + 75% U. Similarly, the root length was also affected by different treatments, an increased root length of 27.4 cm and 22.8 cm was observed in seedlings treated with a mixture of 25% H + 75% U and seedlings grown only in 100% ULAE, respectively, when compared to 22.8 cm root length of the control (Fig. 2).
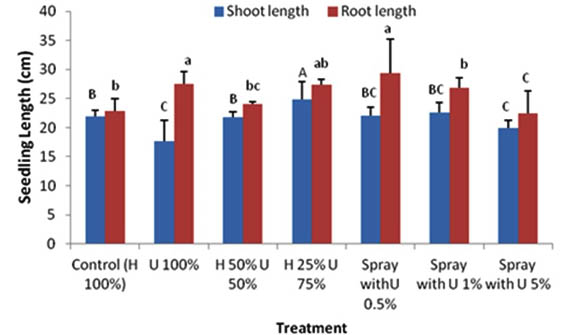
Fig. 2. Effect of applying different concentrations of ULAE in the growth medium or as foliar spray on Shoot and Root lengths of 14-day-old Zea mays seedlings. Values are means±SD. Different letters above each column indicate a significant difference at probability level ≤ 0.05 according to LSD
In foliar application experiment, the use of ULAE significantly promoted the growth and physiology of Zea mays. The treatments applied as a foliar spray of ULAE at 0.5% or 1% displayed an increase in the growth and biochemical parameters. However, the inhibitory effect was also observed when seedlings were sprayed with the higher concentration of ULAE (5%). A significant increase in the seedling length was observed when ULAE applied as a foliar spray at 0.5% and 1% treatment concentration, which may be attributed to the increase in root length rather than shoot length. The maximum effect was found with 0.5% treatment where the seedling length reached 51.4 cm compared to the control (44.7 cm). However, there was a reduction in the seedling length when the seedlings were sprayed with 5% ULAE, the reduction was mainly due to the reduction of root rather than shoot length.
Fresh and dry weight
The fresh weight of the control seedlings was 3.45 g. The seedlings grown in 100% ULAE and 50% H+50% U had relatively lower fresh weights than the control. The seedlings grown in 25% H + 75% U had a significantly higher fresh weight (4.15 g) compared to the control and the percent increase was 16.8%.
The different applications of ULAE treatments had no effect on the dry weights of seedlings (Fig.3).
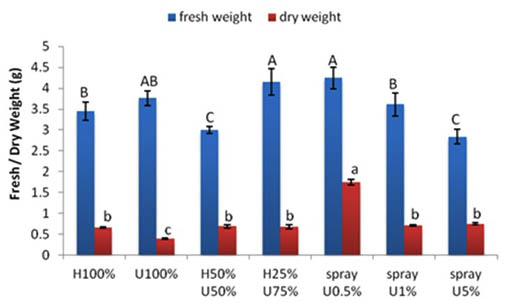
Fig. 3. Effect of applying different concentrations of ULAE in the growth medium or as foliar spray on fresh weight, dry weight of 14-day-old Zea mays seedlings. Values are means±SD. Different letters above each column indicate a significant difference at probability level ≤ 0.05 according to LSD
The differences in fresh and dry weights showed similar responses to foliar applications of ULAE as of seedlings length. A decrease in the fresh weight (2.84 g) of seedlings was observed with the higher concentration of ULAE (5%) compared to the corresponding control (3.45 g); however, an increase in fresh weight was found in other two treatments. The maximum increase was obtained at a concentration of 0.5% of ULAE where fresh weight reached 4.25 g with a percentage increase of 18.8% compared to the control. The dry weight of seedlings was significantly enhanced by all treatments of ULAE applied by foliar spray when compared to the control plants.
Leaf area
The highest leaf area of 16.4 cm2 of Zea mays was recorded for the seedlings receiving H25%+U75% treatment, whereas, seedlings sprayed with 5% extract had the lowest leaf area (10.33 cm2).
Photosynthetic pigments
However, a significant decrease in total photosynthetic pigment content of Zea mays seedlings was recorded for those received different treatments (Table 2).
Table (2):
The variations in the mean concentration of different pigment fractions (mg/g.f.wt.) of 14-day-old Zea mays seedlings as affected by different concentrations of ULAE added to the growth medium or used as a foliar spray. The data are means of three replicates
Treatment |
Chl. a |
Chl. b |
Carotenoids |
Total pigments |
Chl. a / Chl. B |
|---|---|---|---|---|---|
H100%(control) |
14.66± 1.35a |
10.37± 1.14a |
2.55± 0.56a |
27.88a |
1.41 |
U100% |
11.11± 1.09b |
9.60± 0.92 a |
0.89± 0.21b |
21.6 a |
1.15 |
H50%U50% |
11.75± 1.80 b |
8.77± 0.88ab |
1.64± 0.32 ab |
22.16a |
1.33 |
H25%U75% |
13.41± 2.04 ab |
10.88± 1.72 a |
0.84± 0.19 b |
25.13 a |
1.23 |
Spray with0.5U |
15.28± 3.66 a |
10.06± 1.05 a |
2.49± 0.43 a |
27.83 a |
1.51 |
Spray with 1%U |
8.82± 1.09c |
8.88± 0.90 ab |
0.14± 0.37c |
17.84b |
0.99 |
Spray with 5%U |
9.95± 1.63c |
7.34± 0.95b |
1.63± 0.25ab |
18.92 b |
1.35 |
P |
0.023* |
0.045* |
0.017* |
0.0201* |
0.087 |
Total photosynthetic pigment content in the control seedlings was 27.88 mg/g.
H: Hoagland’s solution, U: Ulva lactuca aqueous extract
Chlorophyll a was higher than that of Chlorophyll b, it was 42% and 34% in seedlings sprayed with 1% and 5% of ULAE, respectively when compared to the corresponding control value. The percentage of reduction in Chlorophyll b reached 11% in 1% ULAE and 27% in 5% ULAE treatments, respectively. Similarly, carotenoid contents decreased significantly in response to different treatments.
Elemental content
The effects of ULAE on the mineral composition of Zea mays are presented in Table 3.
Table (3):
The variations in the concentration of some nutrient elements (mg /g. d.wt.) of 14-day-old Zea mays seedlings as affected by different concentrations of ULAE added to the growth medium or as a foliar spray. The data are means of three replicates
Sample |
Cu |
Fe |
Ca |
Zn |
Mn |
Cd |
Mg |
Na |
K |
|---|---|---|---|---|---|---|---|---|---|
H100% |
39.9240 |
74.4704 |
572.3791 |
86.9636 |
50.1901 |
1.5209 |
390.6029 |
430.4 726 |
1200.5160 |
U100% |
6.4387 |
219.3542 |
2212.1895 |
81.8303 |
23.8250 |
1.7959 |
289.8357 |
304.1269 |
826.7864 |
H50%
U50% |
4.1219 |
50.6400 |
1016.7435 |
144.2908 |
28.4178 |
2.3041 |
374.5776 |
389.0681 |
1120.7629 |
H25%
U75% |
4.4005 |
112.8424 |
1070.1841 |
82.2407 |
34.6430 |
2.1554 |
334.7328 |
342.7256 |
1011.5177 |
Spray
0.5%U |
2.8042 |
66.0847 |
1415.5291 |
64.7619 |
53.5185 |
2.8571 |
382.7249 |
383.5185 |
1151.2169 |
Spray
1%U |
6.1754 |
77.2632 |
1416.1404 |
72.7368 |
42.7368 |
3.9298 |
506.2456 |
476.1053 |
1552.9825 |
Spray
5%U |
3.4726 |
95.5675 |
1286.8154 |
73.0096 |
50.8752 |
3.1621 |
411.6318 |
447.5155 |
1232.9475 |
Furthermore, the total photosynthetic pigment content of seedlings sprayed with 5% ULAE was significantly reduced, and the percent reduction observed was 36% as compared to the control. This reduction in total photosynthetic pigment content of the seedlings sprayed with different concentrations of ULAE could be attributed to the decrease in both Chlorophyll a and Chlorophyll b. The percentage of reduction in H: Hoagland’s solution, U: Ulva lactuca aqueous extract
The maximum concentration of Ca (2212.18 mg/g) and Fe (219.3 mg/g) was recorded in seedlings that were grown in 100% U with a percentage increase of 74% and 66%, respectively, as compared to the control. However, the concentration of copper (Cu) was significantly reduced in all treatments tested. The seedlings that received foliar spray with 1% ULAE exhibited the highest content of K, Mg, Na, and Cd as compared to the corresponding control. A significantly increased concentration of Ca was recorded on the application of ULAE compared to the control, where the percent increase was 60%, 59%, and 55% in seedlings sprayed with 0.5, 1, and 5% ULAE. Respectively (Fig. 4).
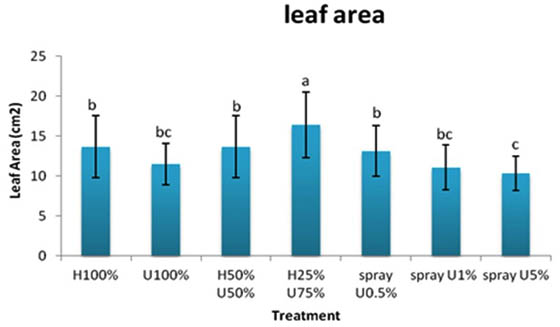
Fig. 4. Effect of applying different concentrations of ULAE in the growth medium or as foliar spray on leaf area of 14-day-old Zea mays seedlings. Values are means±SD. Different letters above each col-umn indicate a significant difference at probability level ≤ 0.05 according to LSD
Protein Profile
The protein profile of 14-day old Zea mays seedlings treated with ULAE was analyzed and the results are shown in Figure 5.
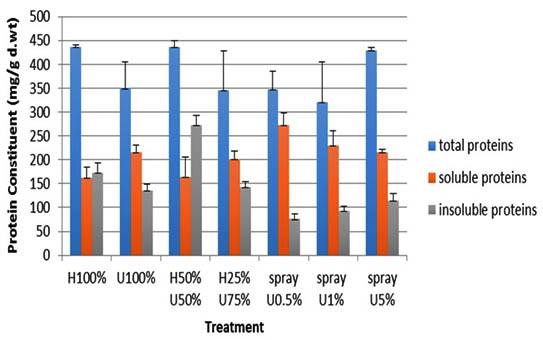
Fig. 5. Effect of ULAE incorporated in the growth medium or applied as foliar spray on protein constitu-ents (mg/g d.wt) of 14 day-old Zea mays seedlings. Values are means±SD. Different letters above each column indicate a significant difference at probability level ≤ 0.05 according to LSD
The soluble protein fraction was relatively higher than the insoluble fraction particularly in seedlings sprayed with different concentrations of ULAE. A decrease in the insoluble protein fraction was recorded in the seedlings treated with 0.5% ULAE, the decrease recorded was 56.2%, when compared with the corresponding control. On the other hand, the seedlings grown in 50%U+50%H showed the highest total protein value among all the treatments, which was due to an increase in the insoluble fraction compared to the soluble one. The percentage increase in the insoluble fraction of those seedlings was 36% compared to the corresponding control.
Rubisco and Rubisco activase
Generally concerning all treatments tested in this work, a high Rubisco enzyme concentration was obtained in seedlings treated with 100% U and those sprayed with 5% ULAE, as compared to the corresponding control. The highest Rubisco activity recorded in seedlings grown in U 100% (Fig. 6).
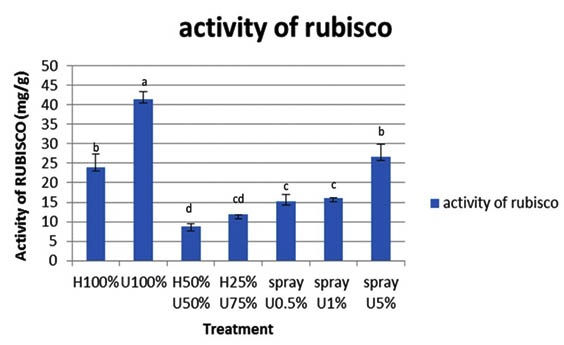
Values are means ±SD. And -Different letters above each column indicate a significant difference at probability level d” 0.05 according to LSD. difference at probability level d” 0.05 according to LSD.
Fig. 6. Effect of ULAE incorporated in the growth medium or applied as foliar spray on activity of Ru-bisco (mg/g) of 14 day-old Zea mays seedlings
Rubisco activase transcripts from Zea mays tended to be less abundant in all treatments compared to the control, suggesting that Rubisco from Zea mays leaves might be less activated. The analysis of the final preparation by agarose gel identified two bands with approx. 372 and 274 bp (Fig 7).
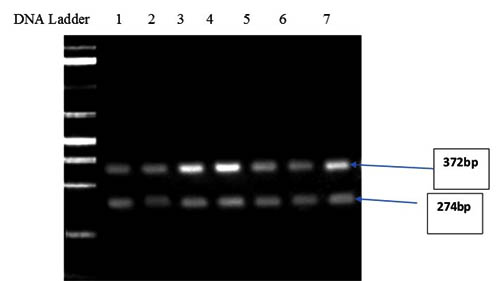
Lanes:1-DNA Marker, 2- Zea mays grown in 50% Hoagland’s solution and 50% of Ulva lactuca extract, 3- Zea mays grown in 25% Hoagland’s solution and 75% of Ulva lactuca extract, 4- Zea mays grown in 100% Hoagland’s solution and sprayed with 1% Ulva lactuca extract as foliar spray, 5- Zea mays grown in 100% Hoagland’s solution and sprayed with 0.5% Ulva lactuca extract as foliar spray, 6-Zea mays grown in 100% of Ulva lactuca extract, 7- Zea mays grown in 100% Hoagland’s solution and sprayed with 5% Ulva lactuca extract as foliar spray, 8- Control treatment 100% half strength Hoagland’s solution
Fig. 7. Agarose gel electrophoresis showing the PCR products of RuBisCO Activase I (rca1) gene with approx. 372 and 274 bp of Zea mays seedlings treated with ULAE added to the growth medium or applied as a foliar spray
Conversely, the supplementation of 100% U tended to promote the accumulation of Rubisco activase transcripts. Even if values of steady-state mRNA content do not reflect the rate of translation or protein synthesis (Boschetti et al., 1990), our results suggest that application of ULAE to the growth medium may either up- or down-regulate the carboxylation by Rubisco through changing the quantity of the enzyme and/or by changing regulation of its activity. The presence of Rubisco activase explains how Rubisco can achieve and maintain a high activation state in vivo at normal levels of CO2 in the presence of millimolar concentrations of RuBP (Salvucci et al., 1985). The occurrence of activase in most plant species is an evidence to its role on Rubisco in higher plants. It was particularly important that activase was detected in maize, a C4 plant, since the CO2 concentration is higher at the site of carboxylation (Hatch, 1971), while RuBP is present in millimolar concentrations. Thus, the occurrence of activase would be anticipated if the major function of activase is to maintain Rubisco in the activated state and prevent RuBP deactivation by catalyzing the activation of the tight binding enzyme-RuBP complex. Our study investigated the effect of different concentrations of ULAE added to the growth medium or applied as a foliar spray on the seedling of Zea mays. The study showed a significant increase in growth due to the application of seaweed extract of Ulva lactuca. Similar results were obtained in earlier studies on Phaseolus vulgaris L. (Munshi and Osman, 2010; Kocira et al., 2013). There was a significant increase in growth and biochemical constituents tested when the seedlings were sprayed with 0.5% or 1% of ULAE, while at the higher concentration seedlings growth was inhibited. Similar results were also reported in spinach, Vigna mungo, and tomatoes Featon (Smith and van Staden, 1983; Ganapathy and Sivakumar, 2013; Hernández-Herrera et al., 2013; Zodape et al., 2011 ; Alothyqic et al., 2016; Bahieldin et al., 2018; El-Helow et al., 2018). The total photosynthetic pigment content of seedlings sprayed with 5% ULAE were significantly reduced, and the percent reduction recorded was 36%. This reduction in total photosynthetic pigment content of the seedlings sprayed with different concentrations of ULAE could be attributed to the decrease in both chlorophyll a and chlorophyll b. An increase in the chlorophyll content in the leaves after the application of extracts from seaweeds was reported by Blunden et al., (1996); however, a negative effect on this trait was recorded by Venkataraman Kumar and Mohan, (1997). More field applications are needed to provide practical evidence on the role of seaweed extracts to growth and development of maize.
In conclusion, the application of ULAE as a foliar spray could be an effective technique to improve the growth of Zea mays.
- Abouseadaa, H. H., Osman, G.H., Ramadan, A. M., Hassanein, S. E., Abdelsattar, M. T., Morsy, Y. B., Alameldin, H. F., El-Ghareeb, D. K., Nour-Eldin, H .A., Salem, R., Gad. A. A., S, Elkhodary, E., Shehata, M. M., Mahfouz, H. M., Eissa, H. F., Bahieldin, A.,. Development of transgenic wheat (Triticum aestivum L.) expressing avidin gene conferring resistance to stored product insects. BMC Plant Biology, 2015;15:183-190.
- Abdel Latif, A., Osman, G.,. Comparison of three genomic DNA extraction methods to obtain high DNA quality from maize. Plant Methods, 2017;13: (1),1-9.
- Abdel Khalik, k., Osman, G., Al-Amoudi, W.,. Genetic diversity and relationships of some Ipomoea species based on analysis of RAPD-PCR and SDS-PAGE of seed proteins. AJCS, 2012; 6: (6), 1088-1093.
- Abdel Khalik, K., Osman, G.,. Genetic analysis of Plectranthus L. (Lamiaceae) in Saudi Arabia based on RAPD and ISSR markers. Pakistan Journal of Botany, 2017;49: (3), 1073-1084.
- Alothyqic, N., Almalki, M., Ebqa’ai, M., Alsamiri, H., Rashdi, M., Ibraheem, F ., Osman, G.,. In Vitro Antibacterial Activity of four Saudi Medicinal Plants. Journal of Microbial & Biochemical Technology, 2016; 8:(2), 83-89.
- Blunden, G., Jenkins, T., Liu, Y.,. Enhanced chlorophyll levels in plants treated with seaweed extract. J. Appl. Phycol, 1996; 8: 535–543.
- AOAC. Official methods of analysis of the AOAC, 15th ed. Methods 932.06, 925.09, 985.29, 923.03. Association of official analytical chemists. Arlington, VA, USA, 1990.
- Cluzet, S., Torregrossa, C., Jacquet, C., Lafitte, C., Fournier, J., Mercier, L., Salamagne, S., Briand, X., Esquerre-Tugaye, M., Dumas, B.,. Gene expression profiling and protection of Medicago truncatula against a fungal infection in response to an elicitor from green algae Ulva spp. Plant Cell and Environ, 2004;27: 917–928.
- Crafts-Brandner, S., Salvucci, M., Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol, 2002;129: 1773–1780.
- Dubey, A., Evolution of cost effective organic fertilizers. Research & Development Centre, Kilpest India Ltd., Govindpura, Bhopal, 462023, (M.P), India, 2010.
- Eissa, H., Hassanien, S. E., Ramadan, A. M., El Shamy, M. M., Saleh, O. M., Shokry, A. M., Abdelsattar, M., Morsy, Y. B., El Maghraby, M. A., Alameldin, H.F., Hassan, S. M., Osman, G.H., Mahfouz, H. T., Gad El Karim, G. A., Madkour, M. A., Bahieldin, A.,. Developing transgenic wheat to encounter rusts and powdery mildew by overexpressing barley chi26 gene for fungal resistance. Plant Methods, 2017;13: :41-48.
- Featonby-Smith, B., van Stadn, J.,. The effect of seaweed concentrate on the growth of tomato plants in nematode-infested soil. Sci Hortic, 1983; 20: 137–146.
- Ganapathy, S., Sivakumar, H.,. Effect of foliar spray from seaweed liquid fertilizer of Ulva reticulata (Forsk.) on Vigna mungo L. and their elemental composition using SEM- energy dispersive spectroscopic analysis. Asian Pacific Journal of Reproduction, 2013; 2:(2), 119–125.
- Hammond, E., Andrews, T., Mott, K., Woodrow, I.,. Regulation of Rubisco activation in antisense plants of tobacco containing reduced levels of Rubisco activase. Plant J, 1998;14: 101–110.
- Hartree, E.,. Determination of protein; A modification of the Lowry method that gives a linear photometric response. Anal. Biochem, 1972;4: 422–427.
- Hatch, M.,. The C4 pathway of photosynthesis. Evidence for an intermediate pool of carbon dioxide and the identity of the donor C4-dicarboxylic acid. Biochem J, 1971;125: 425–431
- Hernández-Herrera, R., Santacruz-Ruvalcaba, F., Ruiz-López, M., NorrieJ, Hernández-Carmona, G.,. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J Appl Phycol, 2013;26: 619–628
- Humphries, E.,.Mineral Components and Ash Analysis Modern Methods of Plant Analysis; 1956:468-502
- Kocira, A., Kornas, R., Kocira, S.,. Effect assessment of Kelpak sl on the Bean yield. Journal of Central European Agriculture, 2013;14:(2), 67–76.
- Lahaye, M ., Robic, A.,. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules, 2007;8:1765–1774.
- Louis, Leitao., Jean-Jose, Maoret., Jean-Philippe. Biolley.,. Changes in PEP Carboxylase, Rubisco and Rubisco activase mRNA levels from maize (Zea mays) exposed to a chronic ozone stress. Biol Res. 2007;40: 137-153.
- Mate, C., Hudson, G., von Caemmerer , S., Evans, J.,. Reduction of Rubisco activase levels in tobacco (Nicotiana tabacum) by antisense RNA reduces Rubisco carbamylation and impairs photosynthesis. Plant Physiol, 1993;102: 1119–1128
- Msuya, F., Neori, A., Ulva reticulata and Gracilaria crassa: macroalgae that can biofilter effluent from tidal fishponds in Tanzania. West. Indian Ocean J. Mar. Sci. 2002;1: 117–126.
- Moran, R.,. Formulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol, 1982; 69:1376–1381.
- Mulbry, W., Westhead, K., Pizarro, C., Sikora, L.,. Recycling of manure nutrients: use of algal biomass from dairy manure treatment as a slow release fertilizer. Bioresource Technol, 2005; 96: 451–458.
- Munshi, A., Osman, G.,. Investigation on molecular phylogeny of some date palm (Phoenix dactylifra L.) cultivars by protein, RAPD and ISSR markers in Saudi Arabia. AJCS, 2010; 4: (1), 23- 28.
- Pengzhan, Y., Quanbin, Z., Ning, L., Zuhong, X., Yanmei, W., Zhi’en, L.,. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol, 2003;15: 21–27.
- Porra, R. J.,. Recent advances and re-assessments in chlorophyll extraction and assay procedures for terrestrial, aquatic, and marine organisms, including recalcitrant algae. In: Scheer H (ed) Chlorophylls, pp 31–57. CRC Press, Boca Raton, Florida,1991.
- Portis, A.,. Regulation of ribulose 1,5-bisphosphate carboxylase oxygenase activity. Annu Rev Plant Physiol, 1992; 43: 415–437
- Portis, A., Li, C., Wang, D., Salvucci, M.,. Regulation of Rubisco activase and its interaction with Rubisco. J Exp Bot, 2008; 59: 1597–1604.
- Portis, A.,. Rubisco activase: Rubisco’s catalytic chaperone. Photosynth Res. 2003;75: 11–27.
- Rathore, S.,. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. South African Journal of Botany. 2009; 75: 351–355.
- Ristic, Z., Momilovi, I., Bukovnik, U. , Prasad, P., Fu, J., Deridder, B., Elthon, T., Mladenov, N.,. Rubisco activase and wheat productivity under heat-stress conditions. J Exp Bot. 2009; 60: 4003–4014
- Osman, G., Munshi, A., Altf, F., Mutawie, H.,. Genetic variation and relationships of Zea mays and Sorghum species using RAPD-PCR and SDS-PAGE of seed proteins. Afr. J. Biotechnol, 2013; 12:(27), 4269-4276.
- Osman, G., Assem, S. K., Alreedy, R. M., El-Ghareeb, D. K., Basry, M. A., Rastogi, A ., Kalaji, H. M.,. Development of insect resistance maize plants expressing the chitinase gene of Spodoptera littoralis. Scientific Reports, 2015, 5:18067 | DOI: 10.1038/srep18067.
- Salvucci, M., Portis, A., ogren, W.,. A soluble chloroplast protein catalyzes ribulosebisphosphate carboxylase/oxygenase activation in vivo. Photosynth Res, 1985; 7: 191–203.
- Sivasankari, S.,. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresource Technology. 2006; 97:1745–1751.
- Sridhar, S., Rengasamy, R.,. Potential of Seaweed Liquid Fertilizers (SLFS) on Some Agricultural Crop with Special Reference to Protein Profile of Seedlings. International Journal of Development Research, 2011; 1:(7), 055–057.
- Vargas-Suárez, M., Ayala-Ochoa, A., Lozano-Franco, J., García-Torres, I., Díaz-Quiñonez, A., Ortíz-Navarrete, V., Sánchez-de-Jiménez, E.,. Rubisco activase chaperone activity is regulated by a post-translational mechanism in maize leaves. J Exp Bot , 2004; 55: 2533–2539.
- Venkataraman, K., Mohan, V.,. Effect of seaweed liquid fertiliser on black gram. Phykos, 19973; 6(2):43–47.
- William, I., Bloom, P.,. Extinction Coefficients of Chlorophyll a and b in N,N-Dimethylformamide and 80% Acetone . Plant Physiol. 1985; 77(2): 483–485.
- Zar, J.,. Biostatistical analysis. Second ed. Prentice-Hall, Inc., Englewood Cliffs, NJ. 718 pp, 1984.
- Zodape, S., Gupta, A., Bhandari, S.,. Foliar application of seaweed sap as biostimulant for enhancement of yield andquality of tomato (Lycopersicon esculentum Mill.). J Sci Ind Res, 2011;70: 215–219.
- Bahieldin Ahmed, Ahmed Atef, Sherif Edris1, Nour O. Gadalla, Ahmed M. Ramadan,Sabah M. HassanSanaa G. Al Attas, Magdy A. Al-Kordy, Abdulrahman S. M. Al-Hajar,Jamal S. M. Sabir, Mahmoud E. Nasr, Gamal H. Osman , Fotouh M. El-Domyati. Multifunctional activities of ERF109 as affected by salt stress in Arabidopsis. Scientific Reports, 2018; 8:6403
- El-Helow Ehab R., Reem Badr,Gamal Osman and Amani Abdel-latif, Molecular Characterization of two Maize Hybrids Based on Primer Bias. Advancin Environmental Biology. 2018;12(1): 1-5.
© The Author(s) 2018. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.


